Mechanisms of Oxidation Degradation of Cr12 Roller Steel during Thermal Fatigue Tests
Abstract
:1. Introduction
2. Materials and Methods
3. Results
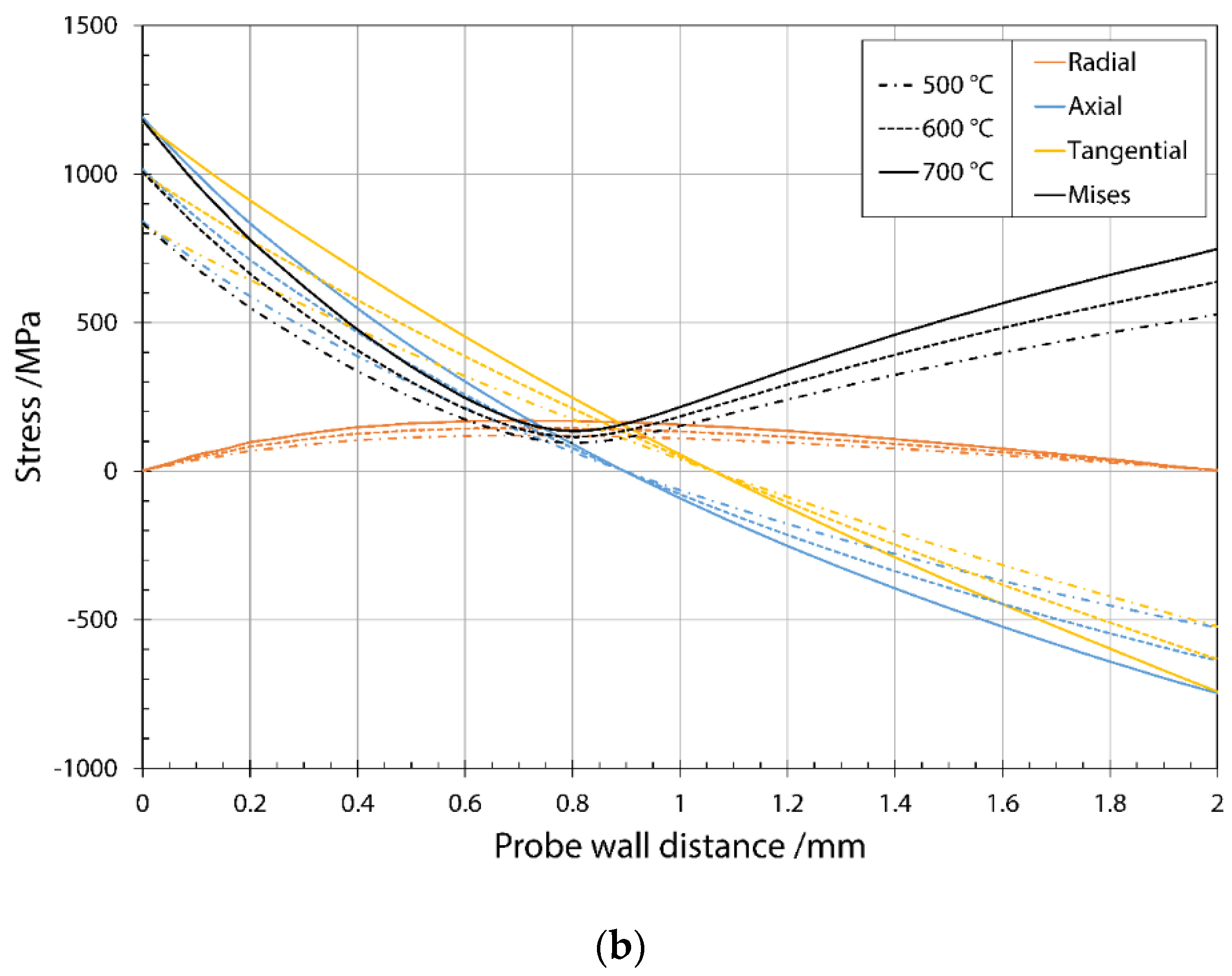
3.1. Stress Distribution during Thermal Fatigue Cycle
3.2. Initial Microstructure
3.3. Oxidation Behaviour and Its Relationship between Microstructure, Thermal Stress and Temperature
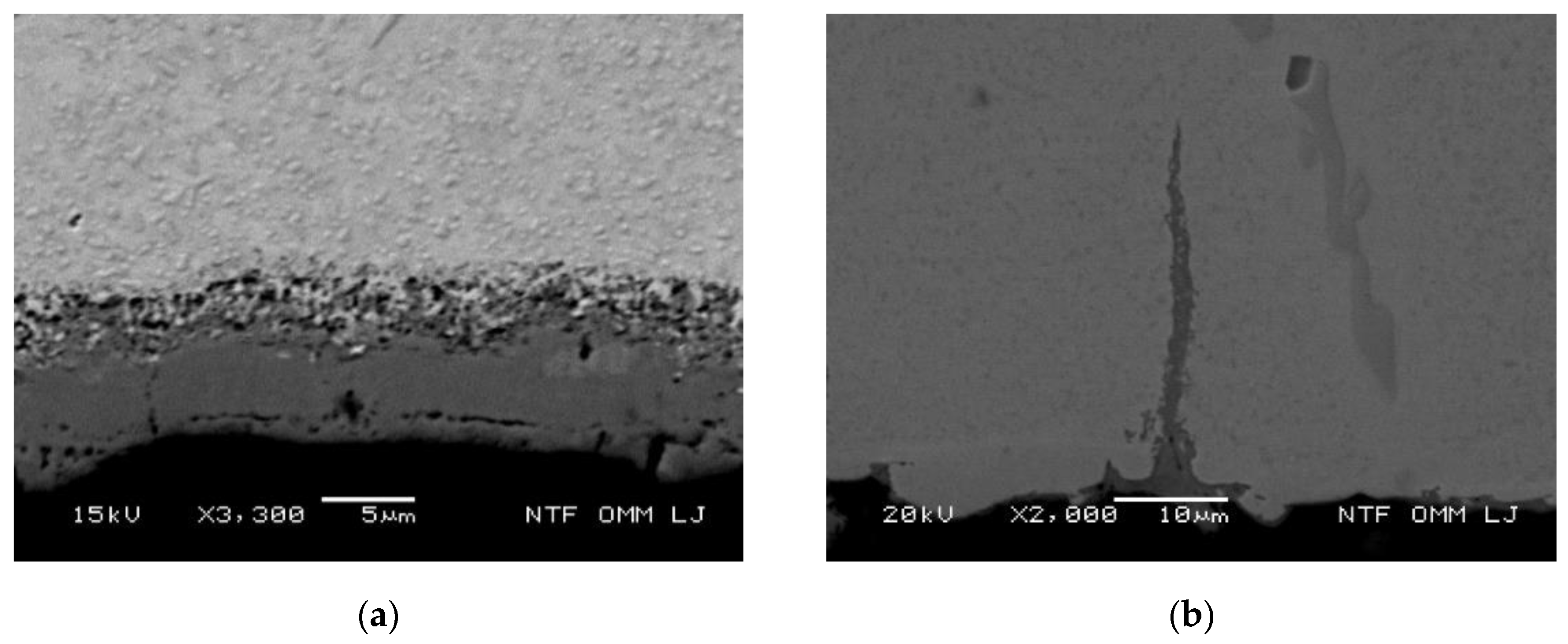
3.3.1. Oxidation Behavior of Uncracked Carbides
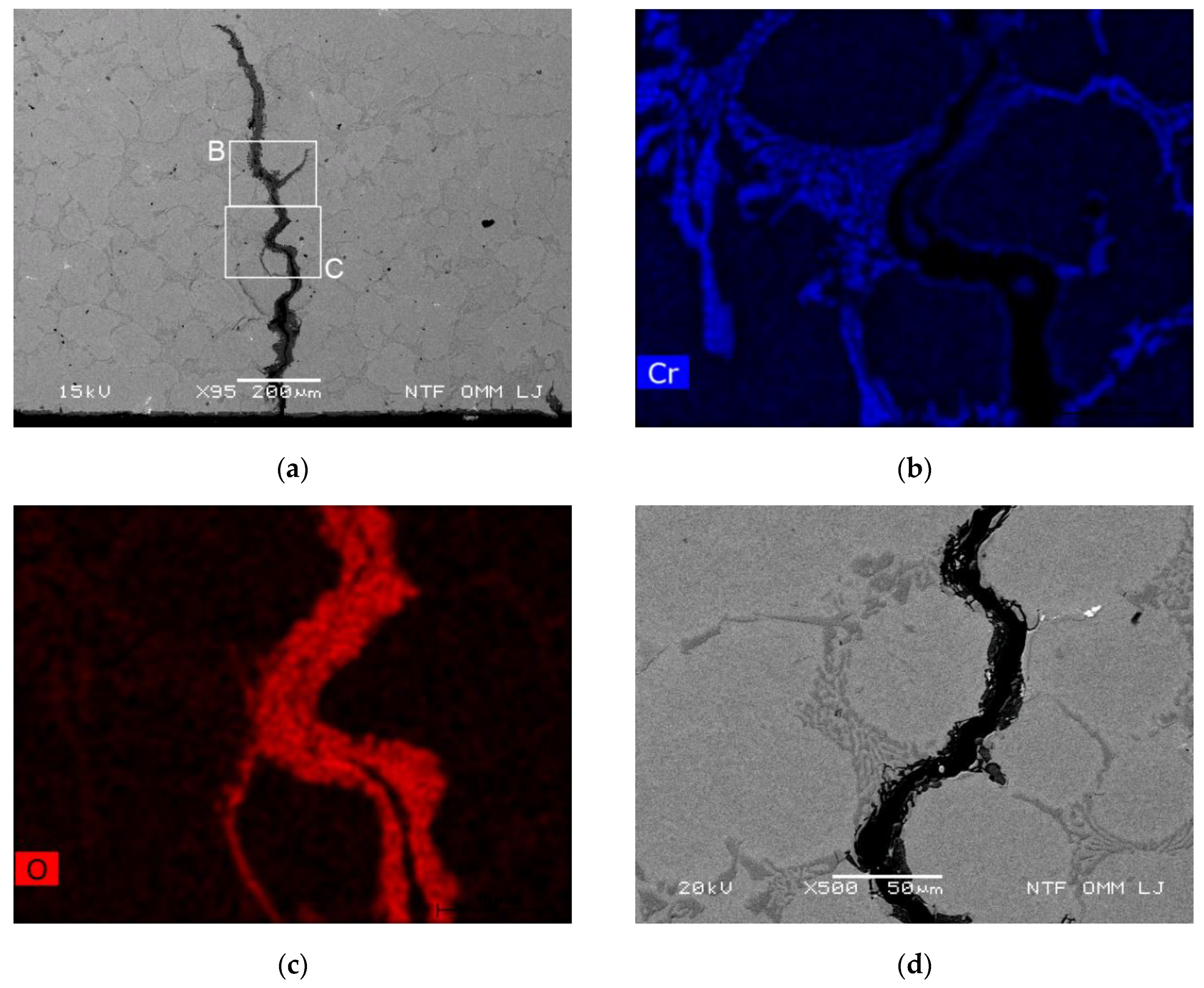
3.3.2. Oxidation Behavior of Cracked Eutectic Carbides at and Close to the Surface
3.3.3. Oxidation of the Matrix at the Cooled Surface and Oxidation Growth Front
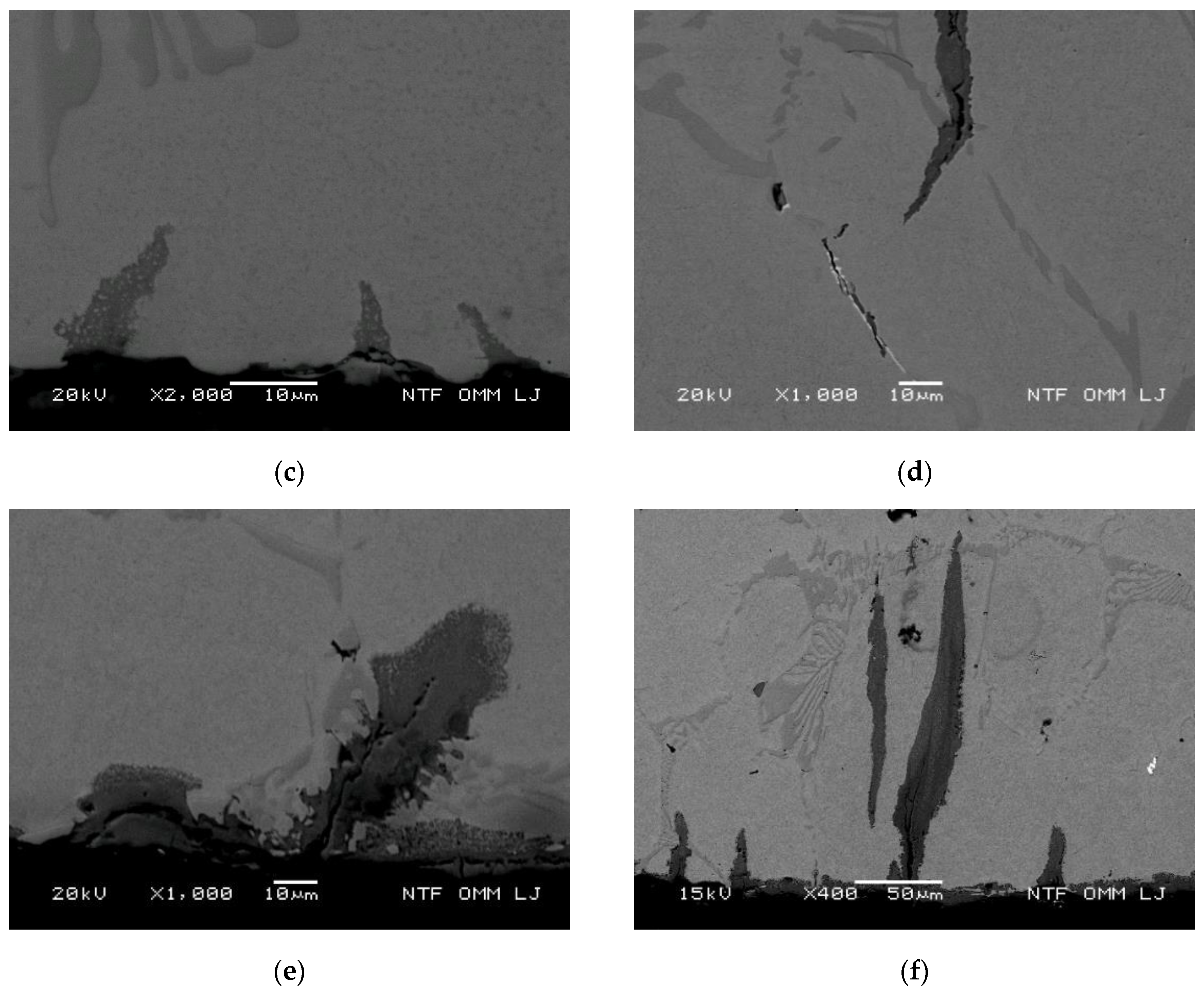
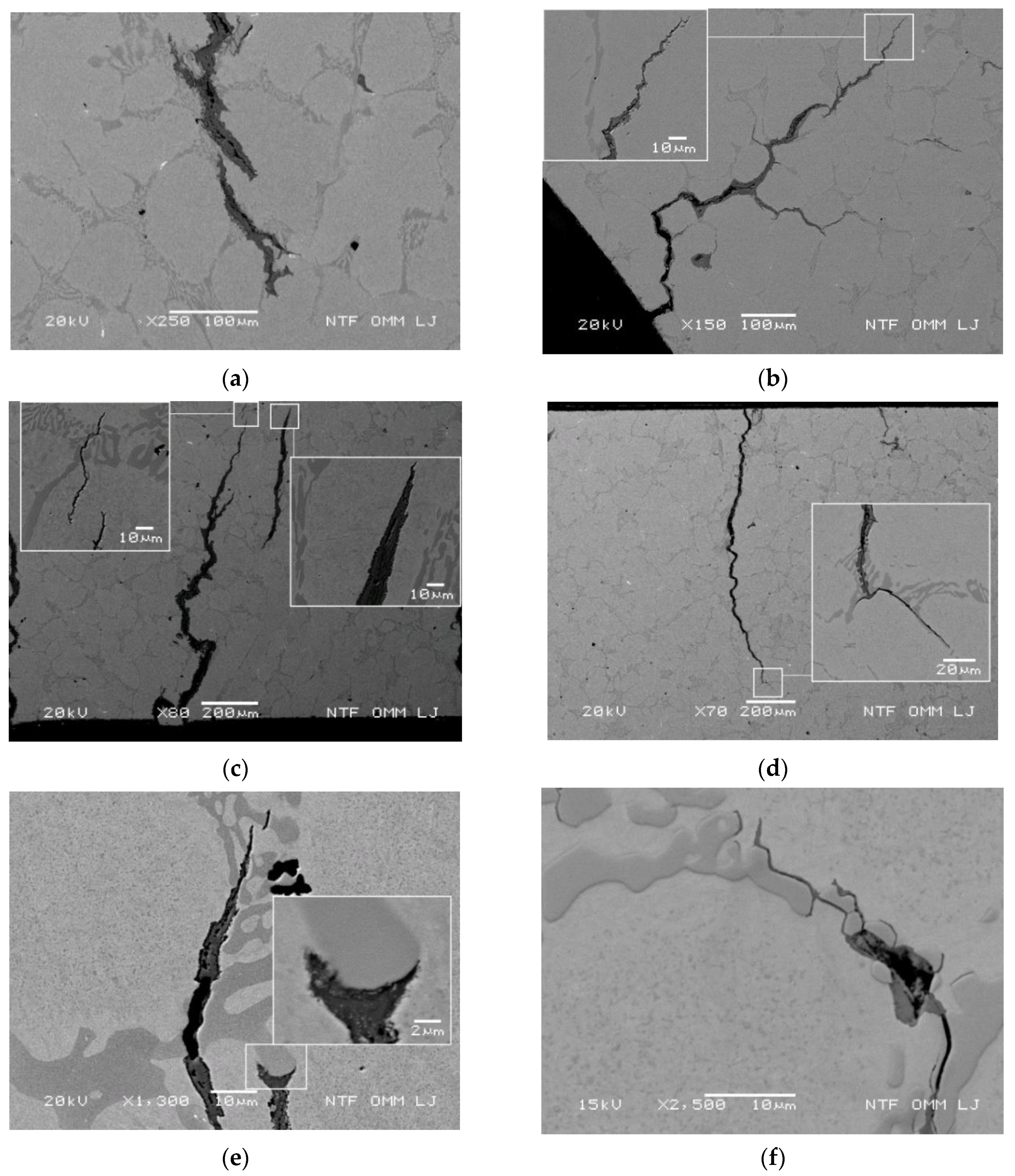
3.3.4. Oxidation Behavior and Crack Growth Direction at High Thermal Stresses
3.3.5. Oxidation Behavior and Crack Growth Direction during Medium Thermal Stress
3.3.6. Oxidation Behavior and Crack Growth Direction at Lower Thermal Stress
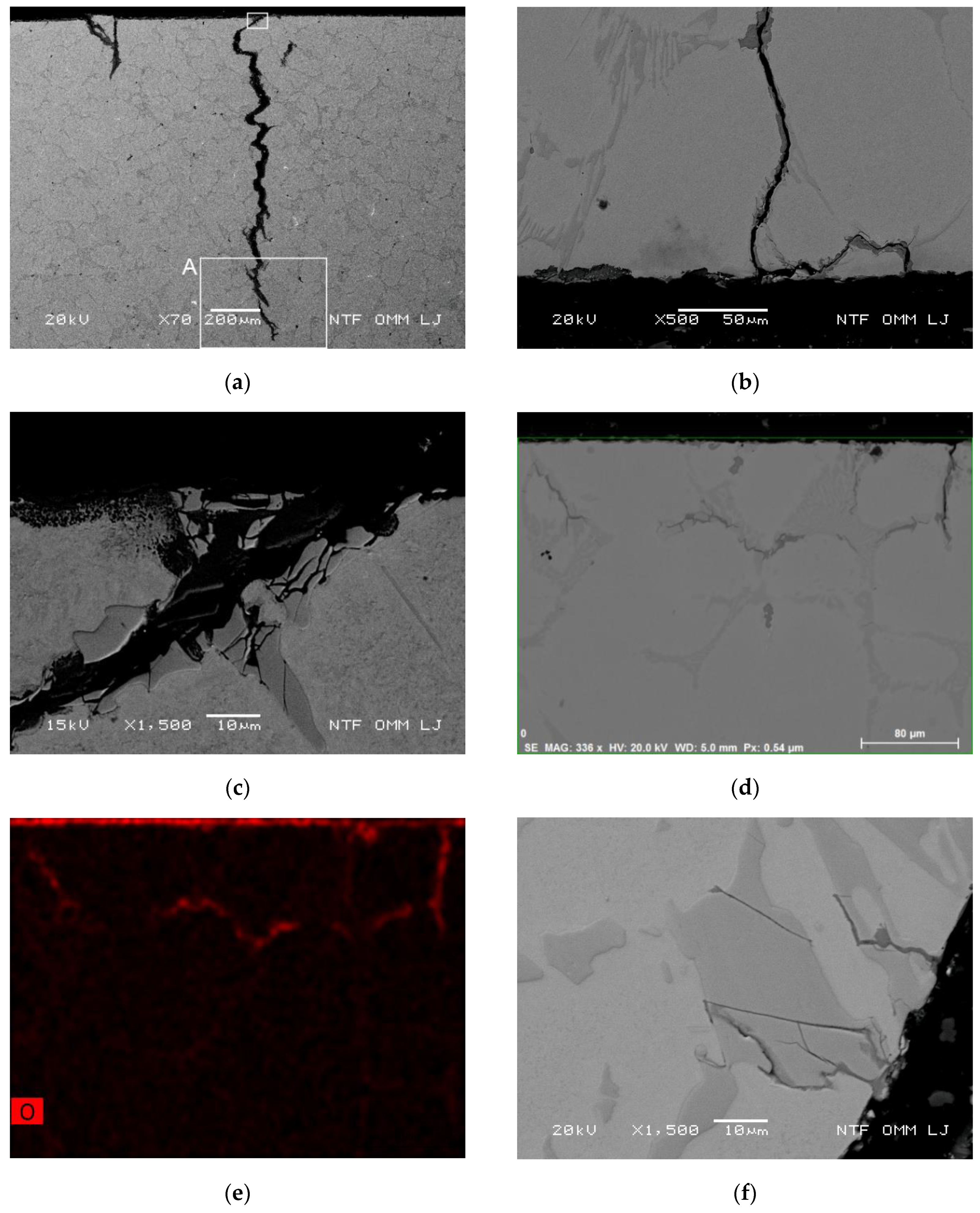
Oxidation Behavior of the Primary Crack Propagating Perpendicular to the Cooled Surface
Oxidation Behavior and Crack Growth Direction in the End Regions of the Secondary Cracks at 500 and 700 °C
Oxidation and Branching of Primary Cracks towards Internal Cracks
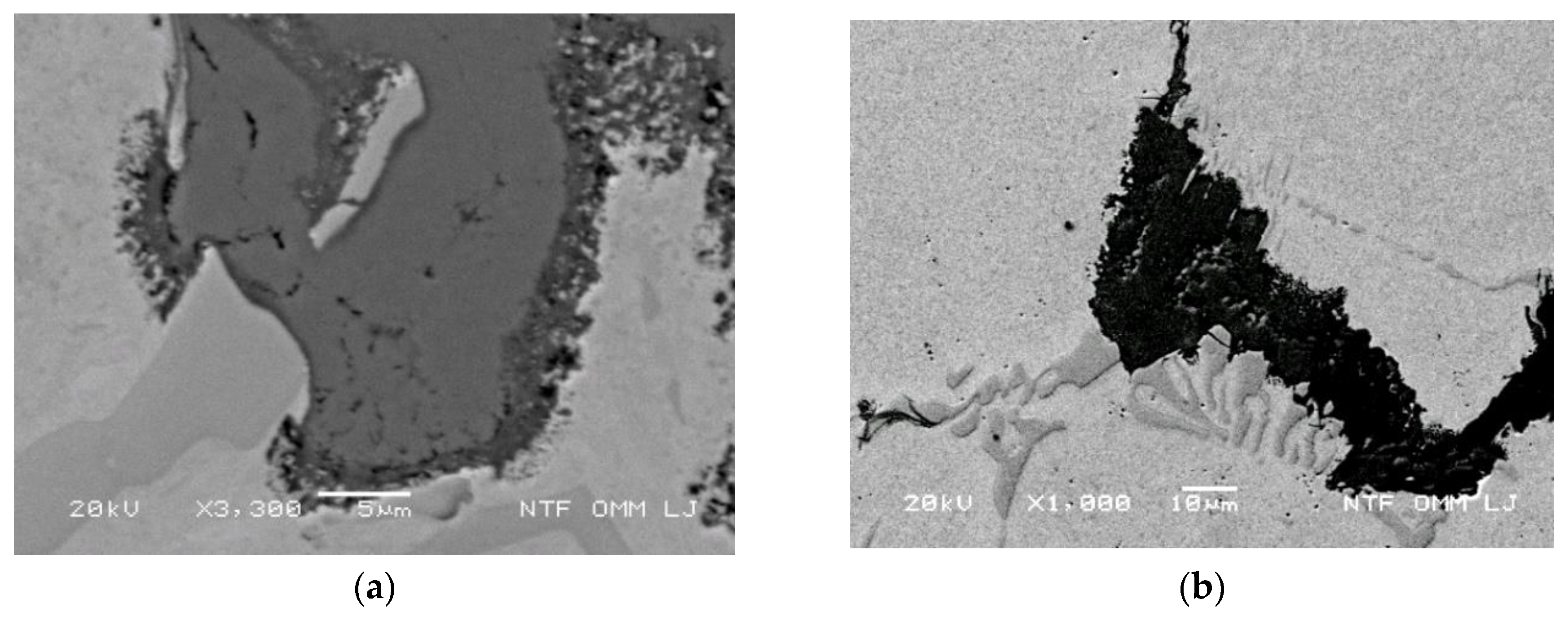
3.3.7. Conditions for an Increased Oxidation Rate of the Matrix along Carbides and Lamellae Eutectics Perpendicular to the Thermal Stresses
3.3.8. Oxidation Progress of Carbides and Matrix Adjacent to Carbides
4. Discussion
5. Conclusions
- The orientation of the carbides embedded in the microstructure is varied, which in combination with the oxidation behavior and the thermal stress influence crack growth.
- Thermal stress depends on the test temperature and the distance from the cooled surface. The FEM simulations were used to determine the change in thermal stress acting through the sample wall and show a strong correlation between temperature and stress value. An effective thermal stress of more than 900 MPa was estimated to be high. The low effective thermal stress is below 700 MPa. Values between low and high were assumed as medium thermal stress.
- Areas with depleted Cr content in the vicinity of eutectic carbides and within a narrow band adjacent to the primary carbides were observed. Areas with reduced Cr are more susceptible to oxidation. This applies to material surrounding eutectic carbide and band adjacent the primary carbides.
- The oxidation behavior is temperature dependent and influences thermal stress as well as carbide and matrix properties. The oxide area grows with higher temperature and thermal stress. The oxidation mechanism is based on diffusion growth, and the oxidation rate of the matrix adjacent to the carbides is generally higher than in the oxidation of carbides. Due to the variation of the local chemical composition, carbides could oxidize faster in special cases.
- The direction of oxidation in the matrix is determined by the direction of the effective thermal stress in combination with the crack tip stress. The oxidation of the eutectic carbides located below the surface is associated with the cracking of eutectic carbides and the formation of cracks from the surface to eutectic carbides, which enabled the diffusion of oxygen in the crack canal.
- Cracking of eutectic carbides is accelerated by thermal stresses, which accelerate the oxidation rates of carbides. The influence of the Mo content on the oxidation of carbides and consequently on crack growth was observed. An increased content of Mo in carbides slows down oxidation around the carbides.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spuzic, S.; Strafford, K.N.; Subramanian, C.; Savage, G. Wear of hot rolling mill rolls: An overview. Wear 1994, 176, 261–271. [Google Scholar] [CrossRef]
- Bataille, C.; Luc, E.; Bigerelle, M.; Deltombe, R.; Dubar, M. Rolls wear characterization in hot rolling process. Tribol. Int. 2016, 100, 328–337. [Google Scholar] [CrossRef]
- Hanlon, D.N.; Rainforth, W.M. The rolling sliding wear response of conventionally processed and spray formed high speed steel at ambient and elevated temperature. Wear 2003, 255, 956–966. [Google Scholar] [CrossRef]
- Zamri, W.F.H.; Kosasih, P.B.; Tieu, A.K.; Zhu, Q.; Zhu, H. Variations in the microstructure and mechanical properties of the oxide layer on high speed steel hot rolling work rolls. J. Mater. Process. Technol. 2012, 212, 2597–2608. [Google Scholar] [CrossRef]
- Bombač, D.; Kugler, G.; Markoli, B.; Terčelj, M. Hot work roller surface layer degradation progress during thermal fatigue in the temperature range 500–700 °C. Int. J. Fatigue 2017, 104, 355–365. [Google Scholar] [CrossRef]
- Bombač, D.; Gintalas, M.; Kugler, G.; Terčelj, M. Thermal fatigue behaviour of Fe-1.7C-11.3Cr-1.9Ni-1.2Mo roller steel in temperature range 500–700 °C. Int. J. Fatigue 2019, 121, 98–111. [Google Scholar] [CrossRef]
- Colás, R.; Ramírez, J.; Sandoval, I.; Morales, J.C.; Leduc, L.A. Damage in hot rolling work rolls. Wear 1999, 230, 56–60. [Google Scholar] [CrossRef]
- Tieu, A.K.; Zhu, Q.; Zhu, H.; Lu, C. An investigation into the tribological behaviour of a work roll material at high temperature. Wear 2011, 273, 43–48. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kim, D. Mechanisms of Surface Deterioration of High-Ni Grain Roll for Hot Strip Rolling. ISIJ Int. 1999, 39, 823–828. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, J.C.; Park, J.W.; Lee, H.C.; Lee, S. Effects of Tempering Temperature on Wear Resistance and Surface Roughness of a High Speed Steel Roll. ISIJ Int. 2001, 41, 859–865. [Google Scholar] [CrossRef]
- Kato, O.; Yamamoto, H.; Ataka, M.; Nakajima, K. Mechanisms of Surface Deterioration of Roll for Hot Strip Rolling. ISIJ Int. 1992, 32, 1216–1220. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.Y.; Zhu, H.T.; Tieu, A.K.; Su, L.H.; Reid, M.; Zhang, L.; Wei, P.T.; Zhao, X.; Wang, H.; Zhang, J.; et al. Theoretical and experimental investigation of thermal and oxidation behaviours of a high speed steel work roll during hot rolling. Int. J. Mech. Sci. 2017, 131–132, 811–826. [Google Scholar] [CrossRef] [Green Version]
- Deng, G.Y.; Zhu, Q.; Tieu, K.; Zhu, H.T.; Reid, M.; Saleh, A.A.; Su, L.H.; Ta, T.D.; Zhang, J.; Lu, C.; et al. Evolution of microstructure, temperature and stress in a high speed steel work roll during hot rolling: Experiment and modelling. J. Mater. Process. Technol. 2017, 240, 200–208. [Google Scholar] [CrossRef]
- Delaunois, F.; Stanciu, V.I.; Sinnaeve, M. Resistance to High-Temperature Oxidation and Wear of Various Ferrous Alloys Used in Rolling Mills. Metall. Mater. Trans. A 2018, 49, 822–835. [Google Scholar] [CrossRef]
- Molinari, A.; Straffelini, G.; Tomasi, A.; Biggi, A.; Corbo, G. Oxidation behaviour of ledeburitic steels for hot rolls. Mater. Sci. Eng., A 2000, 280, 255–262. [Google Scholar] [CrossRef]
- Joos, O.; Boher, C.; Vergne, C.; Gaspard, C.; Nylen, T.; Rezaï-Aria, F. Assessment of oxide scales influence on wear damage of HSM work rolls. Wear 2007, 263, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Huang, Y.; Zhou, Q.; Wang, J.; Jin, X.; Keer, L.M. Parametric study on stressed volume and its application to the quantification of rolling contact fatigue performance of heterogeneous material. Tribol. Int. 2017, 107, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Hamraoui, M. Thermal behaviour of rollers during the rolling process. Appl. Therm. Eng. 2009, 29, 2386–2390. [Google Scholar] [CrossRef]
- Mercado-Solis, R.D.; Beynon, J.H. Simulation of thermal fatigue in hot strip mill work rolls. Scand. J. Metall. 2005, 34, 175–191. [Google Scholar] [CrossRef]
- Belzunce, F.J.; Ziadi, A.; Rodriguez, C. Structural integrity of hot strip mill rolling rolls. Eng. Fail. Anal. 2004, 11, 789–797. [Google Scholar] [CrossRef]
- Günen, A.; Kanca, E.; Karakaş, M.S.; Koç, V.; Gök, M.S.; Kanca, Y.; Çürük, A.; Demir, M. High temperature wear behavior of the surface-modified externally cooled rolls. Surf. Coat. Technol. 2018, 348, 130–141. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhu, H.T.; Tieu, A.K.; Reid, M.; Zhang, L.C. In-situ investigation of oxidation behaviour in high-speed steel roll material under dry and humid atmospheres. Corros. Sci. 2010, 52, 2707–2715. [Google Scholar] [CrossRef]
- Ramírez-Ramírez, J.H.; Colás, R.; Garza-Montes-de-Oca, N.F. High Temperature Oxidation of a Work Roll Grade High-Chromium White Cast Iron. J. Iron. Steel Res. Int. 2013, 20, 122–129. [Google Scholar] [CrossRef]
- Garza-Montes-de-Oca, N.F.; Colás, R.; Rainforth, W.M. On the damage of a work roll grade high speed steel by thermal cycling. Eng. Fail. Anal. 2011, 18, 1576–1583. [Google Scholar] [CrossRef]
- Kang, Y.J.; Oh, J.C.; Lee, H.C.; Lee, S. Effects of carbon and chromium additions on the wear resistance and surface roughness of cast high-speed steel rolls. Metall. Mater. Trans. A 2001, 32, 2515–2525. [Google Scholar] [CrossRef] [Green Version]
- Pellizzari, M.; Molinari, A.; Straffelini, G. Tribological behaviour of hot rolling rolls. Wear 2005, 259, 1281–1289. [Google Scholar] [CrossRef]
- Lao, Y.; Du, H.; Xiong, T.; Wang, Y. Evolution Behaviors of Oxides in Severely Plastic Deformed Region of AISI 52100 Steel during Dry Sliding Wear. J. Mater. Sci. Technol. 2017, 33, 330–337. [Google Scholar] [CrossRef]
- Cho, S.; Jo, I.; Kim, H.; Kwon, H.-T.; Lee, S.-K.; Lee, S.-B. Effect of TiC addition on surface oxidation behavior of SKD11 tool steel composites. Appl. Surf. Sci. 2017, 415, 155–160. [Google Scholar] [CrossRef]
- Min, Y.; Jens, B.; Wu, X.; Xu, L. Oxidation and Thermal Fatigue Behaviors of Two Type Hot Work Steels during Thermal Cycling. J. Iron. Steel Res. Int. 2013, 20, 90–97. [Google Scholar] [CrossRef]
- Molinari, A.; Pellizzari, M.; Tremea, A.; Biggi, A.; Corbo, G. Effect of matrix microhardness on thermal fatigue behaviour of spincast high speed steels for hot rolls. Mater. Sci. Technol. 2005, 21, 352–356. [Google Scholar] [CrossRef]
- Molinari, A.; Tremea, A.; Pellizzari, M.; Biggi, A.; Corbo, G. High speed steels for hot rolls with improved impact and thermal fatigue resistance. Mater. Sci. Technol. 2002, 18, 1574–1580. [Google Scholar] [CrossRef]
- Pellizzari, M.; Cescato, D.; Flora, M.G. De Hot friction and wear behaviour of high speed steel and high chromium iron for rolls. Wear 2009, 267, 467–475. [Google Scholar] [CrossRef]
- Oksanen, V.; Valtonen, K.; Andersson, P.; Vaajoki, A.; Laukkanen, A.; Holmberg, K.; Kuokkala, V.T. Comparison of laboratory rolling–sliding wear tests with in-service wear of nodular cast iron rollers against wire ropes. Wear 2015, 340–341, 73–81. [Google Scholar] [CrossRef]
- Kim, H.-H.; Lim, J.-W.; Lee, J.-J. Oxidation Behavior of High-speed Steels in Dry and Wet Atmospheres. ISIJ Int. 2003, 43, 1983–1988. [Google Scholar] [CrossRef]
- Pellizzari, M.; Molinari, A.; Cescato, D.; Tremea, A.; Corbo, G.; Biggi, A. Wear and friction behaviour of high chromium iron and high speed steels for hot rolls. In Proceedings of the International Conference on Abrasion 2005, Sao Paulo, Brazil, 14–17 August 2005; Volume 2005, pp. 189–198. [Google Scholar]
- Monteiro, M.J.; Saunders, S.R.J.; Rizzo, F.C. The Effect of Water Vapour on the Oxidation of High Speed Steel, Kinetics and Scale Adhesion. Oxid. Met. 2011, 75, 57–76. [Google Scholar] [CrossRef]
- Garza-Montes-de-Oca, N.F.; Colás, R.; Rainforth, W.M. High Temperature Oxidation of a Work Roll Grade High Speed Steel. Oxid. Met. 2011, 76, 451. [Google Scholar] [CrossRef]
- Molinari, A.; Pellizzari, M.; Biggi, A.; Corbo, G.; Tremea, A. Primary carbides in spincast HSS for hot rolls and their effect on the oxidation behaviour. In Proceedings of the Proceedings 6th International Tooling Conference, Karlstadt, Germany, 10–13 September 2002; pp. 365–377. [Google Scholar]
- Garza-Montes-de-Oca, N.F.; Rainforth, W.M. Wear mechanisms experienced by a work roll grade high speed steel under different environmental conditions. Wear 2009, 267, 441–448. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Wang, G. New Method for Evaluating Thermal Wear of Rolls in Rolling Process. J. Iron. Steel Res. Int. 2008, 15, 52–55. [Google Scholar] [CrossRef]
- Pellizzari, M.; Flora, M.G. De Influence of laser hardening on the tribological properties of forged steel for hot rolls. Wear 2011, 271, 2402–2411. [Google Scholar] [CrossRef]
- Sano, Y.; Hattori, T.; Haga, M. Characteristics of High-carbon High Speed Steel Rolls for Hot Strip Mill. ISIJ Int. 1992, 32, 1194–1201. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, H.C.; Lee, S. Composition, microstructure, hardness, and wear properties of high-speed steel rolls. Metall. Mater. Trans. A 1999, 30, 399–409. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, S.-E.; Gustafsson, T. The influence of rolling temperature on roll wear, investigated in a new high temperature test rig. J. Mater. Process. Technol. 1994, 42, 239–291. [Google Scholar] [CrossRef]
- Hanlon, D.N.; Rainforth, W.M.; Sellars, C.M. The rolling/sliding wear response of conventionally processed and spray formed high chromium content cast iron at ambient and elevated temperature. Wear 1999, 225–229, 587–599. [Google Scholar] [CrossRef]
- Xu, L.; Fan, X.; Wei, S.; Liu, D.; Zhou, H.; Zhang, G.; Zhou, Y. Microstructure and wear properties of high-speed steel with high molybdenum content under rolling-sliding wear. Tribol. Int. 2017, 116, 39–46. [Google Scholar] [CrossRef]
- Xu, L.; Xing, J.; Wei, S.; Zhang, Y.; Long, R. Investigation on wear behaviors of high-vanadium high-speed steel compared with high-chromium cast iron under rolling contact condition. Mater. Sci. Eng. A 2006, 434, 63–70. [Google Scholar] [CrossRef]
- Kang, M.; Suh, Y.; Oh, Y.-J.; Lee, Y.-K. The effects of vanadium on the microstructure and wear resistance of centrifugally cast Ni-hard rolls. J. Alloys Compd. 2014, 609, 25–32. [Google Scholar] [CrossRef]
- Mercado-Solis, R.D.; Talamantes-Silva, J.; Beynon, J.H.; Hernandez-Rodriguez, M.A.L. Modelling surface thermal damage to hot mill rolls. Wear 2007, 263, 1560–1567. [Google Scholar] [CrossRef]
- Xu, L.; Wei, S.; Xing, J.; Long, R. Effects of carbon content and sliding ratio on wear behavior of high-vanadium high-speed steel (HVHSS) under high-stress rolling–sliding contact. Tribol. Int. 2014, 70, 34–41. [Google Scholar] [CrossRef]
- Vergne, C.; Boher, C.; Gras, R.; Levaillant, C. Influence of oxides on friction in hot rolling: Experimental investigations and tribological modelling. Wear 2006, 260, 957–975. [Google Scholar] [CrossRef] [Green Version]
- Hokkirigawa, K.; Kato, T.; Fukuda, T.; Shinooka, M. Analysis of Sliding Wear Mechanism of Oxide Films on Hot Roll Surfaces Based On In-Situ Observation of Wear Process by the CCD Microscope Tribosystem. J. Jpn. Soc. Tribol. 1997, 42, 777–784. [Google Scholar]
- Zhu, H.; Zhu, Q.; Tieu, A.K.; Kosasih, B.; Kong, C. A simulation of wear behaviour of high-speed steel hot rolls by means of high temperature pin-on-disc tests. Wear 2013, 302, 1310–1318. [Google Scholar] [CrossRef]
- Ren, X.; Fu, H.; Xing, J.; Yi, Y. Research on high-temperature dry sliding friction wear behavior of CaTi modified high boron high speed steel. Tribol. Int. 2019, 132, 165–176. [Google Scholar] [CrossRef]
- Hao, L.; Wu, H.; Wei, D.; Cheng, X.; Zhao, J.; Luo, S.; Jiang, L.; Jiang, Z. Wear and friction behaviour of high-speed steel and indefinite chill material for rolling ferritic stainless steels. Wear 2017, 376–377, 1580–1585. [Google Scholar] [CrossRef] [Green Version]
- Torres, H.; Varga, M.; Widder, F.; Cihak-Bayr, U.; Viskovic, O.; Ripoll, M.R. Experimental simulation of high temperature sliding contact of hot rolled steel. Tribol. Int. 2016, 93, 745–754. [Google Scholar] [CrossRef]
- Savage, G.; Boelen, R.; Horti, A.; Morikawa, H.; Tsujimoto, Y. Hot wear testing of roll alloys. In Proceedings of the Mechanical Working and Steel Processing Conference Proceedings, Cleveland, OH, USA, 13–16 October 1996; pp. 333–340. [Google Scholar]












| C | Si | Mn | Cr | Mo | Ni | V | Co | S | P |
|---|---|---|---|---|---|---|---|---|---|
| 1.651 | 0.662 | 0.731 | 11.282 | 1.171 | 1.941 | 0.262 | 0.017 | 0.009 | 0.017 |
| Microstructural Feature | Cr | Mo | V |
|---|---|---|---|
| Primary M7C3 | 42–50 | 3–8 | 1.5–1.9 |
| Eutectic M7C3 | 44–52 | 2.1–2.9 | 1.5–1.9 |
| Mo2C | 4–7 | 50–56 | 0.4–0.7 |
| Matrix | 7–9 | 0.45–0.75 | 0.1–0.12 |
| Carbide/matrix band | 4–5 | 0.45–0.7 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bombač, D.; Gintalas, M.; Kugler, G.; Terčelj, M. Mechanisms of Oxidation Degradation of Cr12 Roller Steel during Thermal Fatigue Tests. Metals 2020, 10, 450. https://doi.org/10.3390/met10040450
Bombač D, Gintalas M, Kugler G, Terčelj M. Mechanisms of Oxidation Degradation of Cr12 Roller Steel during Thermal Fatigue Tests. Metals. 2020; 10(4):450. https://doi.org/10.3390/met10040450
Chicago/Turabian StyleBombač, David, Marius Gintalas, Goran Kugler, and Milan Terčelj. 2020. "Mechanisms of Oxidation Degradation of Cr12 Roller Steel during Thermal Fatigue Tests" Metals 10, no. 4: 450. https://doi.org/10.3390/met10040450
APA StyleBombač, D., Gintalas, M., Kugler, G., & Terčelj, M. (2020). Mechanisms of Oxidation Degradation of Cr12 Roller Steel during Thermal Fatigue Tests. Metals, 10(4), 450. https://doi.org/10.3390/met10040450





