A Bibliometric Analysis of the Publications on In Doped ZnO to be a Guide for Future Studies
Abstract
:1. Introduction
2. Materials and Methods
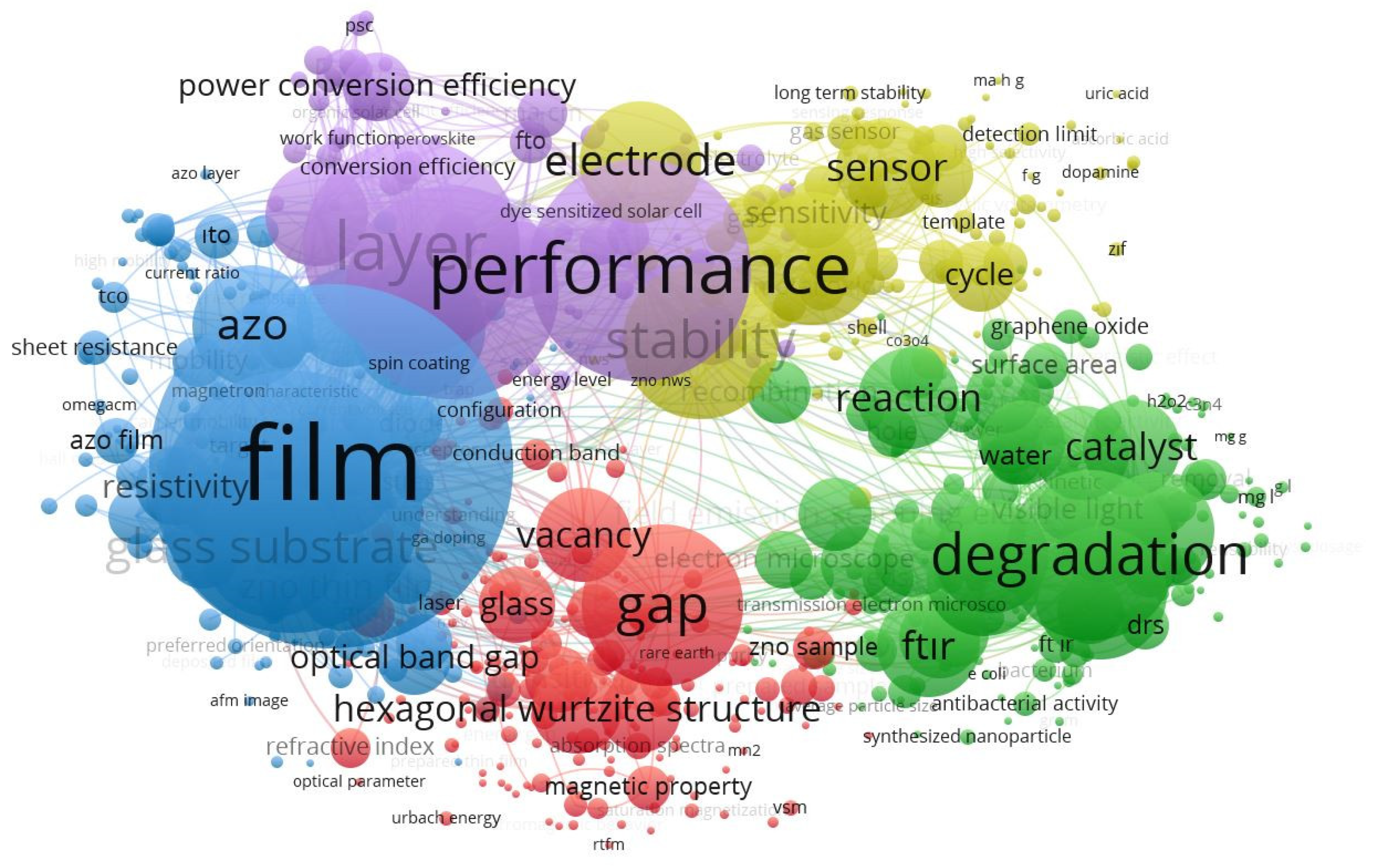
3. Results
- Generally, increasing crystallite size is linked with the ionic radii of the dopant and host ions. That is, increased crystallite sizes are likely to occur frequently, especially when larger atoms are used as the dopant. In this case, larger grains will be expected because hosted atoms with larger size replace the Zn atoms in the lattice. Additionally, some researchers mentioned that the morphology of the samples varies because of particle agglomeration due to indium content and therefore bigger particles were obtained [32,35,36,39,52].
4. Conclusions
- The studies mostly focus on some characteristics of In doped ZnO thin films such as structural, optical and electrical features. It is suggested that authors also pay attention to studies on the magnetic properties of ZnO. It is advised that the evaluation of ZnO’s characteristics should also be made taking growth techniques into consideration.
- For researchers who want to examine the sensitivity of ZnO to ammonia, ethanol, acetone and water vapors, it may be suggested to work with (100) directed ZnO films due to the fact that films with (100) preferred orientation have much higher conductivity and higher sensing performance.
- Research on In doped ZnO generally focuses on its synthesis and characterization in its thin film form. However, studies on In-doped ZnO nanostructures are limited. Therefore, it is important for researchers to study this subject to fill the gap in the field.
- In doped ZnO films have been used in device applications as interlayer structures. Thus, it is recommended that the authors should concentrate on studies investigating the performance of non-shaped indium doped ZnO structures in device applications.
- Maldonado’s studies on Indium doped ZnO entitled "Indium-doped ZnO thin films deposited by the sol-gel technique [63]” and “Characterization of indium-doped zinc oxide films deposited by pyrolytic spray with different indium compounds as dopant [64]” have the most citations. Researchers can use these studies and can also examine other works published in the same journal.
- The studies of Major, Minami and Ozgur could contribute to researchers’ future studies on this subject. Researchers can use these studies as a relevant source in future studies.
- Articles entitled "Zinc oxide thin films by the spray pyrolysis method [65]” and “Highly Transparent and Conducting Indium-Doped Zinc-Oxide Films by Spray Pyrolysis [66]” have more citations than the others in Thin Solid Films. Researchers can use these studies as a source reference in future studies.
- Applied Physics Letters, Thin Solid Films, Journal of Applied Physics, Physical Review, Applied Surface Science are leader journals with their studies on IZO. However, Solar Energy, Ceramics International, Applied Physics-A, Optik, Material Research Express, ACS Applied Materials and Interfaces, Optical Materials published more studies from 2016 to the present. Therefore, it is suggested that all these journals can contribute to the future study of the researchers on this subject.
- The studies entitled "Large and abrupt optical band gap variation in In-doped ZnO [67]" and "ZnO nanowires and nanobelts: Shape selection and thermodynamic modeling [68]" received more citations than others in the Applied Physics Letters. Authors could use these studies as a main source when writing a literature discussion in their future works.
- The countries with the highest number of documents on In doped ZnO are China, India, South Korea, USA and Japan. Researchers working in this field can undertake post-doctoral studies in the aforementioned countries. In addition, international projects can be produced by collaborating with these countries.
- In Mexico, Australia, Austria, Ukraine, Thailand, Egypt, Belgium, Netherlands, Iraq and Morocco, it is seen that the studies on indium doped ZnO are very few. It is recommended that these countries collaborate with the leading countries in the field (China and India) to produce more publications.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ranu, B.C.; Mandal, T. Indium(I) Iodide-Promoted Cleavage of Diaryl Diselenides and Disulfides and Subsequent Condensation with Alkyl or Acyl Halides. One-Pot Efficient Synthesis of Diorganyl Selenides, Sulfides, Selenoesters, and Thioesters. J. Org. Chem. 2004, 69, 5793–5795. [Google Scholar] [CrossRef] [PubMed]
- Schneider, U.; Dao, H.T.; Kobayashi, S. Unusual Carbon−Carbon Bond Formations between Allylboronates and Acetals or Ketals Catalyzed by a Peculiar Indium(I) Lewis Acid. Org. Lett. 2010, 12, 2488–2491. [Google Scholar] [CrossRef] [PubMed]
- Habashi, F. Indium, Physical and Chemical Properties. In Encyclopedia of Metalloproteins; Springer: New York, NY, USA, 2013; pp. 981–982. [Google Scholar]
- Choubey, P.K.; Jha, M.K.; Gupta, D.; Jeong, J.; Lee, J.-C. Recovery of Rare Metal Indium (In) from Discarded LCD Monitors. In Rare Metal Technology 2014; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 37–42. ISBN 9781118888827. [Google Scholar]
- Hofmann, M.; Hofmann, H.; Hagelüken, C.; Hool, A. Critical raw materials: A perspective from the materials science community. Sustain. Mater. Technol. 2018, 17, e00074. [Google Scholar] [CrossRef]
- European Commıssıon Commıttee and the Commıttee of the Regıons on the 2017 list of Critical Raw Materials for the EU. Commun. From Comm. Eur. Parliam. Counc. Eur. Econ. Soc. Comm. Comm. Reg. 2017, XIV, 293.
- Yilmaz, M.; Grilli, M.L. The modification of the characteristics of nanocrystalline ZnO thin films by variation of Ta doping content. Philos. Mag. 2016, 96, 2125–2142. [Google Scholar] [CrossRef]
- Yilmaz, M.; Tatar, D.; Sonmez, E.; Cirak, C.; Aydogan, S.; Gunturkun, R. Investigation of Structural, Morphological, Optical, and Electrical Properties of Al Doped ZnO Thin Films Via Spin Coating Technique. Synth. React. Inorganic, Met. Nano-Metal Chem. 2016, 46, 489–494. [Google Scholar] [CrossRef]
- Yilmaz, M.; Turgut, G.; Aydin, S.; Ertugrul, M. Electrochemical deposition of ZnO thin films on to tin(IV) oxide:Fluorine. Asian J. Chem. 2012, 24, 3371–3374. [Google Scholar]
- Sun, X.; Li, Q.; Jiang, J.; Mao, Y. Morphology-tunable synthesis of ZnO nanoforest and its photoelectrochemical performance. Nanoscale 2014, 6, 8769–8780. [Google Scholar] [CrossRef]
- Kahraman, S.; Çakmak, H.M.; Çetinkaya, S.; Bayansal, F.; Çetinkara, H.A.; Güder, H.S. Characteristics of ZnO thin films doped by various elements. J. Cryst. Growth 2013, 363, 86–92. [Google Scholar] [CrossRef]
- Nunes, P.; Braz Fernandes, F.M.; Silva, R.J.C.; Fortunato, E.; Martins, R. Structural Characterisation of Zinc Oxide Thin Films Produced by Spray Pyrolysis. Key Eng. Mater. 2002, 230, 599–602. [Google Scholar] [CrossRef]
- Rashed, H.A.; Umran, N.M. The stability and electronic properties of Si-doped ZnO nanosheet: A DFT study. Mater. Res. Express 2019, 6, 045044. [Google Scholar] [CrossRef]
- Habashyani, S.; Özmen, A.; Aydogan, S.; Yilmaz, M. An examination of correlation between characteristic and device performance of ZnO films as a function of La content. Vacuum 2018, 157, 497–507. [Google Scholar] [CrossRef]
- Feng, T.-H.; Xia, X.-C. Characteristics of doping controllable ZnO films grown by photo-assisted metal organic chemical vapor deposition. Opt. Mater. Express 2017, 7, 1281. [Google Scholar] [CrossRef]
- Sukee, A.; Kantarak, E.; Singjai, P. Preparation of Aluminum doped Zinc Oxide Thin Films on Glass Substrate by Sparking Process and Their Optical and Electrical Properties. J. Phys. Conf. Ser. 2017, 901, 012153. [Google Scholar] [CrossRef]
- Arici, F.; Yildirim, P.; Caliklar, Ş.; Yilmaz, R.M. Research trends in the use of augmented reality in science education: Content and bibliometric mapping analysis. Comput. Educ. 2019, 142, 103647. [Google Scholar] [CrossRef]
- Yilmaz, R.M.; Topu, F.B.; Takkaç Tulgar, A. An examination of the studies on foreign language teaching in pre-school education: A bibliometric mapping analysis. Comput. Assist. Lang. Learn. 2019, 1–24. [Google Scholar] [CrossRef]
- Liao, H.; Tang, M.; Luo, L.; Li, C.; Chiclana, F.; Zeng, X.-J. A Bibliometric Analysis and Visualization of Medical Big Data Research. Sustainability 2018, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Melkers, J. Bibliometrics as a Tool for Analysis of R&D Impacts. In Evaluating R&D Impacts: Methods and Practice; Springer: Boston, MA, USA, 1993; pp. 43–61. [Google Scholar]
- Silva-Lopez, H.E.; Marcelino, B.S.; Guillen-Cervantes, A.; Zelaya-Angel, O.; Ramirez-Bon, R. Physical Properties of Sputtered Indium-doped ZnO Films Deposited on Flexible Transparent Substrates. Mater. Res. 2018, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Biswal, R.; Maldonado, A.; Vega-Pérez, J.; Acosta, D.R.; De La Luz Olvera, M. Indium Doped Zinc Oxide Thin Films Deposited by Ultrasonic Chemical Spray Technique, Starting from Zinc Acetylacetonate and Indium Chloride. Materials 2014, 7, 5038–5046. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Liu, B.; Li, L.; Kong, X.; Zheng, C.; Lin, H.; Tong, Q.; Tao, S.; Zhang, X. Excimer emission induced intra-system self-absorption enhancement – a novel strategy to realize high efficiency and excellent stability ternary organic solar cells processed in green solvents. J. Mater. Chem. A 2018, 6, 23840–23855. [Google Scholar] [CrossRef]
- Ting, C.-C.; Chang, S.-P.; Li, W.-Y.; Wang, C.-H. Enhanced performance of indium zinc oxide thin film transistor by yttrium doping. Appl. Surf. Sci. 2013, 284, 397–404. [Google Scholar] [CrossRef]
- Moradi-Haji Jafan, M.; Zamani-Meymian, M.-R.; Rahimi, R.; Rabbani, M. The effect of solvents and the thickness on structural, optical and electrical properties of ITO thin films prepared by a sol–gel spin-coating process. J. Nanostructure Chem. 2014, 4, 89. [Google Scholar] [CrossRef] [Green Version]
- Wade, K.; Banister, A. The Chemistry of Aluminium, Gallium, Indium and Thallium: Comprehensive Inorganic Chemistry; Pergamon Press: Oxford, UK, 2016. [Google Scholar]
- Shaheera, M.; Girija, K.G.; Kaur, M.; Geetha, V.; Debnath, A.K.; Vatsa, R.K.; Muthe, K.P.; Gadkari, S.C. Characterization and device application of indium doped ZnO homojunction prepared by RF magnetron sputtering. Opt. Mater. 2020, 101, 109723. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, B.; He, Y.; Cao, B.; Ma, W.; Chang, L. Oxygen defect-rich In-doped ZnO nanostructure for enhanced visible light photocatalytic activity. Mater. Chem. Phys. 2020, 244, 122672. [Google Scholar] [CrossRef]
- Bhatia, S.; Verma, N. Gas Sensing Performance of Dip-Coated Indium-Doped ZnO Films. J. Electron. Mater. 2018, 47, 6450–6457. [Google Scholar] [CrossRef]
- Kyaw, A.K.K.; Wang, Y.; Zhao, D.W.; Huang, Z.H.; Zeng, X.T.; Sun, X.W. The properties of sol-gel processed indium-doped zinc oxide semiconductor film and its application in organic solar cells. Phys. Status Solidi 2011, 208, 2635–2642. [Google Scholar] [CrossRef]
- El Filali, B.; Jaramillo Gomez, J.A.; Torchynska, T.V.; Casas Espinola, J.L.; Shcherbyna, L. Band-edge emission, defects, morphology and structure of in-doped ZnO nanocrystal films. Opt. Mater. 2019, 89, 322–328. [Google Scholar] [CrossRef]
- Khalfallah, B.; Chaabouni, F.; Abaab, M. Some physical investigations on In-doped ZnO films prepared by RF magnetron sputtering using powder compacted target. J. Mater. Sci. Mater. Electron. 2015, 26, 5209–5216. [Google Scholar] [CrossRef]
- Thambidurai, M.; Kim, J.Y.; Kang, C.M.; Muthukumarasamy, N.; Song, H.J.; Song, J.; Ko, Y.; Velauthapillai, D.; Lee, C. Enhanced photovoltaic performance of inverted organic solar cells with In-doped ZnO as an electron extraction layer. Renew. Energy 2014, 66, 433–442. [Google Scholar] [CrossRef]
- Benhaliliba, M.; Benouis, C.E.; Mouffak, Z.; Ocak, Y.S.; Tiburcio-Silver, A.; Aida, M.S.; Garcia, A.A.; Tavira, A.; Sanchez Juarez, A. Preparation and characterization of nanostructures of in-doped ZnO films deposited by chemically spray pyrolysis: Effect of substrate temperatures. Superlattices Microstruct. 2013, 63, 228–239. [Google Scholar] [CrossRef]
- Benouis, C.E.; Benhaliliba, M.; Sanchez Juarez, A.; Aida, M.S.; Chami, F.; Yakuphanoglu, F. The effect of indium doping on structural, electrical conductivity, photoconductivity and density of states properties of ZnO films. J. Alloys Compd. 2010, 490, 62–67. [Google Scholar] [CrossRef]
- Lee, C.; Lim, K.; Song, J. Highly textured ZnO thin films doped with indium prepared by the pyrosol method. Sol. Energy Mater. Sol. Cells 1996, 43, 37–45. [Google Scholar] [CrossRef]
- Rambu, A.P.; Sırbu, D.; Sandu, A.V.; Prodan, G.; Nıca, V. Influence of In doping on electro-optical properties of ZnO films. Bull. Mater. Sci. 2013, 36, 231–237. [Google Scholar] [CrossRef]
- Tang, K.; Gu, S.; Liu, J.; Ye, J.; Zhu, S.; Zheng, Y. Effects of indium doping on the crystallographic, morphological, electrical, and optical properties of highly crystalline ZnO films. J. Alloys Compd. 2015, 653, 643–648. [Google Scholar] [CrossRef]
- Jongthammanurak, S.; Cheawkul, T.; Witana, M. Morphological differences in transparent conductive indium-doped zinc oxide thin films deposited by ultrasonic spray pyrolysis. Thin Solid Film. 2014, 571, 114–120. [Google Scholar] [CrossRef]
- ILICAN, S.; İlgü Büyük, G. ZnO:Eu Filmlerinin Mikroyapısal ve Optik Özellikleri. Karadeniz Fen Bilim. Derg. 2018, 8, 141–153. [Google Scholar] [CrossRef]
- Marouf, S.; Beniaiche, A.; Guessas, H.; Azizi, A. Morphological, Structural and Optical Properties of ZnO Thin Films Deposited by Dip Coating Method. Mater. Res. 2016, 20, 88–95. [Google Scholar] [CrossRef] [Green Version]
- Karakaya, S. Annealing Effect on Structural and Optical Properties of ZnO Films Prepared by Ultrasonic Spray Pyrolysis. ANADOLU Univ. J. Sci. Technol. A Appl. Sci. Eng. 2016, 17, 670–676. [Google Scholar] [CrossRef] [Green Version]
- Purohit, A.; Chander, S.; Sharma, A.; Nehra, S.P.; Dhaka, M.S. Impact of low temperature annealing on structural, optical, electrical and morphological properties of ZnO thin films grown by RF sputtering for photovoltaic applications. Opt. Mater. 2015, 49, 51–58. [Google Scholar] [CrossRef]
- Ye, J.; Gu, S.; Zhu, S.; Chen, T.; Hu, L.; Qin, F.; Zhang, R.; Shi, Y.; Zheng, Y. The growth and annealing of single crystalline ZnO films by low-pressure MOCVD. J. Cryst. Growth 2002, 243, 151–156. [Google Scholar] [CrossRef]
- Prasada Rao, T.; Santhoshkumar, M.C. Highly oriented (100) ZnO thin films by spray pyrolysis. Appl. Surf. Sci. 2009, 255, 7212–7215. [Google Scholar] [CrossRef]
- Francombe, M.H.; Satō, H. Single Crystal Films; Pergamon Press: London, UK, 1964. [Google Scholar]
- Ilıcan, S.; Çağlar, Y.; Çağlar, M. X-ray Diffraction Studies of Undoped and in-Doped Cd0.22Zn0.78S Films Deposited by Spray Pyrolysis. Cankaya Univ. J. Arts Sci. 2005, 1, 85–94. [Google Scholar]
- Dimitrov, O.; Nesheva, D.; Blaskov, V.; Stambolova, I.; Vassilev, S.; Levi, Z.; Tonchev, V. Gas sensitive ZnO thin films with desired (002) or (100) orientation obtained by ultrasonic spray pyrolysis. Mater. Chem. Phys. 2014, 148, 712–719. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, N.; Mehra, R.M.; Kapoor, A.; Purohit, L.P.; Swart, H.C. Role of film thickness on the properties of ZnO thin films grown by sol-gel method. Thin Solid Films 2013, 539, 161–165. [Google Scholar] [CrossRef]
- Liu, F.-C.; Li, J.-Y.; Chen, T.-H.; Chang, C.-H.; Lee, C.-T.; Hsiao, W.-H.; Liu, D.-S. Effect of Silver Dopants on the ZnO Thin Films Prepared by a Radio Frequency Magnetron Co-Sputtering System. Materials 2017, 10, 797. [Google Scholar] [CrossRef]
- Obeid, M.M.; Jappor, H.R.; Al-Marzoki, K.; Al-Hydary, I.A.; Edrees, S.J.; Shukur, M.M. Unraveling the effect of Gd doping on the structural, optical, and magnetic properties of ZnO based diluted magnetic semiconductor nanorods. RSC Adv. 2019, 9, 33207–33221. [Google Scholar] [CrossRef] [Green Version]
- Khashan, K.S.; Mahdi, M. Preparation of indium-doped zinc oxide nanoparticles by pulsed laser ablation in liquid technique and their characterization. Appl. Nanosci. 2017, 7, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Hamberg, I.; Granqvist, C.G. Evaporated Sn-doped In 2O3 films: Basic optical properties and applications to energy-efficient windows. J. Appl. Phys. 1986, 60, R123–R160. [Google Scholar] [CrossRef]
- Singh, A.; Chaudhary, S.; Pandya, D.K. High conductivity indium doped ZnO films by metal target reactive co-sputtering. Acta Mater. 2016, 111, 1–9. [Google Scholar] [CrossRef]
- Cao, Y.; Miao, L.; Tanemura, S.; Tanemura, M.; Kuno, Y.; Hayashi, Y.; Mori, Y. Optical Properties of Indium-Doped ZnO Films. Jpn. J. Appl. Phys. 2006, 45, 1623–1628. [Google Scholar] [CrossRef]
- Aydoğan, Ş.; Grilli, M.L.; Yilmaz, M.; Çaldiran, Z.; Kaçuş, H. A facile growth of spray based ZnO films and device performance investigation for Schottky diodes: Determination of interface state density distribution. J. Alloys Compd. 2017, 708, 55–66. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, S.M.; Kim, H.-S.; Kim, H.Y.; Park, J.; Jung, S.-B.; Park, G.C.; Kim, J.; Joo, J. Synergistic effect of Indium and Gallium co-doping on growth behavior and physical properties of hydrothermally grown ZnO nanorods. Sci. Rep. 2017, 7, 41992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-S.; Tai, W.-P.; Shu, S.-J. Effect of preheating temperature on structural and optical properties of ZnO thin films by sol–gel process. Thin Solid Films 2005, 491, 153–160. [Google Scholar] [CrossRef]
- Lim, S.Y.; Brahma, S.; Liu, C.-P.; Wang, R.-C.; Huang, J.-L. Effect of indium concentration on luminescence and electrical properties of indium doped ZnO nanowires. Thin Solid Film. 2013, 549, 165–171. [Google Scholar] [CrossRef]
- Hori, Y.; Shiota, Y.; Ida, T.; Yoshizawa, K.; Mizuno, M. Local structures and electronic properties of In atoms in In-doped ZnO. Thin Solid Film. 2019, 685, 428–433. [Google Scholar] [CrossRef]
- Chirakkara, S.; Nanda, K.K.; Krupanidhi, S.B. Pulsed laser deposited ZnO: In as transparent conducting oxide. Thin Solid Films 2011, 519, 3647–3652. [Google Scholar] [CrossRef]
- Caglar, M.; Caglar, Y.; Ilican, S. Electrical and optical properties of undoped and In-doped ZnO thin films. Phys. Status Solidi c 2007, 4, 1337–1340. [Google Scholar] [CrossRef]
- Kumar, P.M.R.; Kartha, C.S.; Vijayakumar, K.P.; Abe, T.; Kashiwaba, Y.; Singh, F.; Avasthi, D.K. On the properties of indium doped ZnO thin films. Semicond. Sci. Technol. 2004, 20, 120–126. [Google Scholar] [CrossRef]
- Edinger, S.; Bansal, N.; Bauch, M.; Wibowo, R.A.; Újvári, G.; Hamid, R.; Trimmel, G.; Dimopoulos, T. Highly transparent and conductive indium-doped zinc oxide films deposited at low substrate temperature by spray pyrolysis from water-based solutions. J. Mater. Sci. 2017, 52, 8591–8602. [Google Scholar] [CrossRef] [Green Version]
- Caglar, Y.; Zor, M.; Caglar, M.; Ilican, S. Influence of the indium incorporation on the structural and electrical properties of zinc oxide films. J. Optoelectron. Adv. Mater. 2006, 8, 1867–1873. [Google Scholar]
- Nasir, M.F.; Hannas, M.; Mamat, M.H.; Rusop, M. Electrical Properties of Indium-Doped Zinc Oxide Nanostructures Doped at Different Dopant Concentrations. In Proceedings of the Nanoscience, Nanotechnology and Nanoengineering: Fundamentals and Applications; Trans Tech Publications Ltd.: Baech, Switzerland, 2015; Volume 1109, pp. 593–597. [Google Scholar]
- Peng, L.; Fang, L.; Zhao, Y.; Wu, W.; Ruan, H.; Kong, C. Growth and characterization of indium doped zinc oxide films sputtered from powder targets. J. Wuhan Univ. Technol. Sci. Ed. 2017, 32, 866–870. [Google Scholar] [CrossRef]
- Mahesh, D.; Kumar, M.C.S. Synergetic effects of aluminium and indium dopants in the physical properties of ZnO thin films via spray pyrolysis. Superlattices Microstruct. 2020, 142, 106511. [Google Scholar] [CrossRef]
- Benzitouni, S.; Zaabat, M.; Mahdjoub, A.; Benaboud, A.; Boudine, B. High transparency and conductivity of heavily In-doped ZnO thin films deposited by dip-coating method. Mater. Sci. 2018, 36, 427–434. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.; Chunlei, W.; Su, W.-B.; Manan, A.; Ahmad, A.S.; Rehman, A.U. Thermoelectric properties of indium-doped zinc oxide sintered in an argon atmosphere. J. Mater. Sci. Mater. Electron. 2019, 30, 4813–4818. [Google Scholar] [CrossRef]
- Winkler, N.; Wibowo, A.; Kubicek, B.; Kautek, W.; Ligorio, G.; List-Kratochvil, E.; Dimopoulos, T. Rapid Processing of In-Doped ZnO by Spray Pyrolysis from Environment-Friendly Precursor Solutions. Coatings 2019, 9, 245. [Google Scholar] [CrossRef] [Green Version]
- Mahesh, D.; Kumar, B.H.; Kumar, M.C.S. Enhanced luminescence property of 1 D nanorods realised by aqueous chemical growth on indium doped zinc oxide thin films. Thin Solid Film. 2019, 686, 137279. [Google Scholar] [CrossRef]
- Kotlyarchuk, B.; Savchuk, V.; Oszwaldowski, M. Preparation of undoped and indium doped ZnO thin films by pulsed laser deposition method. Cryst. Res. Technol. 2005, 40, 1118–1123. [Google Scholar] [CrossRef]
- Wang, B.; Callahan, M.J.; Xu, C.; Bouthillette, L.O.; Giles, N.C.; Bliss, D.F. Hydrothermal growth and characterization of indium-doped-conducting ZnO crystals. J. Cryst. Growth 2007, 304, 73–79. [Google Scholar] [CrossRef]
- Ye, Z.-Z.; Tang, J.-F. Transparent conducting indium doped ZnO films by dc reactive S-gun magnetron sputtering. Appl. Opt. 1989, 28, 2817. [Google Scholar] [CrossRef]
- Joseph, B.; Manoj, P.K.; Vaidyan, V.K. Studies on preparation and characterization of indium doped zinc oxide films by chemical spray deposition. Bull. Mater. Sci. 2005, 28, 487–493. [Google Scholar] [CrossRef]
- Qin, G.-P.; Zhang, H.; Ruan, H.-B.; Wang, J.; Wang, D.; Kong, C.-Y. Effect of Post-Annealing on Structural and Electrical Properties of ZnO: In Films. Chin. Phys. Lett. 2019, 36, 047301. [Google Scholar] [CrossRef]
- Barquinha, P.; Gonçalves, G.; Pereira, L.; Martins, R.; Fortunato, E. Effect of annealing temperature on the properties of IZO films and IZO based transparent TFTs. Thin Solid Film. 2007, 515, 8450–8454. [Google Scholar] [CrossRef]
- Özmen, A.; Aydogan, S.; Yilmaz, M. Fabrication of spray derived nanostructured n-ZnO/p-Si heterojunction diode and investigation of its response to dark and light. Ceram. Int. 2019, 45, 14794–14805. [Google Scholar] [CrossRef]
- Luna-Arredondo, E.J.; Maldonado, A.; Asomoza, R.; Acosta, D.R.; Meléndez-Lira, M.A.; Olvera, M. de la L. Indium-doped ZnO thin films deposited by the sol–gel technique. Thin Solid Film. 2005, 490, 132–136. [Google Scholar] [CrossRef]
- Gómez, H.; Maldonado, A.; Asomoza, R.; Zironi, E.P.; Cañetas-Ortega, J.; Palacios-Gómez, J. Characterization of indium-doped zinc oxide films deposited by pyrolytic spray with different indium compounds as dopants. Thin Solid Film. 1997, 293, 117–123. [Google Scholar] [CrossRef]
- Krunks, M.; Mellikov, E. Zinc oxide thin films by the spray pyrolysis method. Thin Solid Films 1995, 270, 33–36. [Google Scholar] [CrossRef]
- Major, S.; Banerjee, A.; Chopra, K.L. Highly transparent and conducting indium-doped zinc oxide films by spray pyrolysis. Thin Solid Film. 1983, 108, 333–340. [Google Scholar] [CrossRef]
- Kim, K.J.; Park, Y.R. Large and abrupt optical band gap variation in In-doped ZnO. Appl. Phys. Lett. 2001, 78, 475–477. [Google Scholar] [CrossRef]
- Fan, H.J.; Barnard, A.S.; Zacharias, M. ZnO nanowires and nanobelts: Shape selection and thermodynamic modeling. Appl. Phys. Lett. 2007, 90, 143116. [Google Scholar] [CrossRef]











| The Study (As a Function of Increasing “In” Rate) | Growth Technique | Crystallite Size (nm) | Optical Band Gap (eV) | Resistivity (Ωcm) |
|---|---|---|---|---|
| Filali et al. [31] | Ultrasonic spray pyrolysis | 26–28; 21–23 | 3.31; 3.27 | - |
| Khalfallah et al. [32] | RF magnetron sputtering | 24.91; 31.06 | 3.27; 3.36 | 5.35 × 10−3 |
| Thambidurai et al. [33] | Sol-gel | 22.4; 7.3 | 3.12; 3.23 | 7.98 × 10−3; 2.40 × 10−3 |
| Benhaliliba et al. [34] | Ultrasonic spray pyrolysis technique | 39; 24 | 3.18; 3.21 | ~385; ~8.35 |
| Benouis et al. [35] | Spray pyrolysis | 26; 55 | 3.28; 3.35 | 17 × 10−3; 6 × 10−3 |
| Lee et al. [36] | Pyrosol method | ~49; ~62 | -- | 1.3 × 10−2; 3.0 × 10-3 |
| Silva-Lopez et al. [21] | RF magnetron sputtering | 16.4; 16.0 | 3.2 ± 0.1 | 2.9 ± 0.7 × 10−3 |
| Rambu et al. [37] | Thermal evaporation | 29; 24 | 3.32; 3.38 | 4.12 × 10−3; 4.26 |
| Tang et al. [38] | MOCVD | 38.2; 20.1 | ~3.25; ~3.18 | ~30; ~6 × 10−3 |
| Jongthammanurak et al. [39] | Ultrasonic spray pyrolysis technique | 18.7; 25.4 | 3.300; 3.293 | 0.521; 0.176 |
| Sample Name | Growing Tech. | Doping Ratio (at%) | Resistivity (Ωcm) |
|---|---|---|---|
| IZO 3 [68] | Spray pyrolysis | 3 | 3.48 × 10−2 |
| IZO33 [69] | Dip coating | 0.33 | 1.48 |
| (Zn1−xInx)O [70] | Solid-state reaction | 0.02 | 1.884 × 10−3 |
| IZO-6 [71] | Ultrasonic spray pyrolysis | 4 | 5.7 × 10−3 |
| In:ZnO [72] | Spray Pyrolysis | 2.5 | 1.77 × 10−2 |
| ZnO:In [73] | PLD | - | 10−1 |
| IZO [54] | Dc reactive co-sputtering | 3.2 | 3.6 × 10−4 |
| In:ZnO [74] | Hydrothermal | 0.55 | 1.5 × 10−2 |
| ZnO-1 [75] | Modified S-gun magnetron sputtering | 6 | 1.08 × 10−3 |
| ZnO:In [36] | Pyrosol spray method | 3 | 3 × 10−3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, M.; Grilli, M.L.; Turgut, G. A Bibliometric Analysis of the Publications on In Doped ZnO to be a Guide for Future Studies. Metals 2020, 10, 598. https://doi.org/10.3390/met10050598
Yilmaz M, Grilli ML, Turgut G. A Bibliometric Analysis of the Publications on In Doped ZnO to be a Guide for Future Studies. Metals. 2020; 10(5):598. https://doi.org/10.3390/met10050598
Chicago/Turabian StyleYilmaz, Mehmet, Maria Luisa Grilli, and Guven Turgut. 2020. "A Bibliometric Analysis of the Publications on In Doped ZnO to be a Guide for Future Studies" Metals 10, no. 5: 598. https://doi.org/10.3390/met10050598





