Characterization of Influences of Steel-Aluminum Dissimilar Joints with Intermediate Zinc Layer
Abstract
:1. Introduction
2. Materials and Experimental Procedure
3. Phase Classification and Results of Tensile Testing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bian, J.; Zhu, Y.; Liu, X.-H.; Wang, G.-d. Development of Hot Dip Galvanized Steel Strip and Its Application in Automobile Industry. J. Iron Steel Res. Int. 2006, 13, 47–50. [Google Scholar] [CrossRef]
- Radscheit, C.R. Laserstrahlfügen von Aluminium mit Stahl; BIAS-Verlag: Bremen, Germany, 1997. [Google Scholar]
- Heumann, T.; Dittrich, S. Über die Kinetik der Reaktion von festem und flüssigem Aluminium mit Eisen. Zeitschrift für Metallkunde 1959, 50, 617–625. [Google Scholar]
- Li, Y.; Liu, Y.; Yang, J. First principle calculations and mechanical properties of the intermetallic compounds in a laser welded steel/aluminum joint. Opt. Laser Technol. 2020, 122, 105875. [Google Scholar] [CrossRef]
- Hoffmann, H.R. Über das Verhalten von Zink und Zink-Kupfer-Titan-Aluminium-Legierungen beim Kriechen unter hohen. Lasten. Dissertation, Technischen Universität Berlin, Berlin, Germany, 1971. [Google Scholar]
- Goecke, S.-F. Energiereduziertes Lichtbogen-Fügeverfahren für Wärmeempfindliche Werkstoffe. In Proceedings of the Schweißen und Schneiden 2005—Vorträge der gleichnamigen Großen Schweißtechnischen Tagung, Essen, Germany, 12–14 September 2005; pp. 44–48. [Google Scholar]
- Jank, N.; Staufer, H.; Bruckner, J. Schweißverbindungen von Stahl mit Aluminium—eine Perspektive für die Zukunft. Berg Hüttenmännische Monatshefte 2008, 153, 189–192. [Google Scholar] [CrossRef]
- Yuce, C.; Karpat, F.; Yavuz, N. Investigations on the microstructure and mechanical properties of laser welded dissimilar galvanized steel–Aluminum joints. Int. J. Adv. Manuf. Technol. 2019, 104, 2693–2704. [Google Scholar] [CrossRef]
- Goldmann, F.; Hahn, O.; Tetzlaff, U.; Kunze, S. Gefügemorphologie beim Widerstandspunktschweißen von Aluminium-Stahl-Verbindungen. Schweißen Schneiden 2015, 67, 238–244. [Google Scholar]
- Leuschen, B. Beitrag zum Tragverhalten von Aluminium- und Aluminium/Stahl-Widerstandspunktschweissverbindungen bei Verschiedenartiger Beanspruchung. Ph.D. Thesis, RWTH, Fak. f. Maschinenwesen, Aachen, Germany, 1984. [Google Scholar]
- Pereira, A.; Cabrinha, A.; Rocha, F.; Marques, P.; Fernandes, F.; Alves de Sousa, R. Dissimilar Metals Laser Welding between DP1000 Steel and Aluminum Alloy 1050. Metals 2019, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Guan, Q.; Long, J.; Yu, P.; Jiang, S.; Huang, W.; Zhou, J. Effect of steel to aluminum laser welding parameters on mechanical properties of weld beads. Opt. Laser Technol. 2019, 111, 387–394. [Google Scholar] [CrossRef]
- Lu, D.Q.; Cui, L.; Chen, H.X.; Chang, Y.Q.; Peng, Z.B.; He, D.Y. Laser-MIG Hybrid Keyhole Welded 6mm Steel/Aluminum Butt Joints. Mater. Sci. Forum 2019, 944, 581–592. [Google Scholar] [CrossRef]
- Matsuda, T.; Adachi, H.; Sano, T.; Yoshida, R.; Hori, H.; Ono, S.; Hirose, A. High-frequency linear friction welding of aluminum alloys to stainless steel. J. Mater. Process. Technol. 2019, 269, 45–51. [Google Scholar] [CrossRef]
- Mrzljak, S.; Gelinski, N.; Hülsbusch, D.; Schumacher, E.; Boehm, S.; Walther, F. Influence of Process Parameters, Surface Topography and Corrosion Condition on the Fatigue Behavior of Steel/Aluminum Hybrid Joints Produced by Magnetic Pulse Welding. Key Eng. Mater. 2019, 809, 197–202. [Google Scholar] [CrossRef]
- Kashani, H.T.; Kah, P.; Martikainen, J. Laser Overlap Welding of Zinc-coated Steel on Aluminum Alloy. Phys. Procedia 2015, 78, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Köster, M.; Schuhmacher, B.; Sommer, D. The influence of the zinc content on the lattice constants and structure of the intermetallic compound Fe2Al5. Steel Res. 2001, 72, 371–375. [Google Scholar] [CrossRef]
- Meco, S.; Ganguly, S.; Williams, S.; McPherson, N. Effect of Laser Processing Parameters on the Formation of Intermetallic Compounds in Fe-Al Dissimilar Welding. J. Mater. Eng. Perform. 2014, 23, 3361–3370. [Google Scholar] [CrossRef] [Green Version]
- Elrefaey, A.; Gouda, M.; Takahashi, M.; Ikeuchi, K. Characterization of Aluminum/Steel Lap Joint by Friction Stir Welding. J. Mater. Eng. Perform. 2005, 14, 10–17. [Google Scholar] [CrossRef]
- Eichhorn, F.; Emonts, M.; Leuschen, B. Widerstandspunktschweißen der Werkstoffkombination Alumnium-Stahl. Schweißen Schneiden 1982, 34, 15–20. [Google Scholar]
- Ozaki, H.; Kutsuna, M. Laser-roll welding of a dissimilar metal joint of low carbon steel to aluminium alloy using 2 kW fibre laser. Weld. Int. 2009, 23, 345–352. [Google Scholar] [CrossRef]
- Ozaki, H.; Kutsuna, M. Dissimilar Metal Joining of Zinc Coated Steel and Aluminum Alloy by Laser Roll Welding. In Welding Processes; Kovacevic, R., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0854-2. [Google Scholar]
- Pardal, G.; Meco, S.; Ganguly, S.; Williams, S.; Prangnell, P. Dissimilar metal laser spot joining of steel to aluminium in conduction mode. Int. J. Adv. Manuf. Technol. 2014, 73, 365–373. [Google Scholar] [CrossRef]
- Engelbrecht, L.; Meier, O.; Ostendorf, A.; Haferkamp, H. Einflüsse auf die mechanischen Eigenschaften lasergelöteter Mischverbindungen aus Stahl und Aluminium. Materialwissenschaft Werkstofftechnik 2006, 37, 272–278. [Google Scholar] [CrossRef]
- Girard, M.; Huneau, B.; Genevois, C.; Sauvage, X.; Racineux, G. Friction stir diffusion bonding of dissimilar metals. Sci. Technol. Weld. Join. 2013, 15, 661–665. [Google Scholar] [CrossRef]
- Meco, S.; Cozzolino, L.; Ganguly, S.; Williams, S.; McPherson, N. Laser welding of steel to aluminium: Thermal modelling and joint strength analysis. J. Mater. Process. Technol. 2017, 247, 121–133. [Google Scholar] [CrossRef]
- Langner, J.; Stonis, M.; Behrens, B.-A. Hybridschmieden eines Druckflansches. Available online: https://www.umformtechnik.net/binary_data/3165637_hybridschmieden-langner.pdf (accessed on 9 January 2020).
- Behrens, B.-A.; Kosch, K.-G. Influence of different alloying elements on the intermetallic phase seam thickness of compound forged steel-aluminum parts. Prod. Eng. Res. Dev. 2011, 5, 517–522. [Google Scholar] [CrossRef]
- Behrens, B.-A.; Odening, D.; Holz, F.; Kosch, K.-G. Finite Elemente Modellierung der induktiven Erwärmung hybrider Stahl-Aluminium Bauteile. Available online: https://www.umformtechnik.net/binary_data/99892_fem_erw_rmung_ifum_kosch.pdf (accessed on 6 January 2020).
- Behrens, B.-A.; Kosch, K.-G. Development of the heating and forming strategy in compound forging of hybrid steel-aluminum parts. Materialwissenschaft Werkstofftechnik 2011, 42, 973–978. [Google Scholar] [CrossRef]
- Behrens, B.-A.; Holz, F. Verbundschmieden hybrider Stahl-Aluminium Bauteile. Materialwissenschaft Werkstofftechnik 2008, 39, 599–603. [Google Scholar] [CrossRef]
- Bick, T. Hybrides Verbundschmieden von Aluminium und Stahl durch Bildung einer Zinkzwischenschicht: Bestimmung der Prozesseinflussgrößen und Verbindungseigenschaften. Masterarbeit; Technische Universität Clausthal: Clausthal, Germany, 2018. [Google Scholar]
- Bick, T.; Treutler, K.; Wesling, V. Charakterisierung der Verbindungseigenschaften hybrid verbundgeschmiedeter Stahl Aluminium Mischverbindungen in Abhängigkeit der Zinkschichtzusammensetzungen; Assistentenseminar Füge- und Schweißtechnik No. 39; DVS Media: Düsseldorf, Germany, 2019. [Google Scholar]
- Hoppe, C.; Ebbert, C.; Grothe, R.; Schmidt, H.C.; Hordych, I.; Homberg, W.; Maier, H.J.; Grundmeier, G. Influence of the Surface and Heat Treatment on the Bond Strength of Galvanized Steel/Aluminum Composites Joined by Plastic Deformation. Adv. Eng. Mater. 2016, 18, 1371–1380. [Google Scholar] [CrossRef]
- Bick, T.; Treutler, K.; Wesling, V. Soldering of Steel Sheets and Zinc Coated Aluminum by Hybrid Composite Forging; 2018. [Google Scholar]
- Ghosh, G. Al-Fe-Zn Ternary Phase Diagram Evaluation. Available online: http://www.msi-eureka.com/full-html/10.17658.3.6/Al-Fe-Zn_Ternary_Phase_Diagram_Evaluation/ (accessed on 20 August 2019).
- Petrov, D.; Watson, A.; Gröbner, J.; Rogl, P.; Tedenac, J.; Bulanova, M.; Turkevich, V.; Lukas, H. Al-Mg-Zn Ternary Phase Diagram Evaluation. Available online: http://www.msi-eureka.com/full-html/10.11491.3.7/Al-Mg-Zn_Ternary_Phase_Diagram_Evaluation/ (accessed on 28 January 2020).
- Suzuki, T. Al-Si-Zn Ternary Phase Diagram Evaluation. Available online: http://www.msi-eureka.com/full-html/10.14605.1.6/Al-Si-Zn_Ternary_Phase_Diagram_Evaluation/ (accessed on 29 January 2020).
- Silvayeh, Z.; Vallant, R.; Sommitsch, C.; Götzinger, B.; Karner, W.; Hartmann, M. Influence of Filler Alloy Composition and Process Parameters on the Intermetallic Layer Thickness in Single-Sided Cold Metal Transfer Welding of Aluminum-Steel Blanks. Metall. Mater. Trans. A 2017, 48, 5376–5386. [Google Scholar] [CrossRef] [Green Version]
- Schuerz, S.; Fleischanderl, M.; Luckeneder, G.H.; Preis, K.; Haunschmied, T.; Mori, G.; Kneissl, A.C. Corrosion behaviour of Zn–Al–Mg coated steel sheet in sodium chloride-containing environment. Corros. Sci. 2009, 51, 2355–2363. [Google Scholar] [CrossRef]
- Springer, H.; Szczepaniak, A.; Raabe, D. On the role of zinc on the formation and growth of intermetallic phases during interdiffusion between steel and aluminium alloys. Acta Mater. 2015, 96, 203–211. [Google Scholar] [CrossRef]

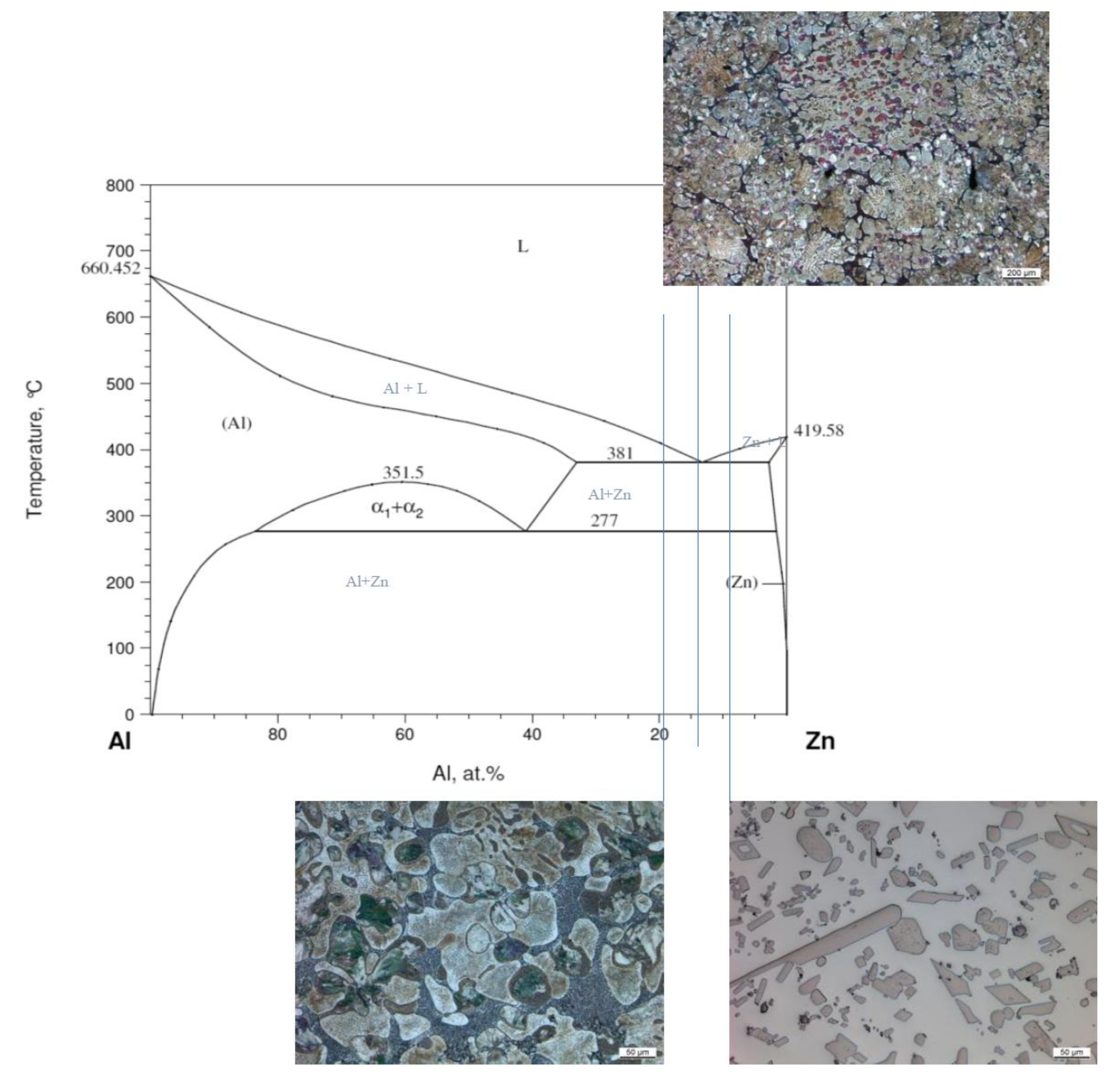
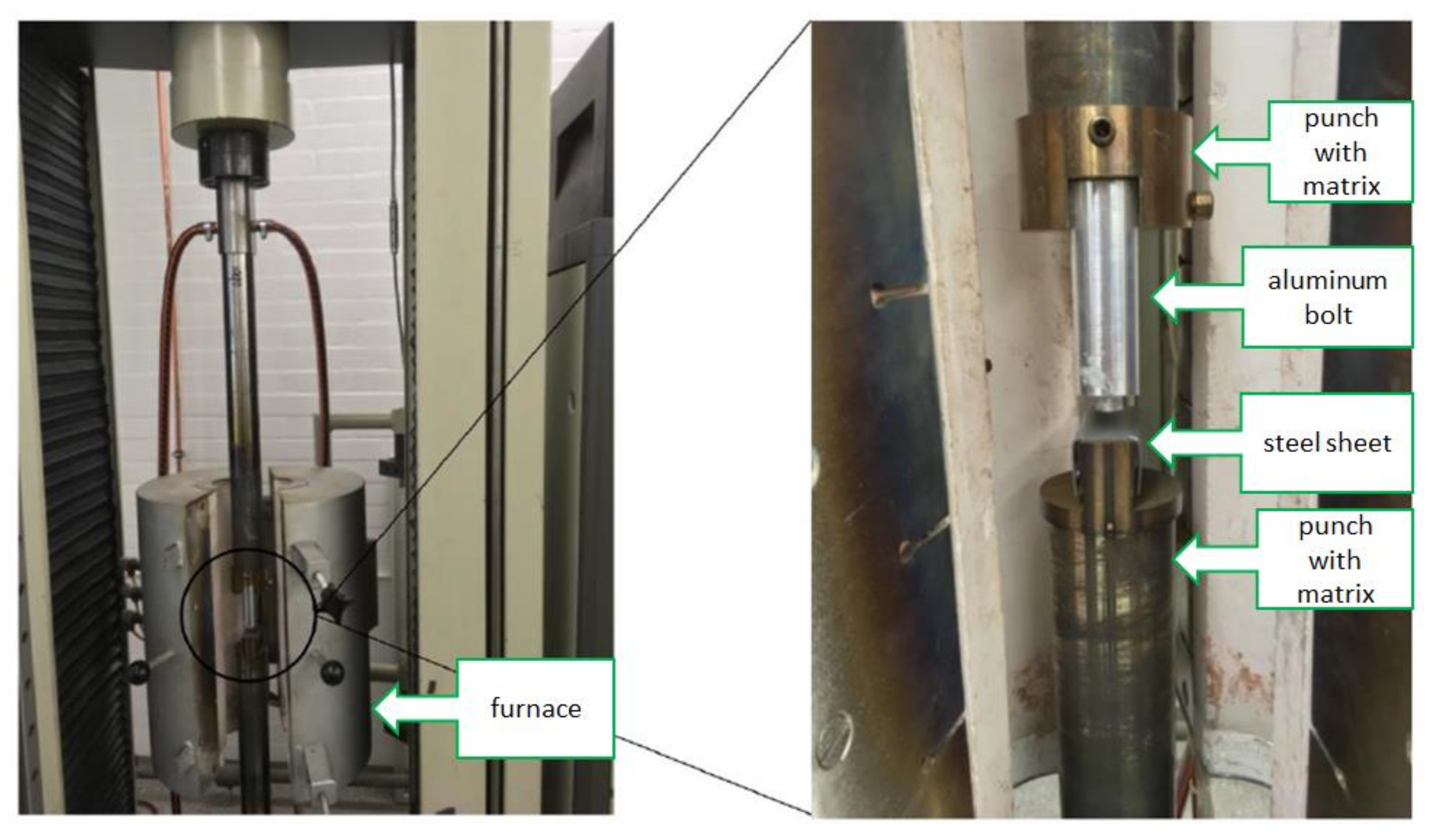
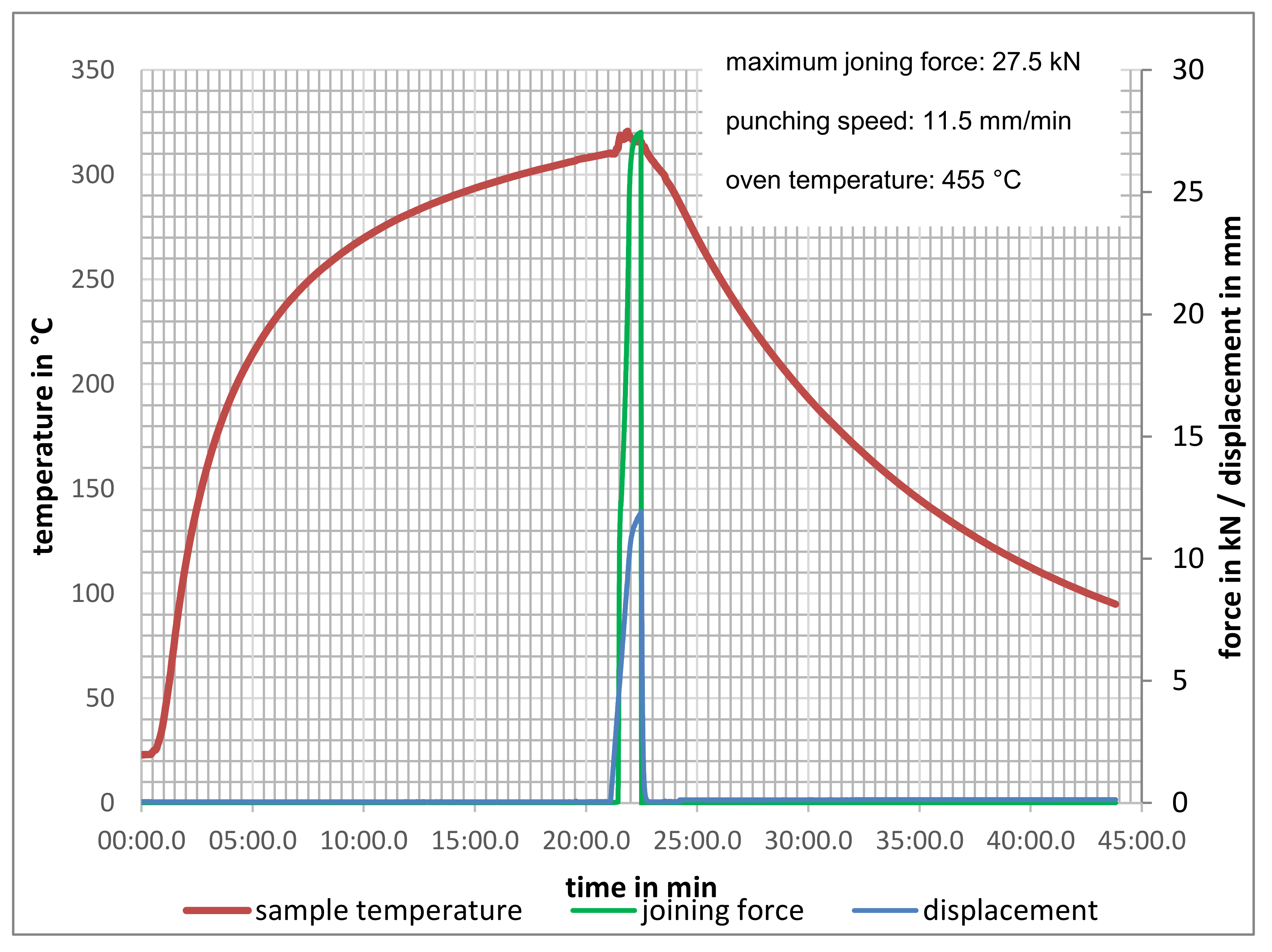
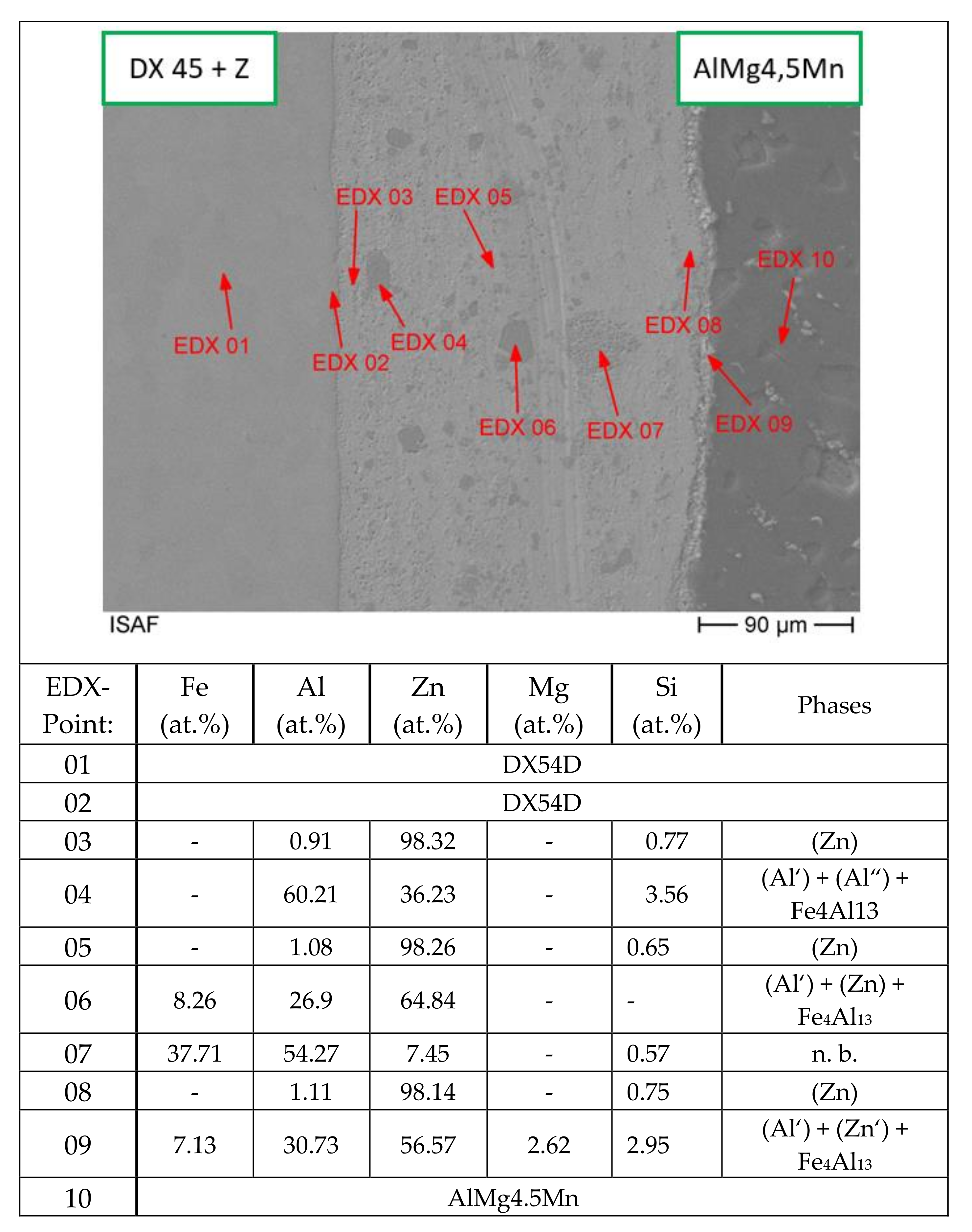
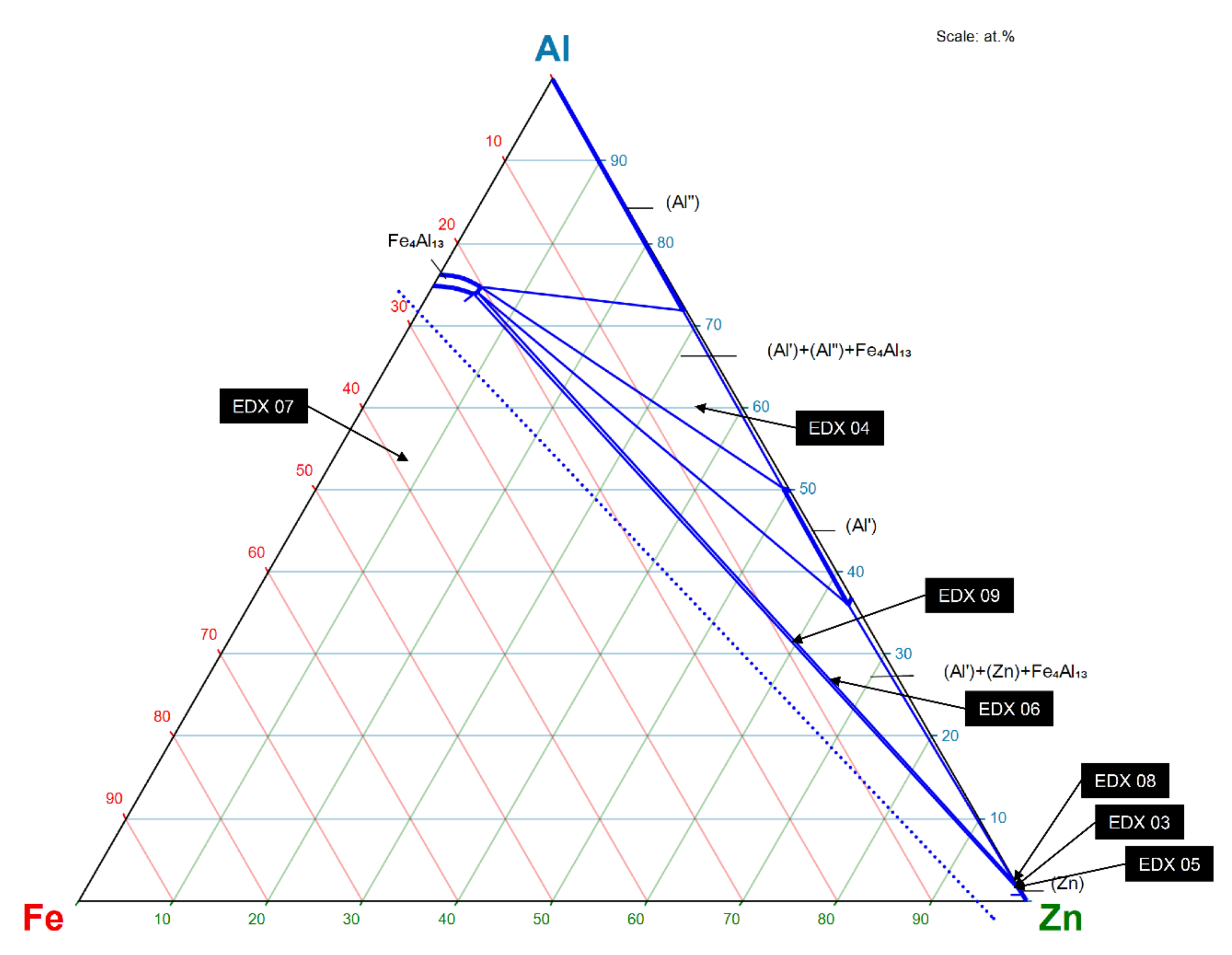

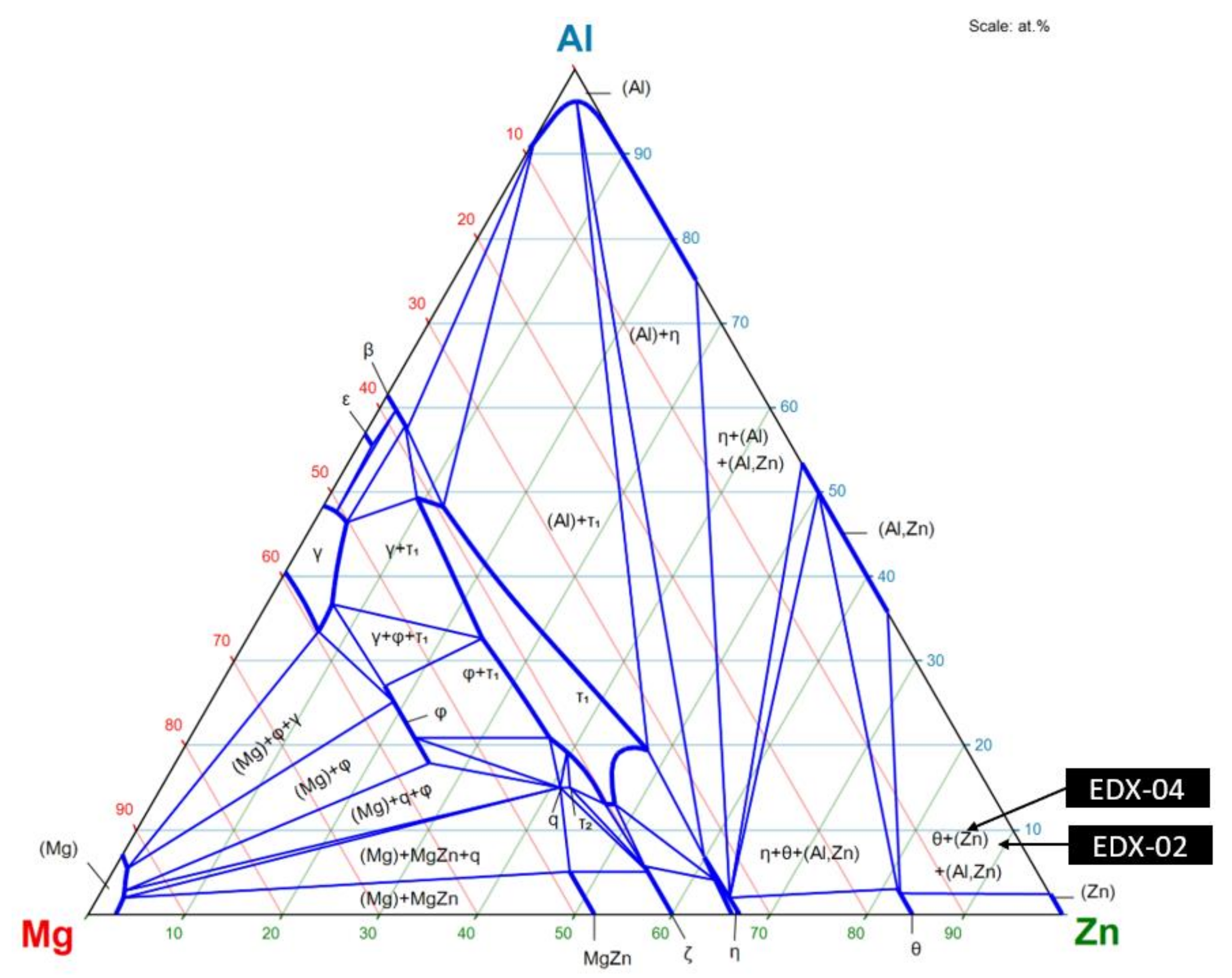
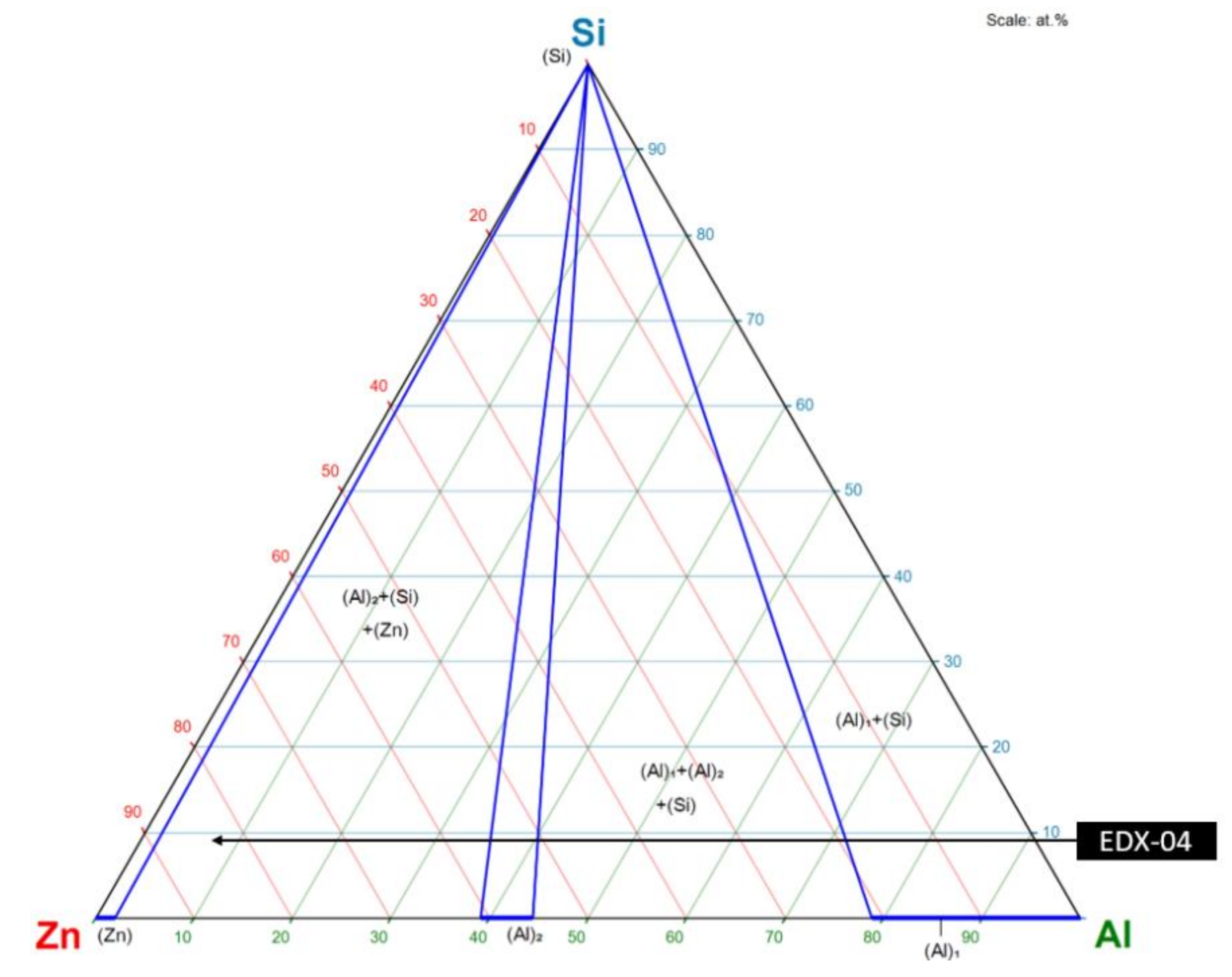



| Material | Tensile Strength | Yield Strength | Elastic Modulus | Elongation at Break | Yield Point |
| AlMg4.5Mn | 329 MPa | 223 MPa | 92,400 MPa | 14.36% | 328 MPa |
| DX54 | 260 MPa | 120 MPa | 210,000 MPa | 36% | - |
| AlMg4.5Mn | |||||||
|---|---|---|---|---|---|---|---|
| Al | Mg | Mn | Fe | Si | Cr | Zn | Cu |
| 94.3 | 4.489 | 0.515 | 0.3042 | 0.1555 | 0.0796 | 0.0470 | 0.0403 |
| DX54 | |||||||
| Fe | C | Si | Mn | P | S | Ti | Al |
| 99.47 | 0.011 | 0.029 | 0.113 | 0.012 | 0.0082 | 0.094 | 0.062 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bick, T.; Heuler, V.; Treutler, K.; Wesling, V. Characterization of Influences of Steel-Aluminum Dissimilar Joints with Intermediate Zinc Layer. Metals 2020, 10, 442. https://doi.org/10.3390/met10040442
Bick T, Heuler V, Treutler K, Wesling V. Characterization of Influences of Steel-Aluminum Dissimilar Joints with Intermediate Zinc Layer. Metals. 2020; 10(4):442. https://doi.org/10.3390/met10040442
Chicago/Turabian StyleBick, Tobias, Verena Heuler, Kai Treutler, and Volker Wesling. 2020. "Characterization of Influences of Steel-Aluminum Dissimilar Joints with Intermediate Zinc Layer" Metals 10, no. 4: 442. https://doi.org/10.3390/met10040442





