Metastable Austenite Transformation Kinetics of Medium-Carbon Silicon-Rich Steel during Partitioning in a Q & P Process
Abstract
:1. Introduction
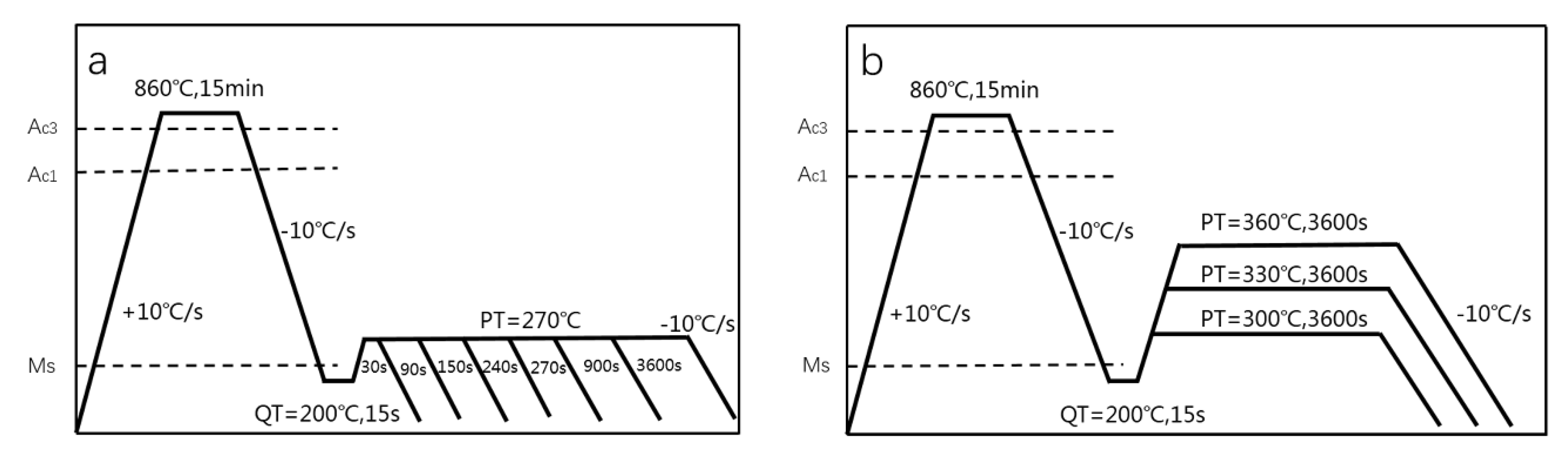
2. Experimental Materials and Methods
3. Results and Discussion
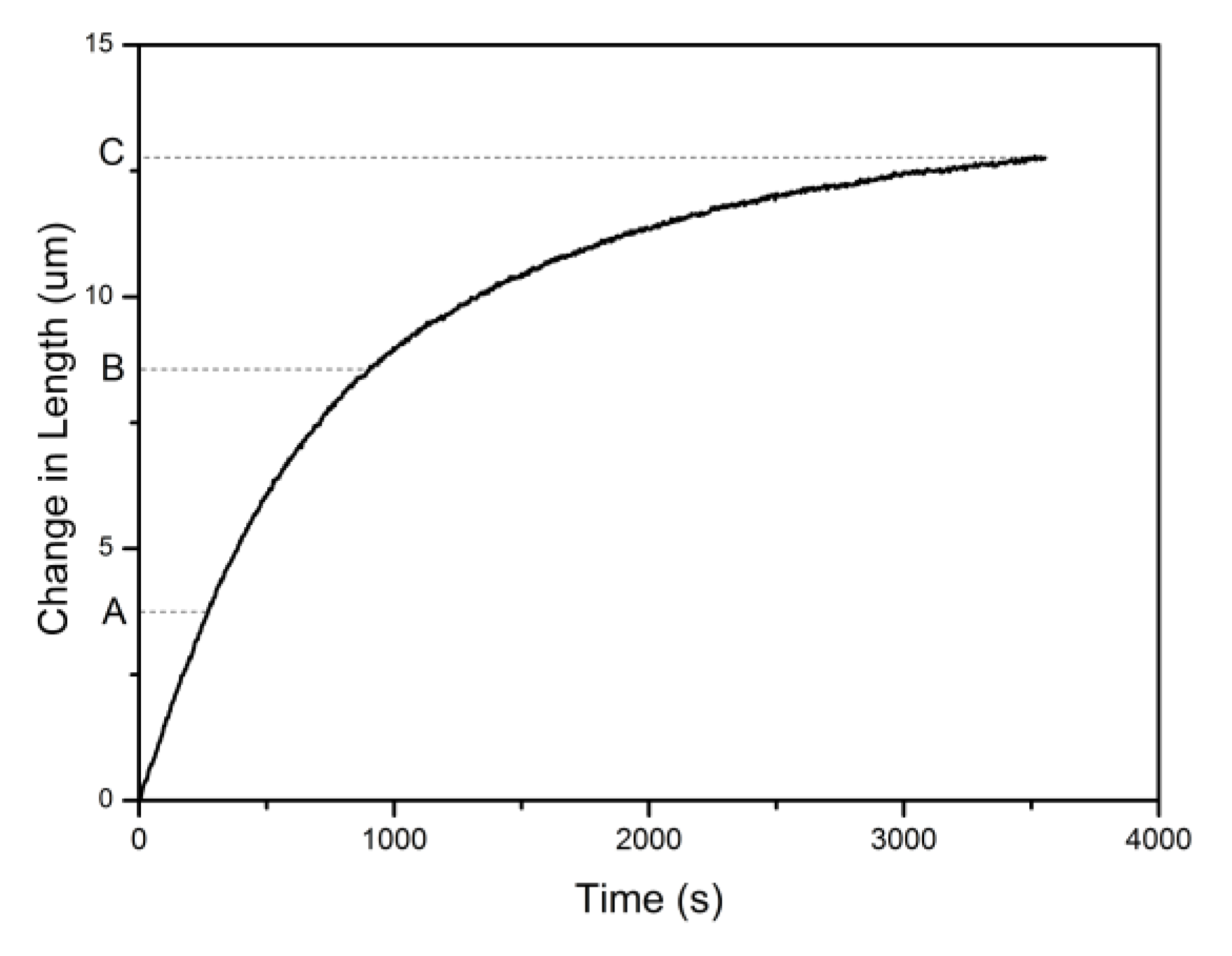
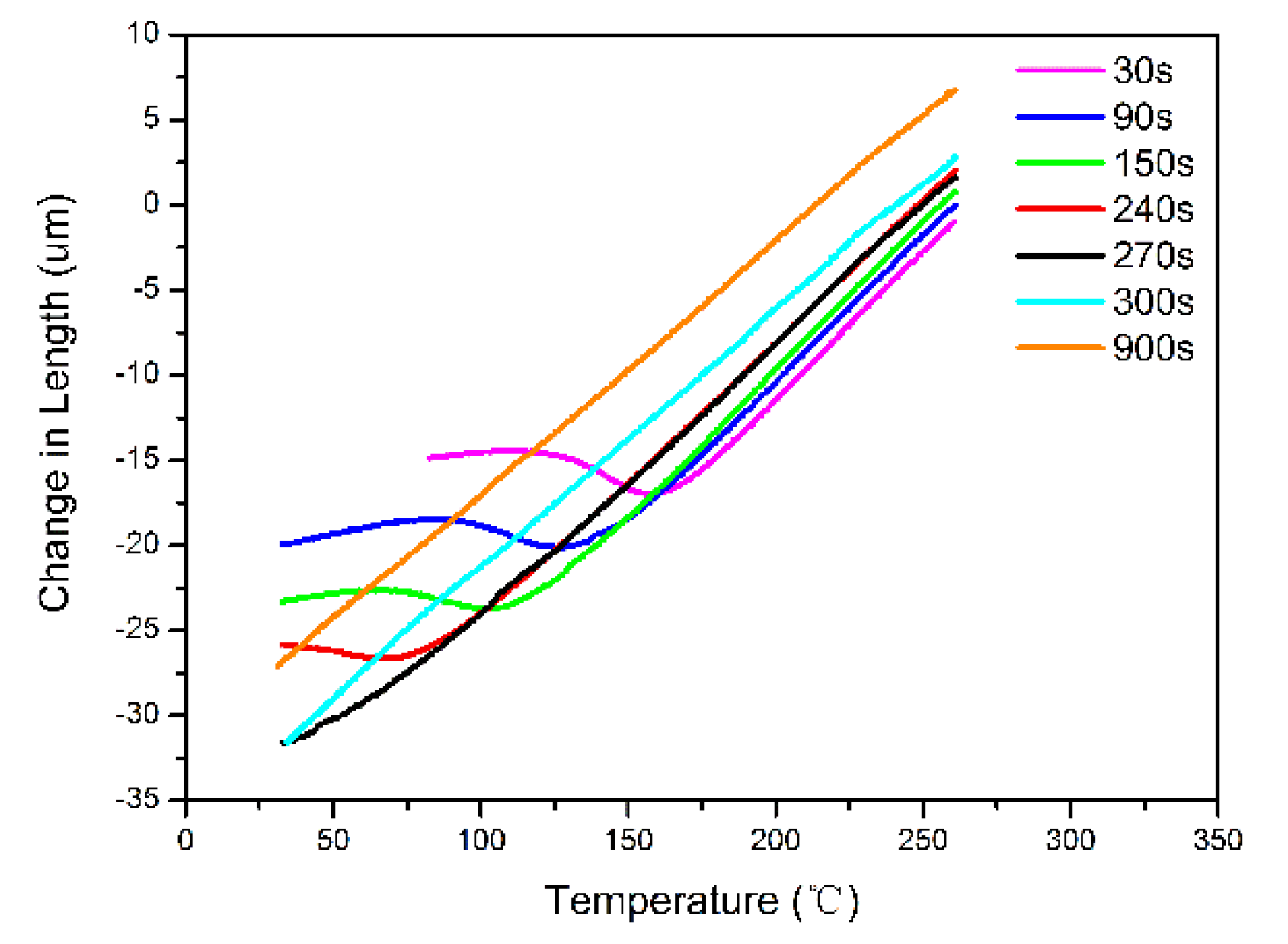
3.1. The Kinetics of Transformation
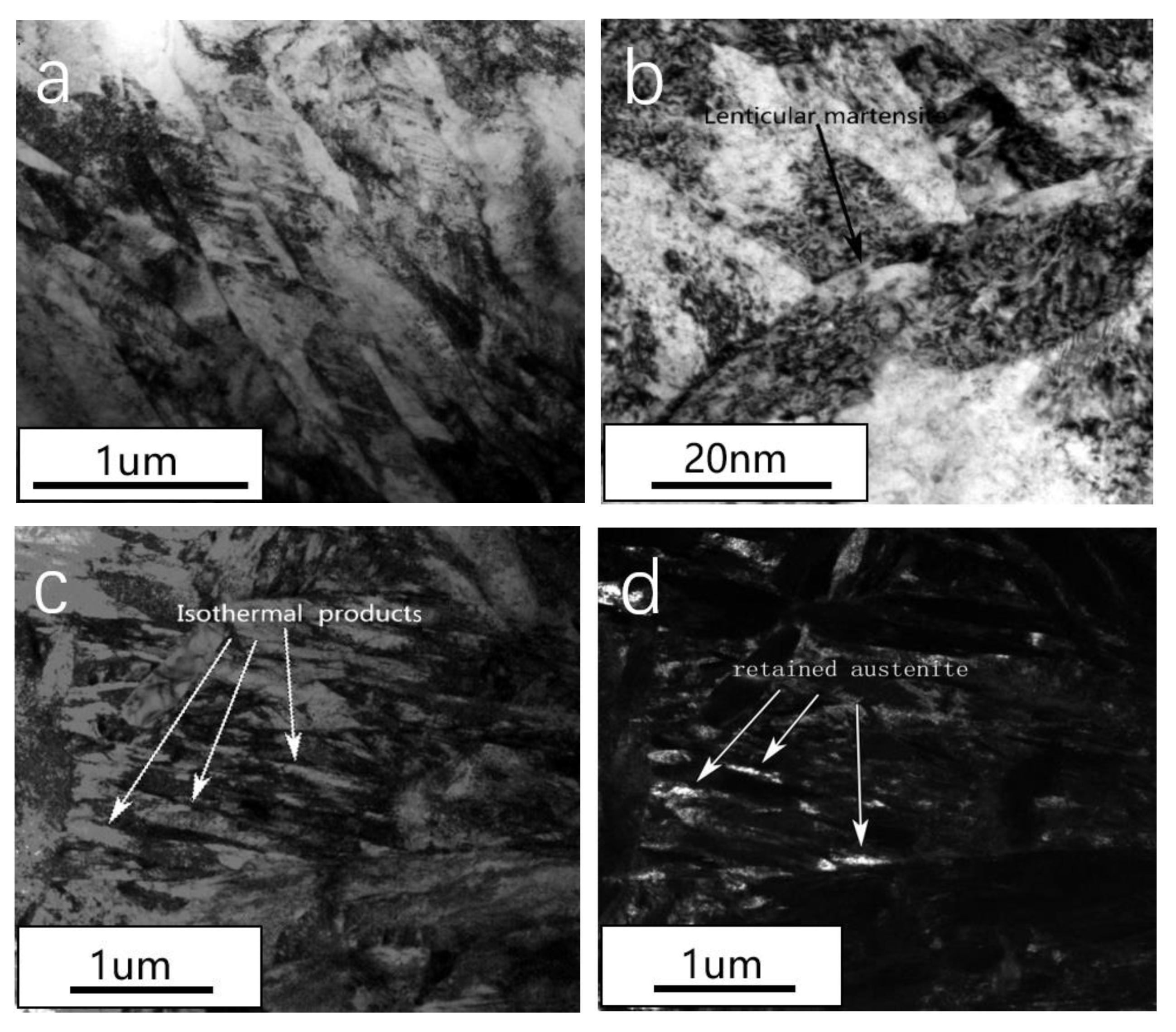
3.2. Microstructure Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Ehrhardt, B.; Gerber, T.; Schaumann, T.W. Approaches to microstructural design of TRIP and TRIP aided cold rolled high strength steels. In Proceedings of the International Conference on Advanced High Strength Sheet Steels for Automotive Applications, Winter Park, CO, USA, 6–9 June 2004; pp. 39–50. [Google Scholar]
- Covarrubias, O.; Guerrero, M.P.; Colas, R.; Petrov, R.; Kestens, L.; Houbaert, Y. Transformation behaviour of Si and Mn bearing low carbon steels. In Proceedings of the International Conference on TRIP-Aided High Strength Ferrous Alloys; GRIPS: Aachen, Germany, 2002; pp. 227–230. [Google Scholar]
- Kim, S.; Lee, C.; Choi, I.; Lee, S. Effects of heat treatment and alloying elements on the microstructures and mechanical properties of 0.15 wt pct C transformation-induced plasticity-aided cold-rolled steel sheets. Metall. Mater. Trans. A 2001, 32, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Lee, S.; De Cooman, B.C. Microstructure of Low C Steel Isothermally Transformed in the M S to M f Temperature Range. Metall. Mater. Trans. A 2012, 43, 4967–4983. [Google Scholar] [CrossRef] [Green Version]
- Van Bohemen, S.M.C.; Santofimia, M.J.; Sietsma, J. Experimental evidence for bainite formation below Ms in Fe–0.66C. Scr. Mater. 2008, 58, 488–491. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C.; Sietsma, J. The kinetics of bainite and martensite formation in steels during cooling. Mater. Sci. Eng. A 2010, 527, 6672–6676. [Google Scholar] [CrossRef]
- Da Silva, E.P.; De Knijf, D.; Xu, W.; Fojer, C.; Houbaert, Y.; Sietsma, J.; Petrov, R. Isothermal transformations in advanced high strength steels below martensite start temperature. Mater. Sci. Technol. 2015, 31, 808–816. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Zebarjad, S.M. Isothermal transformation of austenite to bainite in high carbon steels. J. Mater. Process. Technol. 2007, 189, 107–113. [Google Scholar] [CrossRef]
- Samanta, S.; Biswas, P.; Giri, S.; Singh, S.B.; Kundu, S. Formation of bainite below the M temperature: Kinetics and crystallography. Acta Mater. 2016, 105, 390–403. [Google Scholar] [CrossRef]
- Somani, M.C.; Porter, D.A.; Karjalainen, L.P.; Misra, R.D.K. On Various Aspects of Decomposition of Austenite in a High-Silicon Steel during Quenching and Partitioning. Metall. Mater. Trans. A 2014, 45, 1247–1257. [Google Scholar] [CrossRef]
- Samanta, S.; Das, S.; Chakrabarti, D.; Samajdar, I.; Singh, S.B.; Haldar, A. Development of Multiphase Microstructure with Bainite, Martensite, and Retained Austenite in a Co-Containing Steel Through Quenching and Partitioning (Q & P) Treatment. Metall. Mater. Trans. A 2013, 44A, 5653–5664. [Google Scholar] [CrossRef]
- Li, Y.J.; Mao, Q.J.; Kang, J.; Wang, X.H.; Yuan, G.; Wang, G.D. Determination of retained austenite using CCE model accounting for isothermal transformation in a low density quenched and partitioned steel. Mater. Lett. 2019, 239, 90–93. [Google Scholar] [CrossRef]
- Chen, S.; Wang, G.; Liu, C.; Wang, C.; Zhao, X.; Xu, W. Correlation of isothermal bainite transformation and austenite stability in quenching and partitioning steels. J. Iron Steel Res. Int. 2017, 24, 1095–1103. [Google Scholar] [CrossRef]
- HajyAkbary, F.; Sietsma, J.; Miyamoto, G.; Furuhara, T.; Santofimia, M.J. Interaction of carbon partitioning, carbide precipitation and bainite formation during the Q & P process in a low C steel. Acta Mater. 2016, 104, 72–83. [Google Scholar] [CrossRef]
- Koistinen, D.P.; Marburger, R.E. A general equation prescribing the extent of the austenite-martensite transformation in pure iron-carbon alloys and plain carbon steels. Acta Metall. 1959, 7, 59–60. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Barlat, F.; Lee, M. Transformation kinetics and density models of quenching and partitioning (Q & P) steels. Acta Mater. 2016, 109, 394–404. [Google Scholar] [CrossRef]
- Kinsman, K.R.; Shyne, J.G. Thermal stabilization of austenite in iron-nickel-carbon alloys. Acta Metall. 1967, 15, 1527–1543. [Google Scholar] [CrossRef]
- Speer, J.G.; Edmonds, D.V.; Rizzo, F.C.; Matlock, D.K. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Curr. Opin. Solid State Mater. Sci. 2004, 8, 219–237. [Google Scholar] [CrossRef]
- Kung, C.; Rayment, J. An examination of the validity of existing empirical formulae for the calculation of ms temperature. Metall. Mater. Trans. A 1982, 13, 328–331. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, N.H.; Butt, A.M.; Zhao, L.; Sietsm, J.; Offerman, S.E.; Wright, J.P.; van der Zwaag, S. Thermal stability of retained austenite in TRIP steels studied by synchrotron X-ray diffraction during cooling. Acta Mater. 2005, 53, 5439–5447. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Petrov, R.; Kwakernaak, C.; Sloof, W.G.; Sietsma, J. Microstructural development during the quenching and partitioning process in a newly designed low-carbon steel. Acta Mater. 2011, 59, 6059–6068. [Google Scholar] [CrossRef]
- Dyson, D.J. Effect of alloying additions on the lattice parameter of austenite. J. Iron Steel Inst. 1970, 208, 469–474. [Google Scholar]
- William, J.; Mehl, R. Reaction kinetics in processes of nucleation and growth. Trans AIME 1939, 135, 416–458. [Google Scholar]
- Avrami, M. Granulation, Phase Change, and Microstructure Kinetics of Phase Change. III. J. Chem. Phys. 1941, 9, 177–184. [Google Scholar] [CrossRef]
- Kempen, A.T.W.; Sommer, F.; Mittemeijer, E.J. Determination and interpretation of isothermal and non-isothermal transformation kinetics; the effective activation energies in terms of nucleation and growth. J. Mater. Sci. 2002, 37, 1321–1332. [Google Scholar] [CrossRef]
- Okamoto, H.; Oka, M. Lower bainite with midrib in hypereutectoid steels. Metall. Trans. A 1986, 17, 1113–1120. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Christian, J.W. Bainite in steels. Metall. Trans. A 1990, 21, 767–797. [Google Scholar] [CrossRef]
- Chang, L.C.; Bhadeshia, H.K.D.H. Austenite films in bainitic microstructures. Mater. Sci. Technol. 1995, 11, 874–882. [Google Scholar] [CrossRef]
- Huang, Q.; De Cooman, B.C.; Biermann, H.; Mola, J. Influence of Martensite Fraction on the Stabilization of Austenite in Austenitic–Martensitic Stainless Steels. Metall. Mater. Trans. A 2016, 47, 1947–1959. [Google Scholar] [CrossRef]
- Cahn, J.W. The kinetics of cellular segregation reactions. Acta Metall. 1959, 7, 18–28. [Google Scholar] [CrossRef]
- Matsuda, H.; Bhadeshia, H.K.D.H. Kinetics of the bainite transformation. Proc. R. Soc. A 2004, 460, 1707–1722. [Google Scholar] [CrossRef]





| C | Si | Cr | V | Mn | P | S |
|---|---|---|---|---|---|---|
| 0.535 | 1.725 | 1.035 | 0.127 | 0.631 | 0.017 | 0.0025 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Han, Y.; Yin, S.; Zhao, F. Metastable Austenite Transformation Kinetics of Medium-Carbon Silicon-Rich Steel during Partitioning in a Q & P Process. Metals 2020, 10, 738. https://doi.org/10.3390/met10060738
Liu Y, Han Y, Yin S, Zhao F. Metastable Austenite Transformation Kinetics of Medium-Carbon Silicon-Rich Steel during Partitioning in a Q & P Process. Metals. 2020; 10(6):738. https://doi.org/10.3390/met10060738
Chicago/Turabian StyleLiu, Yuan, Yan Han, Sheng Yin, and Fei Zhao. 2020. "Metastable Austenite Transformation Kinetics of Medium-Carbon Silicon-Rich Steel during Partitioning in a Q & P Process" Metals 10, no. 6: 738. https://doi.org/10.3390/met10060738




