Thermodynamic Analysis of the Formation of FCC and BCC Solid Solutions of Ti-Based Ternary Alloys by Mechanical Alloying
Abstract
:1. Introduction
2. Thermodynamic Model
2.1. Gibbs Free Energy of Mixing,
2.2. Miedema’s Model Theory
2.3. Extended Miedema’s Model for Ternary Alloys
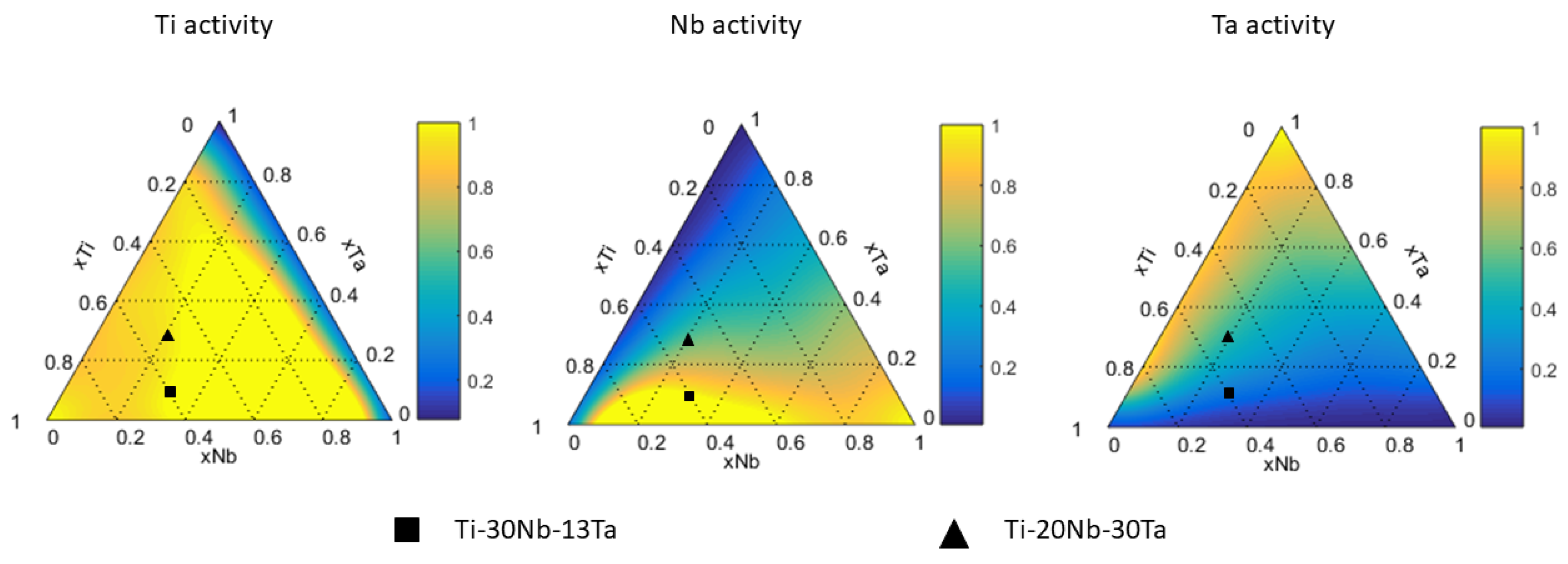
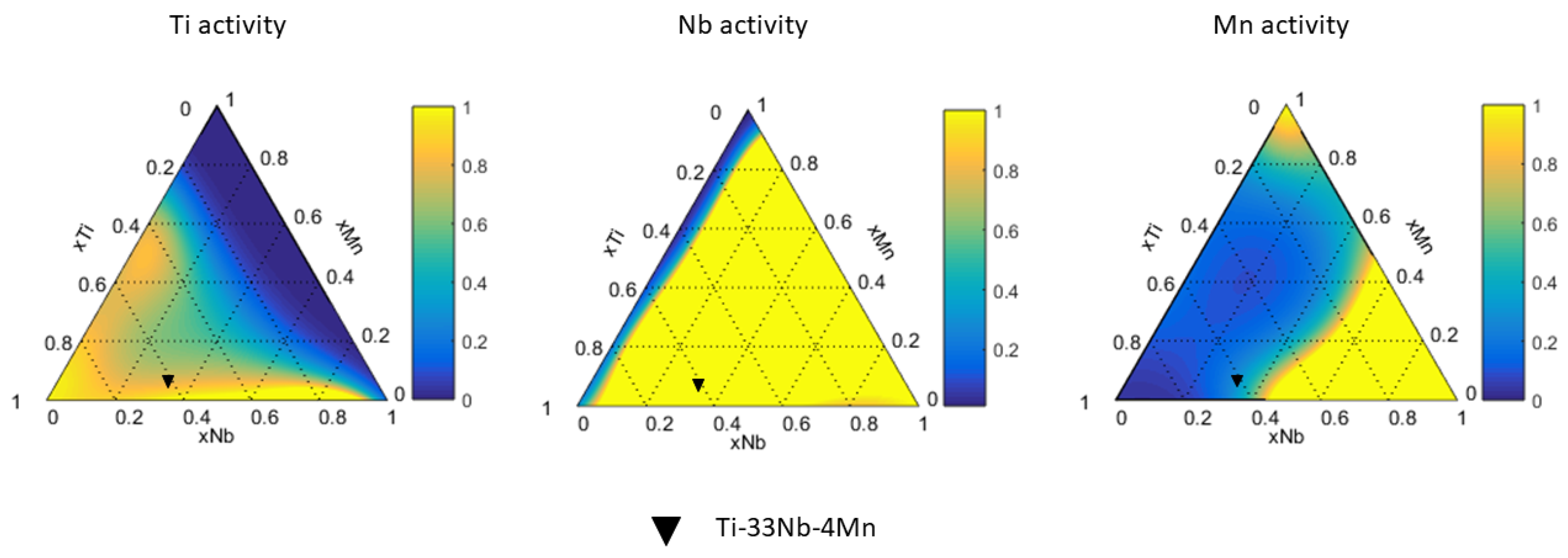
2.4. Calculation of Activity
2.5. Estimation of Storage Energy Produced by Crystalline Defects
3. Materials and Methods
3.1. Ti-Ta-Sn System
3.2. Ti-Nb-Ta System
3.3. Ti-Nb-Mn System
4. Results and Discussion
4.1. Ti-Ta-Sn System
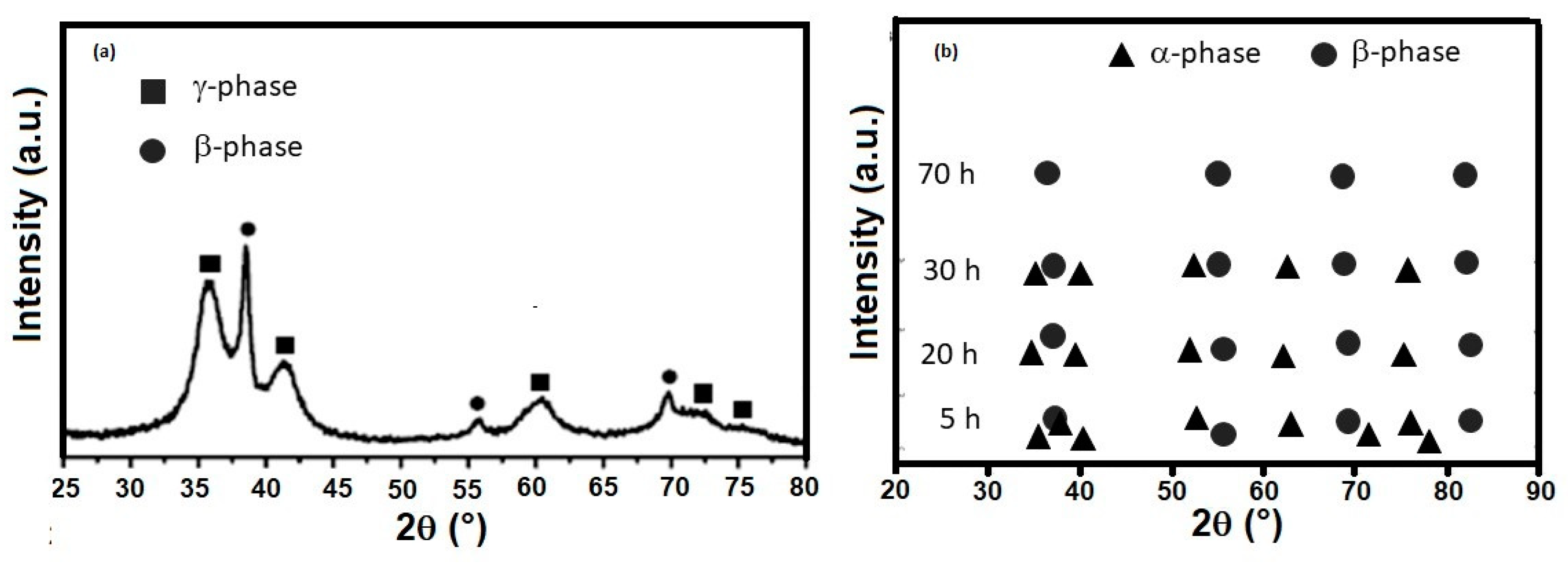
4.2. Ti-Nb-Ta System
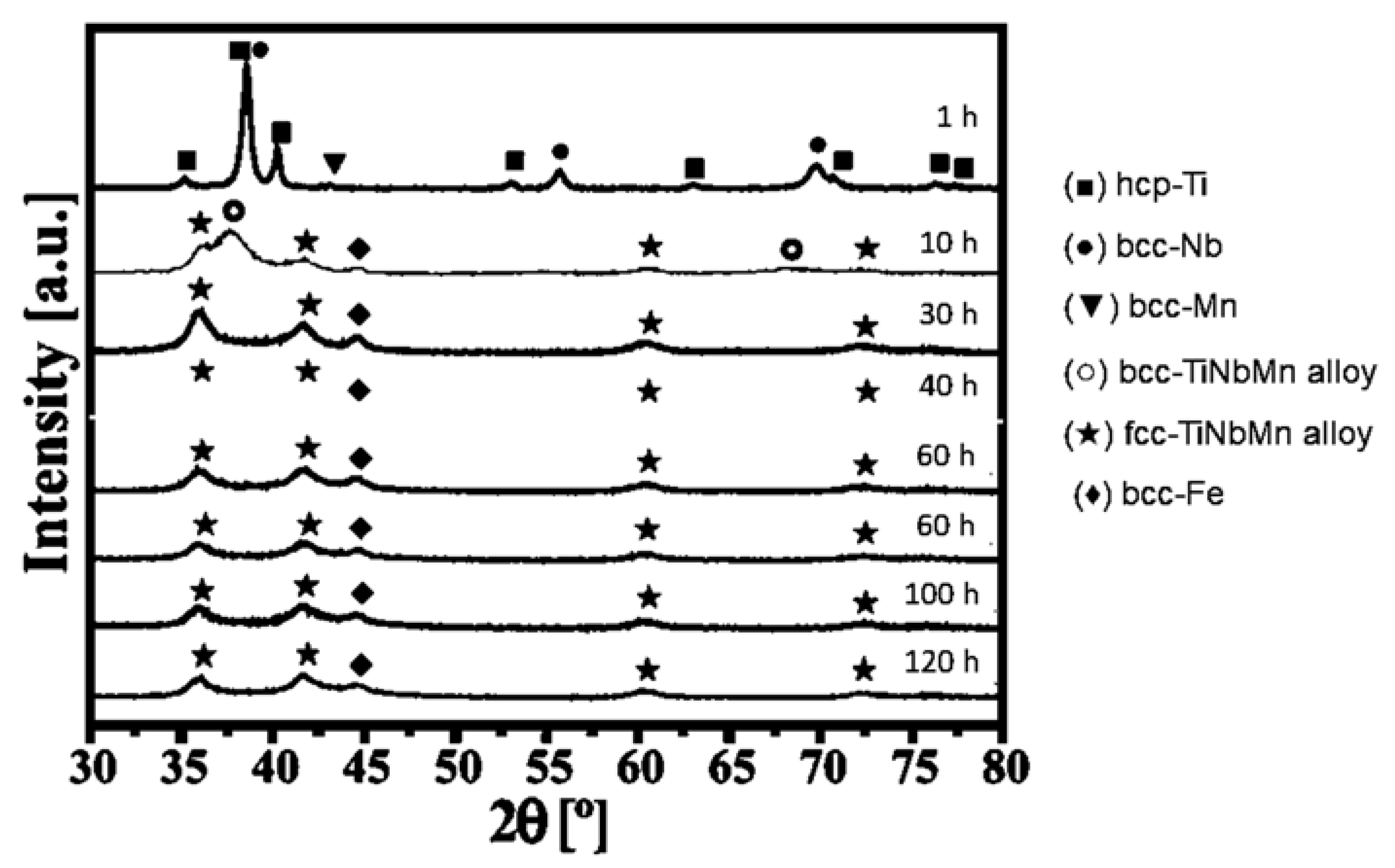
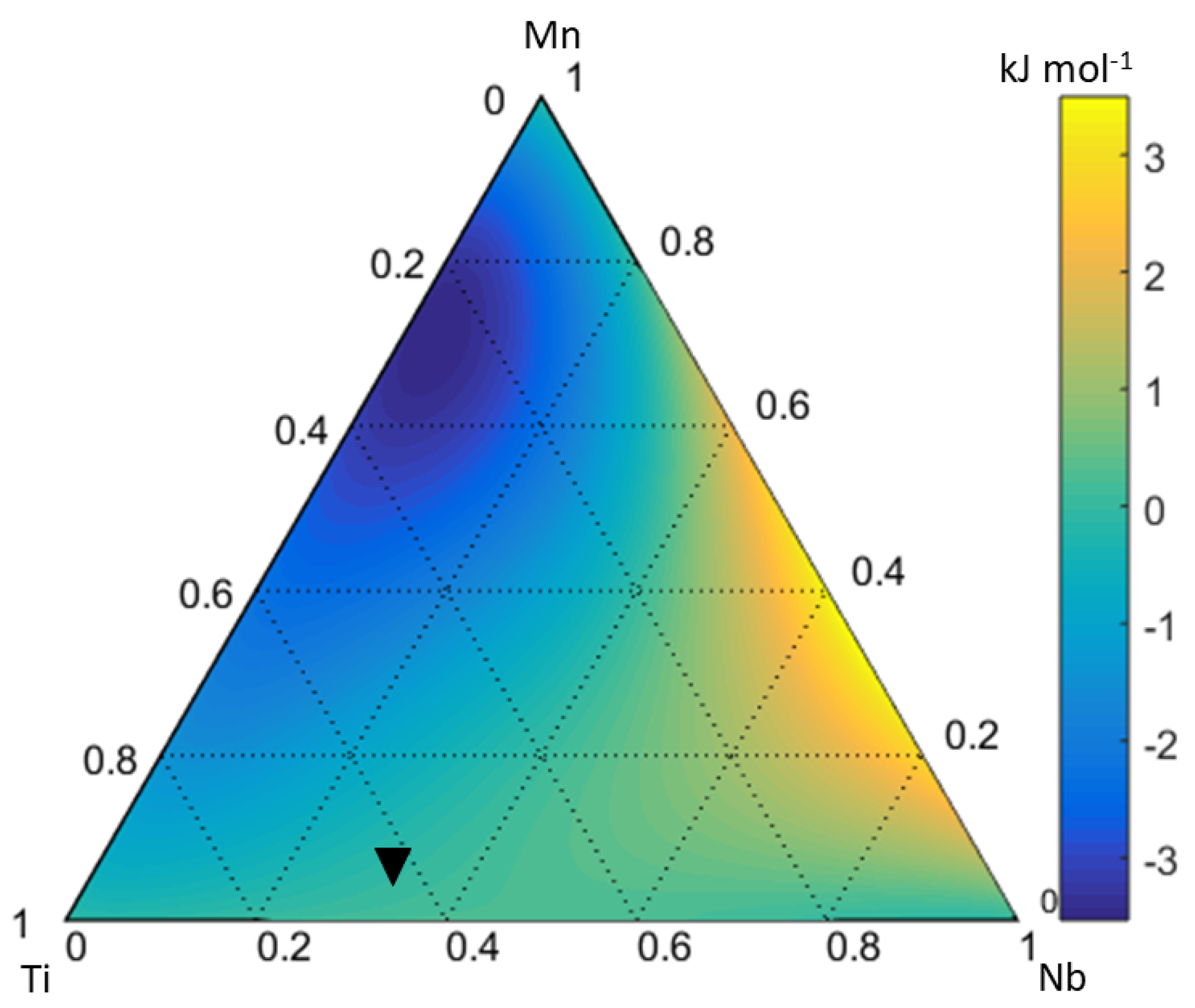
4.3. Ti-Nb-Mn System
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peters, M.; Leyens, C. (Eds.) Titanium and Titanium Alloys; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 3527305343. [Google Scholar]
- Bhaskar, P.; Dasgupta, A.; Sarma, V.S.; Mudali, U.K.; Saroja, S. Mechanical properties and corrosion behaviour of nanocrystalline Ti-5Ta-1.8Nb alloy produced by cryo-rolling. Mater. Sci. Eng. A 2014, 616, 71–77. [Google Scholar] [CrossRef]
- Veiga, C.; Loureiro, A.J.R.; Davim, J.P. Proprties and applications of titanium alloys. Rev. Adv. Mater. Sci. 2012, 32, 133–148. [Google Scholar]
- Ning, Y.Q.; Xie, B.C.; Fu, M.W.; Liang, H.Q.; Yao, Z.K.; Guo, H.Z. Microstructure and superplastic deformation for aerospace Ti-alloys associated with α-phase curing behavior. Aerosp. Sci. Technol. 2015, 45, 416–421. [Google Scholar] [CrossRef]
- Sun, K.; Yi, X.; Sun, B.; Yin, X.; Meng, X.; Cai, W.; Zhao, L. Microstructure and mechanical properties of Ti–V–Al–Cu shape memory alloy by tailoring Cu content. Mater. Sci. Eng. A 2020, 771, 138641. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Yu, L.; Liang, H.; Huang, Y.; Ma, Z. Microstructures and tensile properties of Ti2AlNb and Mo-modified Ti2AlNb alloys fabricated by hot isostatic pressing. Mater. Sci. Eng. A 2020, 776, 139043. [Google Scholar] [CrossRef]
- Antunes, R.A.; de Oliveiraa, M.C.L.; Salvador, C.A.F. Materials selection of optimized titanium alloys for aircraft applications. Mater. Res. 2018, 21, 2. [Google Scholar] [CrossRef] [Green Version]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Cleveland, OH, USA, 1988; ISBN 0871706865. [Google Scholar]
- Aguilar, C.; Guerra, C.; Lascano, S.; Guzman, D.; Rojas, P.A.; Thirumurugan, M.; Bejar, L.; Medina, A. Synthesis and characterization of Ti-Ta-Nb-Mn foams. Mater. Sci. Eng. C 2016, 58, 420–431. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of advanced manufactured titanium implants-A review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [Green Version]
- Boyce, B.F.; Elder, H.Y.; Elliot, H.L.; Fogelman, I.; Fell, G.S.; Junor, B.J.; Beastall, G.; Boyle, I.T. Hypercalcaemic osteomalacia due to aluminium toxicity. Lancet 1982, 320, 1009–1013. [Google Scholar] [CrossRef]
- Khadija, G.; Saleem, A.; Akhtar, Z.; Naqvi, Z.; Gull, M.; Masood, M.; Mukhtar, S.; Batool, M.; Saleem, N.; Rasheed, T.; et al. Short term exposure to titanium, aluminum and vanadium (Ti-6Al-4V) alloy powder drastically affects behavior and antioxidant metabolites in vital organs of male albino mice. Toxicol. Rep. 2018, 5, 765–770. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M. Link between aluminum and the pathogenesis of Alzheimer’s disease: The integration of the aluminum and amyloid cascade hypotheses. Int. J. Alzheimers. Dis. 2011, 2011, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Xiong, J.; Wong, C.S.; Hodgson, P.D.; Wen, C. Ti6Ta4Sn alloy and subsequent scaffolding for bone tissue engineering. Tissue Eng. Part A 2009, 15, 3151–3159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okazaki, Y. A new Ti-15Zr-4Nb-4Ta alloy for medical applications. Curr. Opin. Solid State Mater. Sci. 2001, 5, 45–53. [Google Scholar] [CrossRef]
- Tan, M.H.C.; Baghi, A.D.; Ghomashchi, R.; Xiao, W.; Oskouei, R.H. Effect of niobium content on the microstructure and Young’s modulus of Ti-xNb-7Zr alloys for medical implants. J. Mech. Behav. Biomed. Mater. 2019, 99, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lee, P.D.; Dashwood, R.J.; Lindley, T.C. Titanium foams for biomedical applications: A review. Mater. Technol. 2010, 25, 127–136. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Xia, Z.S. Effect of alloying time and composition on the mechanical properties of Ti alloy. Mater. Sci. Eng. A 2005, 394, 220–228. [Google Scholar] [CrossRef]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.A.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef]
- Yu, Q.; Kacher, J.; Gammer, C.; Traylor, R.; Samanta, A.; Yang, Z.; Minor, A.M. In situ TEM observation of FCC Ti formation at elevated temperatures. Scr. Mater. 2017, 140, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Heerden, D.V.A.N.; Josell, D.; Shecthman, D. The formation of f.c.c. titanium in titanium-aluminum multilayers. Acta Metall. 1996, 44, 297–306. [Google Scholar] [CrossRef]
- Prasanthi, T.N.; Sudha, C.; Ravikirana; Saroja, S. Formation and reversion of metastable fcc phase in a Ti-5Ta-2Nb explosive clad. Mater. Charact. 2016, 116, 24–32. [Google Scholar] [CrossRef]
- Zhang, D.L.; Ying, D.Y. Formation of fcc titanium during heating high-energy, ball-milled Al-Ti powders. Mater. Lett. 2001, 50, 149–153. [Google Scholar] [CrossRef]
- Ahuja, R.; Fraser, H.L. Structure and mechanical properties of nanolaminated Ti-Al thin films. JOM 1994, 46, 35–39. [Google Scholar] [CrossRef]
- Ren, J.; Sun, Q.; Xiao, L.; Ding, X.; Sun, J. Size-dependent of compression yield strength and deformation mechanism in titanium single-crystal nanopillars orientated [0001] and [1120]. Mater. Sci. Eng. A 2014, 615, 22–28. [Google Scholar] [CrossRef]
- Lindemann, I.; Schmidt, R.; Pilz, S.; Gebel, B.; Teresiak, A.; Gebert, A. Ultrafine-grained Ti-40Nb prepared by reactive milling of the elements in hydrogen. J. Alloys Compd. 2017, 729, 1244–1249. [Google Scholar] [CrossRef]
- Suryanarayana, C. Nanocrystalline materials. Int. Mater. Rev. 1995, 40, 41–64. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Dercz, G.; Matuła, I.; Zubko, M.; Kazek-Kęsik, A.; Maszybrocka, J.; Simka, W.; Dercz, J.; Świec, P.; Jendrzejewska, I. Synthesis of porous Ti–50Ta alloy by powder metallurgy. Mater. Charact. 2018, 142, 124–136. [Google Scholar] [CrossRef]
- García-Garrido, C.; Gutiérrez-González, C.; Torrecillas, R.; Pérez-Pozo, L.; Salvo, C.; Chicardi, E. Manufacturing optimisation of an original nanostructured (beta + gamma)-TiNbTa material. J. Mater. Res. Technol. 2019, 8, 2573–2585. [Google Scholar] [CrossRef]
- Chicardi, E.; Gutiérrez-gonzález, C.F.; Sayagués, M.J.; García-garrido, C. Development of a novel TiNbTa material potentially suitable for bone replacement implants. Mater. Des. 2018, 145, 88–96. [Google Scholar] [CrossRef]
- Aguilar, C.; Guzman, P.; Lascano, S.; Parra, C.; Bejar, L.; Medina, A.; Guzman, D. Solid solution and amorphous phase in Ti–Nb–Ta–Mn systems synthesized by mechanical alloying. J. Alloys Compd. 2016, 670, 346–355. [Google Scholar] [CrossRef]
- Salvo, C.; Aguilar, C.; Cardoso-Gil, R.; Medina, A.; Bejar, L.; Mangalaraja, R.V. Study on the microstructural evolution of Ti-Nb based alloy obtained by high-energy ball milling. J. Alloys Compd. 2017, 720, 254–263. [Google Scholar] [CrossRef]
- Chicardi, E.; García-Garrido, C.; Sayagués, M.J.; Torres, Y.; Amigó, V.; Aguilar, C. Development of a novel fcc structure for an amorphous-nanocrystalline Ti-33Nb-4Mn (at.%) ternary alloy. Mater. Charact. 2018, 135, 46–56. [Google Scholar] [CrossRef]
- Zhang, R.F.; Zhang, S.H.; He, Z.J.; Jing, J.; Sheng, S.H. Miedema Calculator: A thermodynamic platform for predicting formation enthalpies of alloys within framework of Miedema’s Theory. Comput. Phys. Commun. 2016, 209, 58–69. [Google Scholar] [CrossRef]
- Bakker, H. Miedema’s semi-empirical model. In Enthalpies in Alloys; Trans Tech Publications Ltd.: Zurich, The Switzerland, 1998. [Google Scholar]
- Miedema, A.R.; Chatel, P.F.; Boer, F.R. Cohesion in alloys-Fundamentals of a semi-empirical model. Phys. B Condens. Matter 1980, 100, 1–28. [Google Scholar] [CrossRef]
- Bakker, H.; Modder, I.W.; Kuin, M.J. Extension of Miedema’s semiempirical model to estimates of the formation enthalpies of point defects in intermetallic compounds with the B2 structure. Intermetallics 1997, 5, 535–546. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef] [Green Version]
- Joo, S.H.; Bae, J.W.; Park, W.Y.; Shimada, Y.; Wada, T.; Kim, H.S.; Takeuchi, A.; Konno, T.J.; Kato, H.; Okulov, I.V. Beating Thermal Coarsening in Nanoporous Materials via High-Entropy Design. Adv. Mater. 2020, 32, 1–9. [Google Scholar] [CrossRef]
- Ray, P.K.; Akinc, M.; Kramer, M.J. Estimation of formation enthalpies using an extended Miedema approach. In Proceedings of the 22nd Annual Conference on Fossil Energy Materials, Pittsburghm, PA, USA, 8–10 July 2008. [Google Scholar]
- Physica, E. Extended Miedema model: Predicting the formation enthalpies of intermetallic phases with more than two elements. Physica B 1996, 228, 289–294. [Google Scholar]
- Wang, W.C.; Li, J.H.; Yan, H.F.; Liu, B.X. A thermodynamic model proposed for calculating the standard formation enthalpies of ternary alloy systems. Scr. Mater. 2007, 56, 975–978. [Google Scholar] [CrossRef]
- Bakker, H.; Zhou, G.F.; Yang, H. Mechanically driven disorder and phase transformations in alloys. Prog. Mater. Sci. 1995, 39, 159–241. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Hongmei, C.; Yifang, O.; Yong, D.U. Amorphous forming ranges of Al-Fe-Nd-Zr system predicted by Miedema and geometrical models. J. Rare Earths 2014, 32, 343–351. [Google Scholar] [CrossRef]
- Rafiei, M.; Enayati, M.H.; Karimzadeh, F. Thermodynamic analysis of solid solution formation in the nanocrystalline Fe-Ti-Al ternary system during mechanical alloying. J. Chem. Thermodyn. 2013, 59, 243–249. [Google Scholar] [CrossRef]
- Hillert, M. Empirical methods of predicting and representing thermodynamic properties of ternary solution phases. Calphad 1980, 4, 1–12. [Google Scholar] [CrossRef]
- Toop, G. Predicting ternary activities using binary data. Mater. Sci. 1965, 233, 850–854. [Google Scholar]
- Aguilar, C.; de Martinez, V.P.; Palacios, J.M.; Ordoñez, S.; Pavez, O. A thermodynamic approach to energy storage on mechanical alloying of the Cu-Cr system. Scr. Mater. 2007, 57, 213–216. [Google Scholar] [CrossRef]
- Dercz, G.; Matuła, I.; Zubko, M. Structure and Mechanical Properties of the New Ti30Ta20Nb Biomedical Alloy. J. Nanosci. Nanotechnol. 2019, 19, 2556–2566. [Google Scholar] [CrossRef]
- Aguilar, C.; Guzmán, D.; Castro, F.; Martínez, V.; De Las Cuevas, F.; Lascano, S.; Muthiah, T. Fabrication of nanocrystalline alloys Cu-Cr-Mo super satured solid solution by mechanical alloying. Mater. Chem. Phys. 2014, 146, 493–502. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A friendly Java program for material analysis using diffraction. Newsl. CDP 1999, 21, 14–15. [Google Scholar]
- Lutterotti, L.; Gialanella, S. X-ray diffraction characterization of heavily deformed metallic specimens. Acta Mater. 1998, 46, 101–110. [Google Scholar] [CrossRef]
- Mccusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Loue, D.; Scardi, P. Rietveld refinement guidelines. J. Appl. Phys. 1999, 32, 36–50. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, D.; Ordoñez, S.; Serafini, D.; Rojas, P.A.; Aguilar, C.; Santander, M. Thermal stability of amorphous Mg50Ni50 alloy produced by mechanical alloying. J. Non-Cryst. Solids 2010, 356, 120–123. [Google Scholar]
- Aguilar, C.; Marín, J.; Ordóñez, S.; Celentano, D.; Castro, F.; Martínez, V. Estudio estructural de polvos Cu-Cr aleados mecánicamente. Rev. Metal 2006, 42, 334–344. [Google Scholar]
- Rodriguez-Carvajal, J. Recent developments of the program FULLPROF, commission on powder diffraction. IUCr Newsl. 2001, 26, 12–19. [Google Scholar]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Snyder, R.L.; Bunge, H.J.; Fiala, J. Defect and Microstucture Analysis by Diffraction, 1st ed.; Oxford Science Publications: Oxford, UK, 1999. [Google Scholar]
- Aguilar, C.; Pio, E.; Medina, A.; Bejar, L.; Guzmán, D. Structural study of novel nanocrystalline fcc Ti-Ta-Sn alloy. Metall. Mater. Trans. A 2019, 50, 2061–2065. [Google Scholar] [CrossRef]
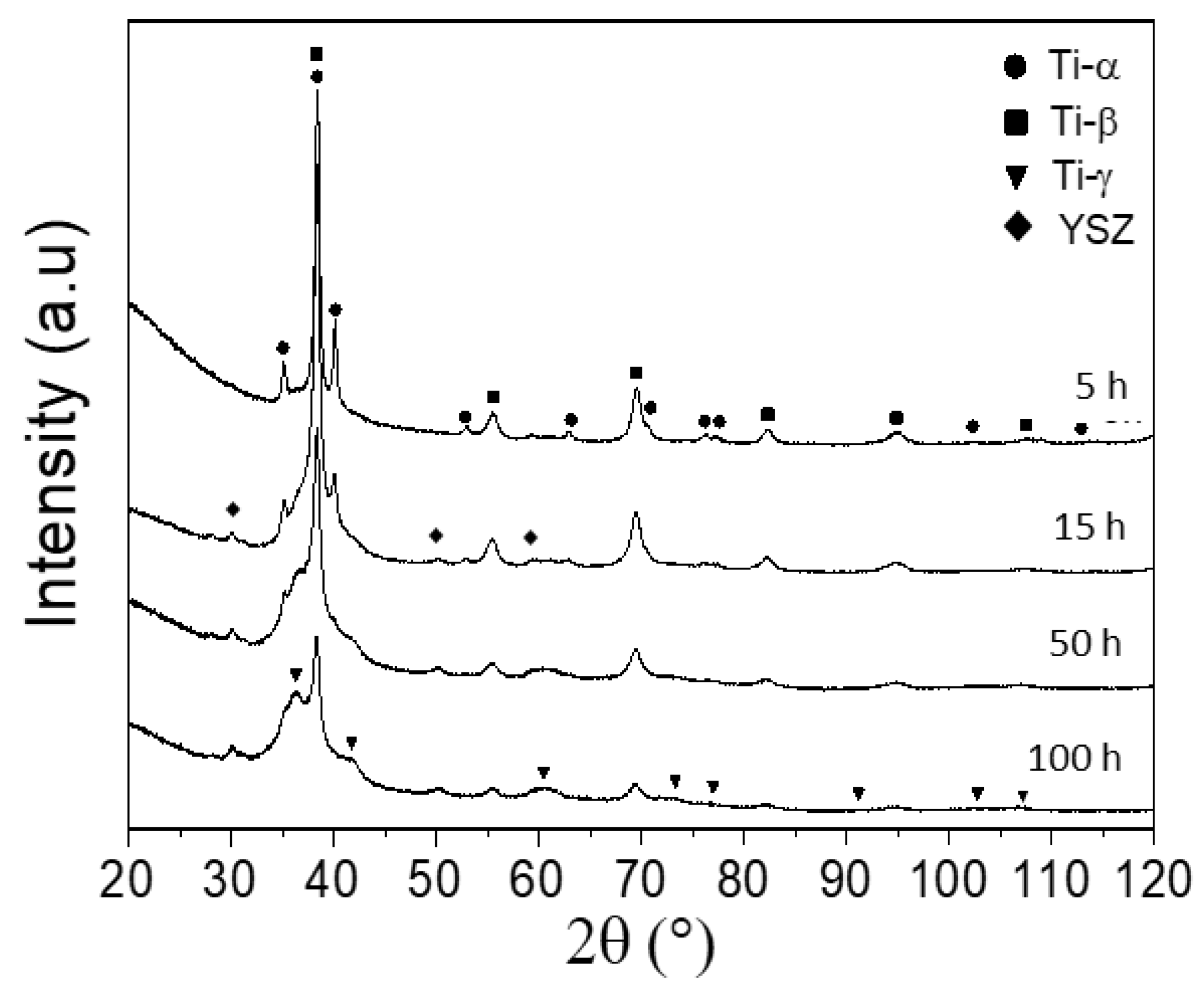
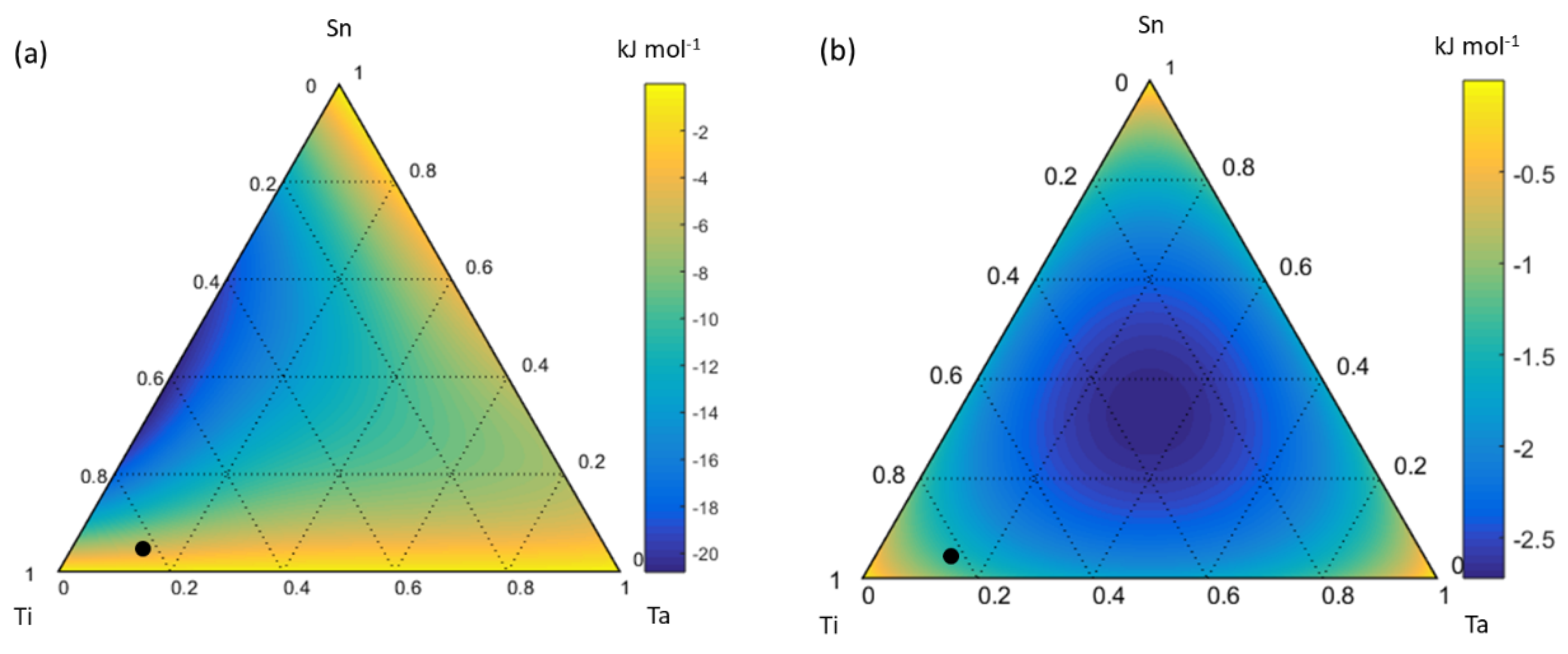
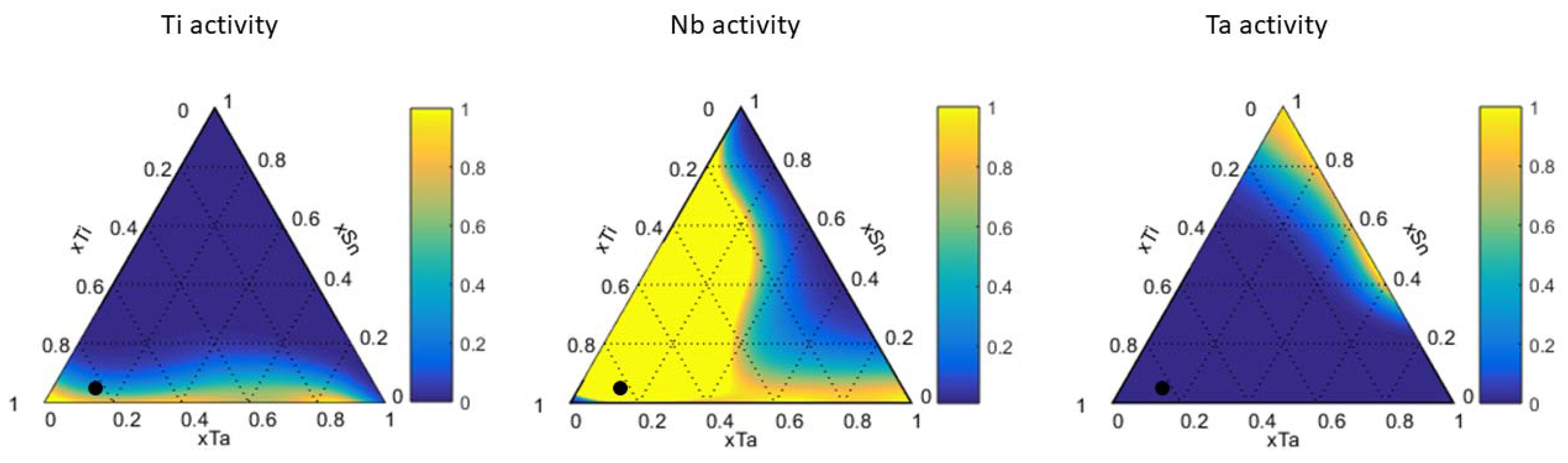
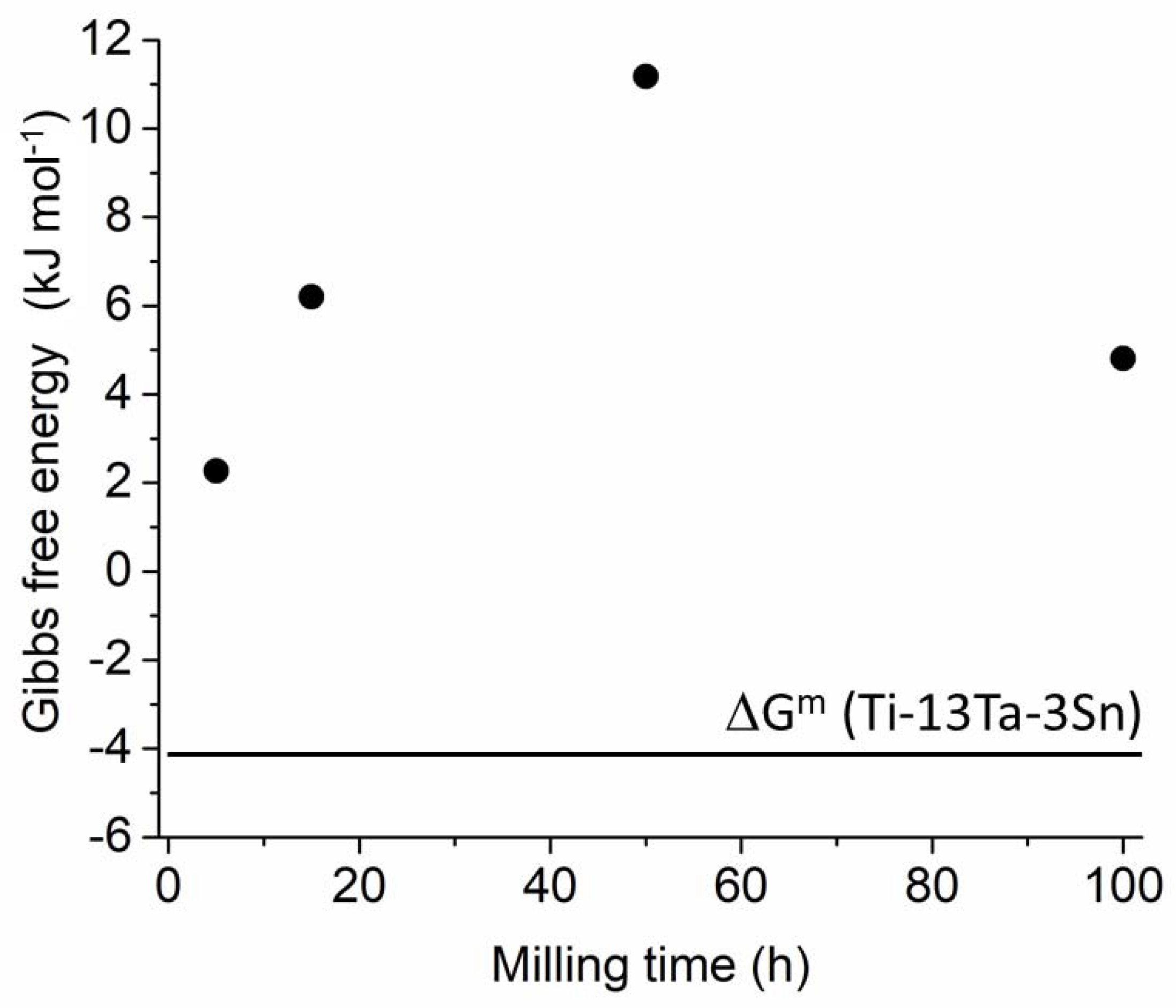



| β-Ti Solid Solution | ||
|---|---|---|
| Milling Time, h | D, nm | <ε2>1/2 × 10−3 |
| 5 | 20.14 | 2.96 |
| 15 | 7.35 | 0.46 |
| 50 | 4.08 | 0.83 |
| 100 | 9.49 | 1.77 |
| Ti-30Nb-13Ta | ||
| β-phase | ||
| Milling time, h | D, nm | <ε2>1/2 × 10−3 |
| 60 | 7.0 | N.I. |
| Ti-20Nb-30Ta | ||
| β−phase | ||
| Milling time, h | D, nm | <ε2>1/2 × 10−3 |
| 5 | 91 | 0.11 |
| 20 | 48 | 0.23 |
| 30 | 27 | 0.28 |
| 70 | 8 | 0.64 |
| Ti-33Nb-4Mn | ||
|---|---|---|
| γ Phase | ||
| Milling Time, h | D, nm | <ε2>1/2 × 10−3 |
| 1 | 54 | 4.55 |
| 10 | 30 | 11.15 |
| 20 | 6.6 | 6.64 |
| 40 | 6.3 | 2.38 |
| 60 | 5.9 | 0.41 |
| 80 | 5.7 | 0.23 |
| 100 | 5.4 | 0.85 |
| 120 | 4.8 | 2.13 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar, C.; Martinez, C.; Tello, K.; Palma, S.; Delonca, A.; San Martín, F.; Alfonso, I. Thermodynamic Analysis of the Formation of FCC and BCC Solid Solutions of Ti-Based Ternary Alloys by Mechanical Alloying. Metals 2020, 10, 510. https://doi.org/10.3390/met10040510
Aguilar C, Martinez C, Tello K, Palma S, Delonca A, San Martín F, Alfonso I. Thermodynamic Analysis of the Formation of FCC and BCC Solid Solutions of Ti-Based Ternary Alloys by Mechanical Alloying. Metals. 2020; 10(4):510. https://doi.org/10.3390/met10040510
Chicago/Turabian StyleAguilar, Claudio, Carola Martinez, Karem Tello, Sergio Palma, Adeline Delonca, Francisca San Martín, and Ismeli Alfonso. 2020. "Thermodynamic Analysis of the Formation of FCC and BCC Solid Solutions of Ti-Based Ternary Alloys by Mechanical Alloying" Metals 10, no. 4: 510. https://doi.org/10.3390/met10040510






