Effects of Zn Content on Hot Tearing Susceptibility of Mg–7Gd–5Y–0.5Zr Alloy
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
3.1. Phase and Microstructure in As-Cast Condition
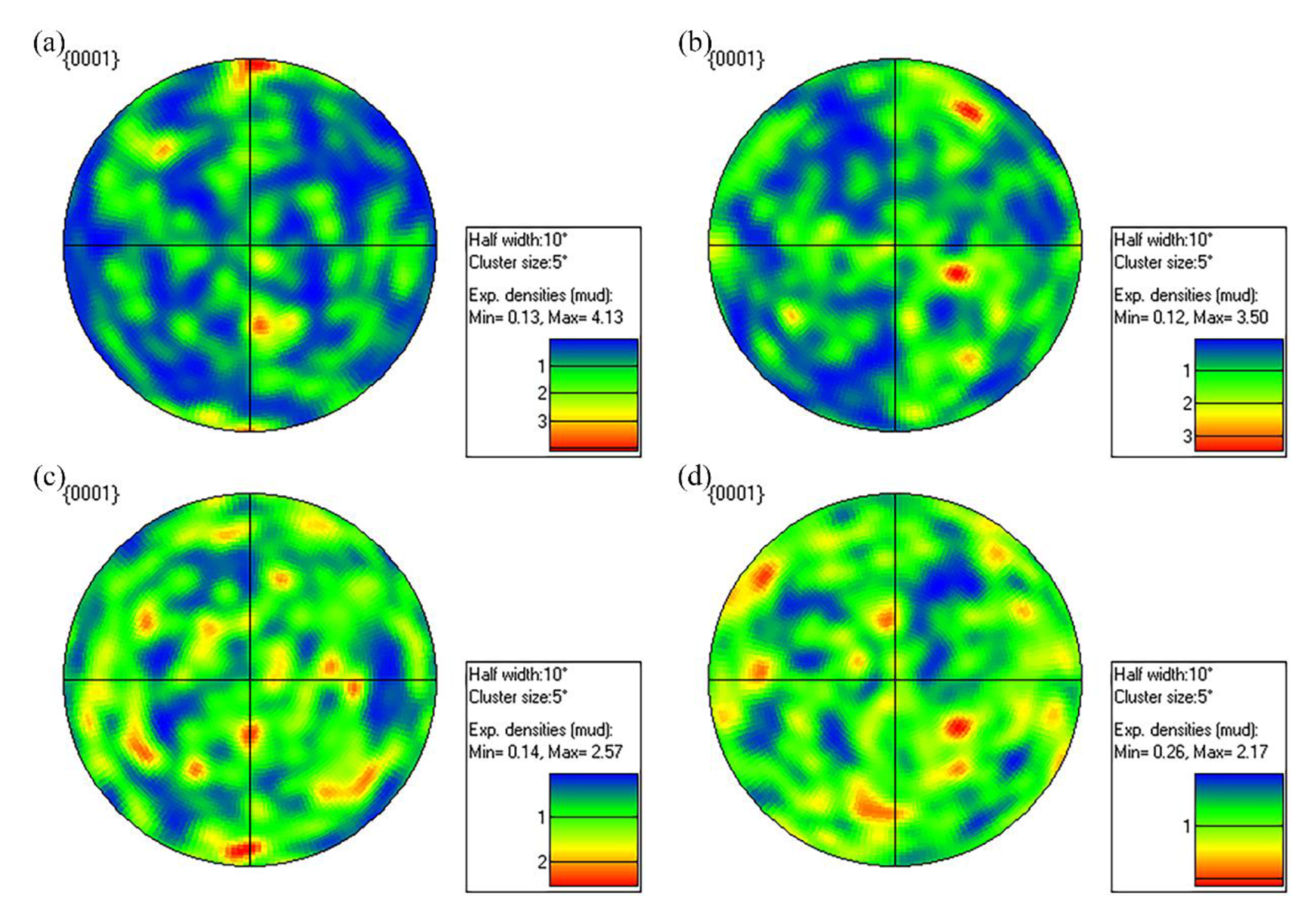
3.1.1. Phase Composition and Microstructure
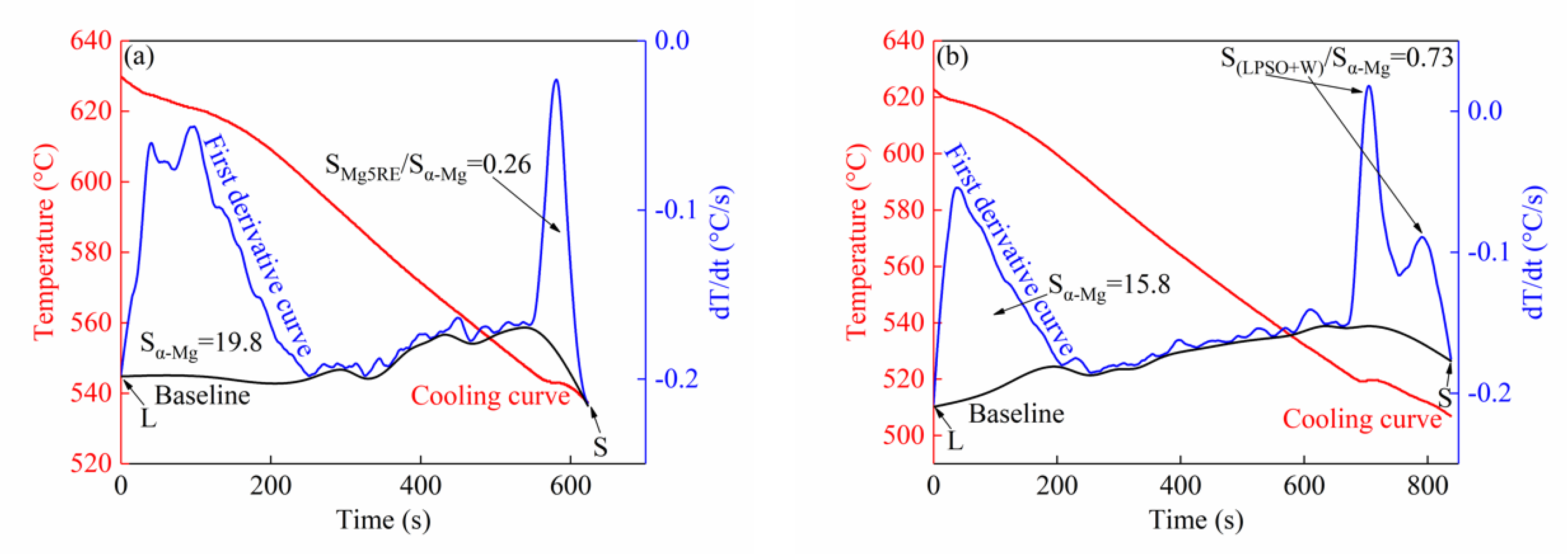
3.1.2. Thermal Analysis Results
3.2. Solidification Characteristics and Hot Tearing Susceptibility
3.2.1. Solidification Characteristics
3.2.2. Hot Tearing Behavior
3.2.3. Hot Tearing Susceptibility
3.3. Fracture Characteristics and Hot Tearing Mechanism
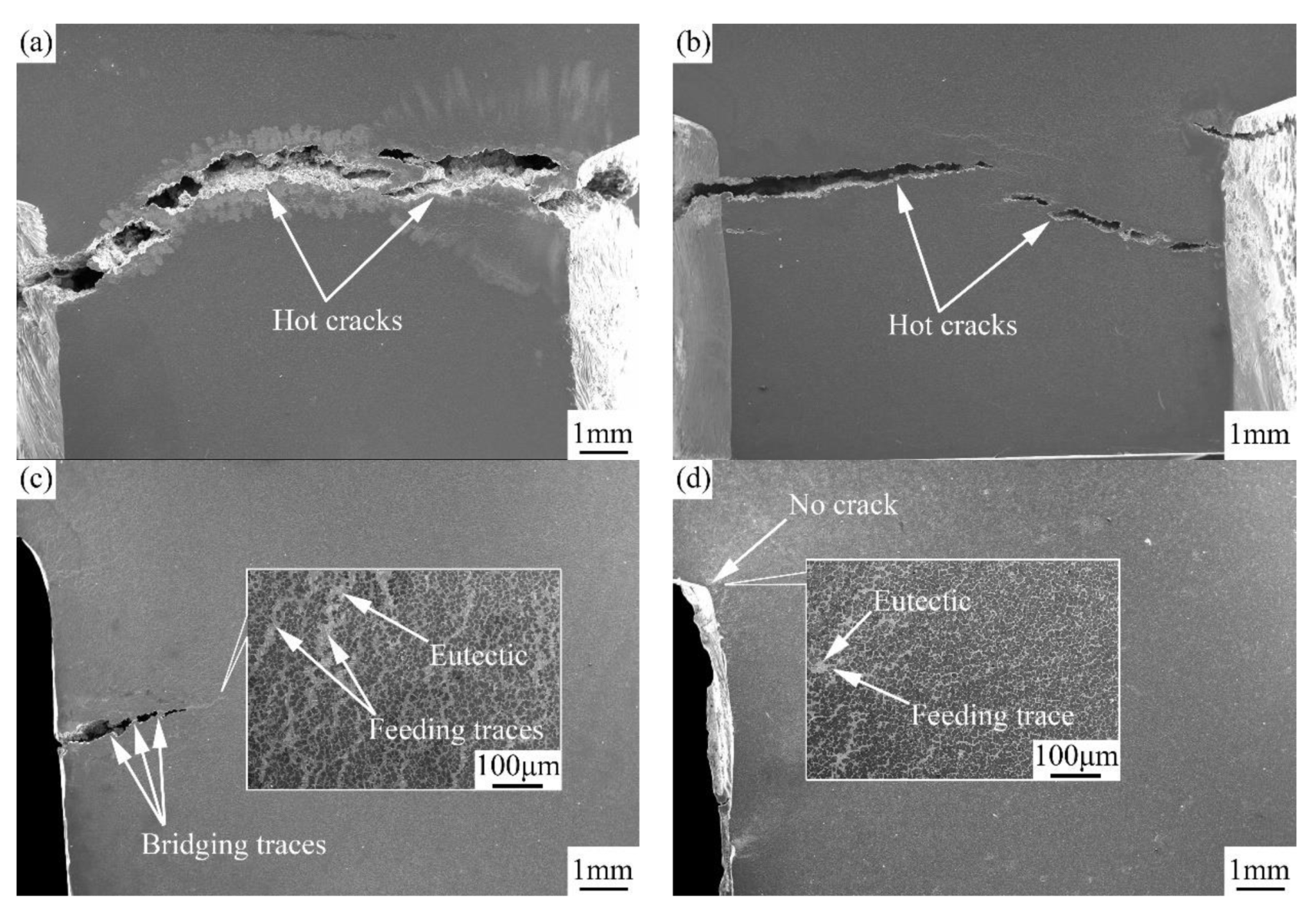
3.3.1. Fracture Characteristics
3.3.2. Effect of α-Mg Crystallization Precipitation on HTS
3.3.3. Effect of Second Phase Precipitation on HTS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, J.F.; Pan, F.S.; Jiang, B.; Atrens, A.; Zhang, M.X.; Lu, Y. A review on hot tearing of magnesium alloys. J. Magnes. Alloy. 2016, 4, 151–172. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Nakata, T.; Fan, G.H.; Li, X.W.; Tang, G.Z.; Kamado, S. Enhancing strength and creep resistance of Mg–Gd–Y–Zn–Zr alloy by substituting Mn for Zr. J. Magnes. Alloy. 2019, 7, 388–399. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.F.; Duan, Y.B.; Wang, K.J.; Wang, Y.T.; Zhang, W.J.; Hu, J. Precipitation processes during the peak-aged and over-aged stages in an Mg–Gd–Y–Zr alloy. J. Alloy. Compd. 2019, 788, 541–548. [Google Scholar] [CrossRef]
- You, C.; Liu, C.M.; Wan, Y.C.; Tang, B.; Wang, B.Z.; Gao, Y.H.; Han, X.Z. Dislocations-induced precipitates and their effect on mechanical properties of Mg–Gd–Y–Zr alloy. J. Magnes. Alloy. 2019, 7, 414–418. [Google Scholar] [CrossRef]
- Xu, C.; Nakata, T.; Fan, G.H.; Li, X.W.; Tang, G.Z.; Geng, L.; Kamado, S. Microstructure and mechanical properties of extruded Mg–Gd–Y–Zn alloy with Mn or Zr addition. J. Mater. Sci. 2019, 54, 10473–10488. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, L.; Wu, G.H.; Liu, W.C.; Ding, W.J. Effect of Y and Gd content on the microstructure and mechanical properties of Mg–Y–RE alloys. J. Magnes. Alloy. 2019, 7, 345–354. [Google Scholar] [CrossRef]
- Li, J.L.; Wu, D.; Chen, R.S.; Han, E.H. Anomalous effects of strain rate on the room-temperature ductility of a cast Mg–Gd–Y–Zr alloy. Acta Mater. 2018, 159, 31–45. [Google Scholar] [CrossRef]
- Ding, Z.B.; Zhao, Y.H.; Lu, R.P.; Yuan, M.N.; Wang, Z.J.; Li, H.J.; Hou, H. Effect of Zn addition on microstructure and mechanical properties of cast Mg–Gd–Y–Zr alloys. Trans. Nonferrous Met. Soc. China 2019, 29, 722–734. [Google Scholar] [CrossRef]
- Li, J.L.; Chen, R.S.; Ma, Y.Q.; Ke, W. Computer-aided cooling curve thermal analysis and microstructural characterization of Mg–Gd–Y–Zr system alloys. Thermochim. Acta 2014, 590, 232–241. [Google Scholar] [CrossRef]
- Luo, S.F.; Yang, G.Y.; Zou, Z.; Ouyang, S.X.; Xiao, L.; Jie, W.Q. Hot tearing susceptibility of binary Mg–Gd alloy castings and influence of grain refinement. Adv. Eng. Mater. 2018, 20, 1800139. [Google Scholar] [CrossRef]
- Srinivasan, A.; Wang, Z.; Huang, Y.D.; Beckmann, F.; Kainer, K.U.; Hort, N. Hot tearing characteristics of binary Mg–Gd alloy castings. Metall. Mater. Trans. A 2013, 44, 2285–2298. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, Y.D.; Srinivasan, A.; Liu, Z.; Beckmann, F.; Kainer, K.U.; Hort, N. Hot tearing susceptibility of binary Mg–Y alloy castings. Mater. Des. 2013, 47, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Wang, C.Q.; Zhang, Z.M. Microstructures, corrosion and mechanical properties of as-cast Mg–Zn–Y–(Gd) alloys. Trans. Nonferrous Met. Soc. China 2015, 25, 2172–2180. [Google Scholar] [CrossRef]
- Shao, J.B.; Chen, Z.Y.; Chen, T.; Hu, Z.; Zhou, X.J.; Liu, C.M. The effect of LPSO on the deformation mechanism of Mg–Gd–Y–Zn–Zr magnesium alloy. J. Magnes. Alloy. 2016, 4, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wang, J.F.; Huang, S.; Gao, S.Q.; Guo, S.F.; Liu, S.J.; Chen, X.H.; Pan, F.S. Enhanced mechanical properties of Mg–Gd–Y–Zn–Mn alloy by tailoring the morphology of long period stacking ordered phase. Mater. Sci. Eng. A 2018, 733, 267–275. [Google Scholar] [CrossRef]
- Xu, C.; Nakata, T.; Qiao, X.G.; Zheng, M.Y.; Wu, K.; Kamado, S. Effect of LPSO and SFs on microstructure evolution and mechanical properties of Mg–Gd–Y–Zn–Zr alloy. Sci. Rep. 2017, 7, 40846. [Google Scholar] [CrossRef]
- Zhou, X.J.; Liu, C.M.; Gao, Y.H.; Jiang, S.N.; Liu, W.H.; Lu, L.W. Microstructure and mechanical properties of extruded Mg–Gd–Y–Zn–Zr alloys filled with intragranular LPSO phases. Mater. Charact. 2018, 135, 76–83. [Google Scholar] [CrossRef]
- Chi, Y.Q.; Xu, C.; Qiao, X.G.; Zheng, M.Y. Effect of trace zinc on the microstructure and mechanical properties of extruded Mg–Gd–Y–Zr alloy. J. Alloy. Compd. 2019, 789, 416–427. [Google Scholar] [CrossRef]
- Wang, Y.F.; Zhang, F.; Wang, Y.T.; Duan, Y.B.; Wang, K.J.; Zhang, W.J.; Hu, J. Effect of Zn content on the microstructure and mechanical properties of Mg–Gd–Y–Zr alloys. Mater. Sci. Eng. A 2019, 745, 149–158. [Google Scholar] [CrossRef]
- Liu, J.H.; Han, E.H.; Song, Y.W.; Shan, D.Y. Effect of twins on the corrosion behavior of Mg–5Y–7Gd–1Nd–0.5Zr Mg alloy. J. Alloy. Compd. 2018, 757, 356–363. [Google Scholar] [CrossRef]
- Liu, J.H.; Song, Y.W.; Chen, J.C.; Chen, P.; Shan, D.Y.; Hou, E.H. The special role of anodic second phases in the micro-galvanic corrosion of EW75 Mg alloy. Electrochim. Acta 2016, 189, 190–195. [Google Scholar] [CrossRef]
- Liu, J.H.; Song, Y.W.; Shan, D.Y.; Hou, E.H. Different microgalvanic corrosion behavior of cast and extruded EW75 Mg alloys. J. Electrochem. Soc. 2016, 163, C856–C863. [Google Scholar] [CrossRef] [Green Version]
- Zhu, R.L.; Jin, Z.; Wen, G. Effect of silane on galvanic corrosion between EW75 Magnesium alloy and TC4 alloy. Rare Metal Mat. Eng. 2015, 44, 1838–1844. [Google Scholar] [CrossRef]
- Gunde, P.; Schiffl, A.; Uggowitzer, P.J. Influence of yttrium additions on the hot tearing susceptibility of magnesium-zinc alloys. Mater. Sci. Eng. A 2010, 527, 7074–7079. [Google Scholar] [CrossRef]
- Bichler, L.; Ravindran, C. New developments in assessing hot tearing in magnesium alloy castings. Mater. Des. 2010, 31, S17–S23. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Liu, Z.; Wang, Y.; Li, X.X. Hot tearing behavior and microstructure mechanism of Mg–6.5Zn–xY–0.5Zr alloys. Mater. Res. Express 2019, 6, 076570. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.B.; Mao, P.L.; Wang, F. Effects of Y on hot tearing mechanism of Mg–Zn–Y–Zr alloys. Mater. Sci. Technol. 2014, 30, 1214–1222. [Google Scholar] [CrossRef]
- Cruz, H.; Gonzalez, C.; Juárez, A.; Herrera, M.; Juarez, J. Quantification of the microconstituents formed during solidification by the newton thermal analysis method. J. Mater. Process Technol. 2006, 178, 128–134. [Google Scholar] [CrossRef]
- Huang, Z.H.; Liang, S.M.; Chen, R.S.; Han, E.H. Solidification pathways and constituent phases of Mg–Zn–Y–Zr alloys. J. Alloy. Compd. 2009, 468, 170–178. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Wang, Y.; Liu, Z. Effects of Zn and Y on hot-tearing susceptibility of Mg–xZn–2xY alloys. Mater. Sci. Technol. 2018, 34, 2001–2007. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.B.; Mao, P.L.; Wang, F. Effects of Y on hot tearing susceptibility of Mg–Zn–Y–Zr alloys. Trans. Nonferrous Met. Soc. China 2014, 24, 907–914. [Google Scholar] [CrossRef]
- Clyne, T.W.; Davies, G.J. The influence of composition on solidification cracking susceptibility in binary alloys. Brit. Found. 1981, 74, 65–73. [Google Scholar]
- Clyne, T.W.; Davies, G.J. A quantitative solidification cracking test for castings and an evaluation of cracking in aluminium–magnesium alloys. Brit. Found. 1975, 68, 238–244. [Google Scholar]
- Lee, J.Y.; Kim, D.H.; Lim, H.K.; Kim, D.H. Effects of Zn/Y ratio on microstructure and mechanical properties of Mg–Zn–Y alloys. Mater. Lett. 2005, 59, 3801–3805. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Liu, Z.; Sheng, X.F.; Wang, Y.; Zhang, Z.L.; Ju, Y.D. Effects of Y and Zn/Y on hot tearing susceptibility of Mg–Zn–Y–Zr alloys. Mater. Sci. Technol. 2019, 35, 1872–1882. [Google Scholar] [CrossRef]
- Davis, T.A.; Bichler, L.; D’Elia, F.; Hort, N. Effect of TiBor on the grain refinement and hot tearing susceptibility of AZ91D magnesium alloy. J. Alloy. Compd. 2018, 759, 70–79. [Google Scholar] [CrossRef]
- Wei, L.Y.; Dunlop, G.L. The solidification behavior of Mg–Al–rare earth alloys. J. Alloy. Compd. 1996, 232, 264–268. [Google Scholar] [CrossRef]
- Arnberg, L.; Chai, G.; Backerud, L. Determination of dendritic coherency in solidifying melts by rheological measurements. Mater. Sci. Eng. A 1993, 173, 101–103. [Google Scholar] [CrossRef]
- Zhang, G.J.; Wang, Y.; Liu, Z.; Liu, S.M. Influence of Al addition on solidification path and hot tearing susceptibility of Mg–2Zn–(3+0.5x)Y–xAl alloys. J. Magnes. Alloy. 2019, 7, 272–282. [Google Scholar] [CrossRef]
- Hao, H.; Maijer, D.M.; Wells, M.A.; Phillion, A.; Cockcroft, S.L. Modeling the stress-strain behavior and hot tearing during direct chill casting of an AZ31 magnesium billet. Metall. Metal. Trans. A 2010, 41, 2067–2077. [Google Scholar] [CrossRef]
- Zhong, H.G.; Li, X.H.; Wang, B.; Wu, T.M.; Zhang, Y.H.; Liu, B.; Zhai, Q.J. Hot tearing of 9Cr3Co3W heat-resistant steel during solidification. Metals 2019, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Li, Y.Z.; Wang, F.; Huang, Y.D.; Song, J.F.; Mao, P.L.; Liu, Z. Hot tearing susceptibility of Mg–xZn–2Y alloys. Trans. Nonferrous Met. Soc. China 2016, 26, 3115–3211. [Google Scholar] [CrossRef]












| Alloy No. | Alloy Composition | Mg | Gd | Y | Zn | Zr |
|---|---|---|---|---|---|---|
| Alloy I | Mg–7Gd–5Y–0.5Zr | Bal. | 7.12 | 4.98 | 0 | 0.45 |
| Alloy II | Mg–7Gd–5Y–3Zn–0.5Zr | Bal. | 7.10 | 4.82 | 3.21 | 0.48 |
| Alloy III | Mg–7Gd–5Y–5Zn–0.5Zr | Bal. | 7.06 | 4.94 | 4.89 | 0.44 |
| Alloy IV | Mg–7Gd–5Y–7Zn–0.5Zr | Bal. | 7.18 | 5.29 | 6.94 | 0.46 |
| Alloy | L→α-Mg | L→α-Mg + Mg5RE | L→α-Mg + LPSO | L→α-Mg + W | Ts |
|---|---|---|---|---|---|
| Alloy I | 630 | 547 | —— | —— | 537 |
| Alloy II | 623 | —— | 523 | 517 | 507 |
| Alloy III | 619 | —— | 516 | 510 | 492 |
| Alloy IV | 619 | —— | —— | 519 | 509 |
| Alloy | Hot Tearing Initiation Time (s) | Hot Tearing Propagation End Time (s) | Hot Tearing Propagation Time Range (s) | Hot Tearing Initiation Temperature (°C) | Hot Tearing Propagation End Temperature (°C) |
|---|---|---|---|---|---|
| Alloy I | 11.6/0.65 | 14.1/0.17 | 2.5/0.62 | 568/3.86 | 546/0.94 |
| Alloy II | 16.6/0.08 | 18.4/0.29 | 1.8/0.24 | 519/0.94 | 514/2.16 |
| Alloy III | 32.5/0.29 | 32.8/0.22 | 0.3/0.17 | 499/3.10 | 498/2.49 |
| Alloy No. | Lattice Constants | ||
|---|---|---|---|
| a (nm) | c (nm) | c/a | |
| Alloy I | 0.32209 | 0.52116 | 1.61806 |
| Alloy II | 0.32162 | 0.52085 | 1.61946 |
| Alloy III | 0.32146 | 0.52081 | 1.62014 |
| Alloy IV | 0.32121 | 0.52078 | 1.62131 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Liu, S.; Liu, Z.; Wang, F.; Mao, P.; Wang, X.; Li, X. Effects of Zn Content on Hot Tearing Susceptibility of Mg–7Gd–5Y–0.5Zr Alloy. Metals 2020, 10, 414. https://doi.org/10.3390/met10030414
Wei Z, Liu S, Liu Z, Wang F, Mao P, Wang X, Li X. Effects of Zn Content on Hot Tearing Susceptibility of Mg–7Gd–5Y–0.5Zr Alloy. Metals. 2020; 10(3):414. https://doi.org/10.3390/met10030414
Chicago/Turabian StyleWei, Ziqi, Shimeng Liu, Zheng Liu, Feng Wang, Pingli Mao, Xiaoxia Wang, and Xingxing Li. 2020. "Effects of Zn Content on Hot Tearing Susceptibility of Mg–7Gd–5Y–0.5Zr Alloy" Metals 10, no. 3: 414. https://doi.org/10.3390/met10030414
APA StyleWei, Z., Liu, S., Liu, Z., Wang, F., Mao, P., Wang, X., & Li, X. (2020). Effects of Zn Content on Hot Tearing Susceptibility of Mg–7Gd–5Y–0.5Zr Alloy. Metals, 10(3), 414. https://doi.org/10.3390/met10030414





