Effects of Travel Speed on the Microstructure and Abrasion Resistance of Hardfacing Alloys Deposited with Composite Powder Particles and Solid Wire
Abstract
1. Introduction
2. Experiments
2.1. The Preparation of Composite Powder Particles
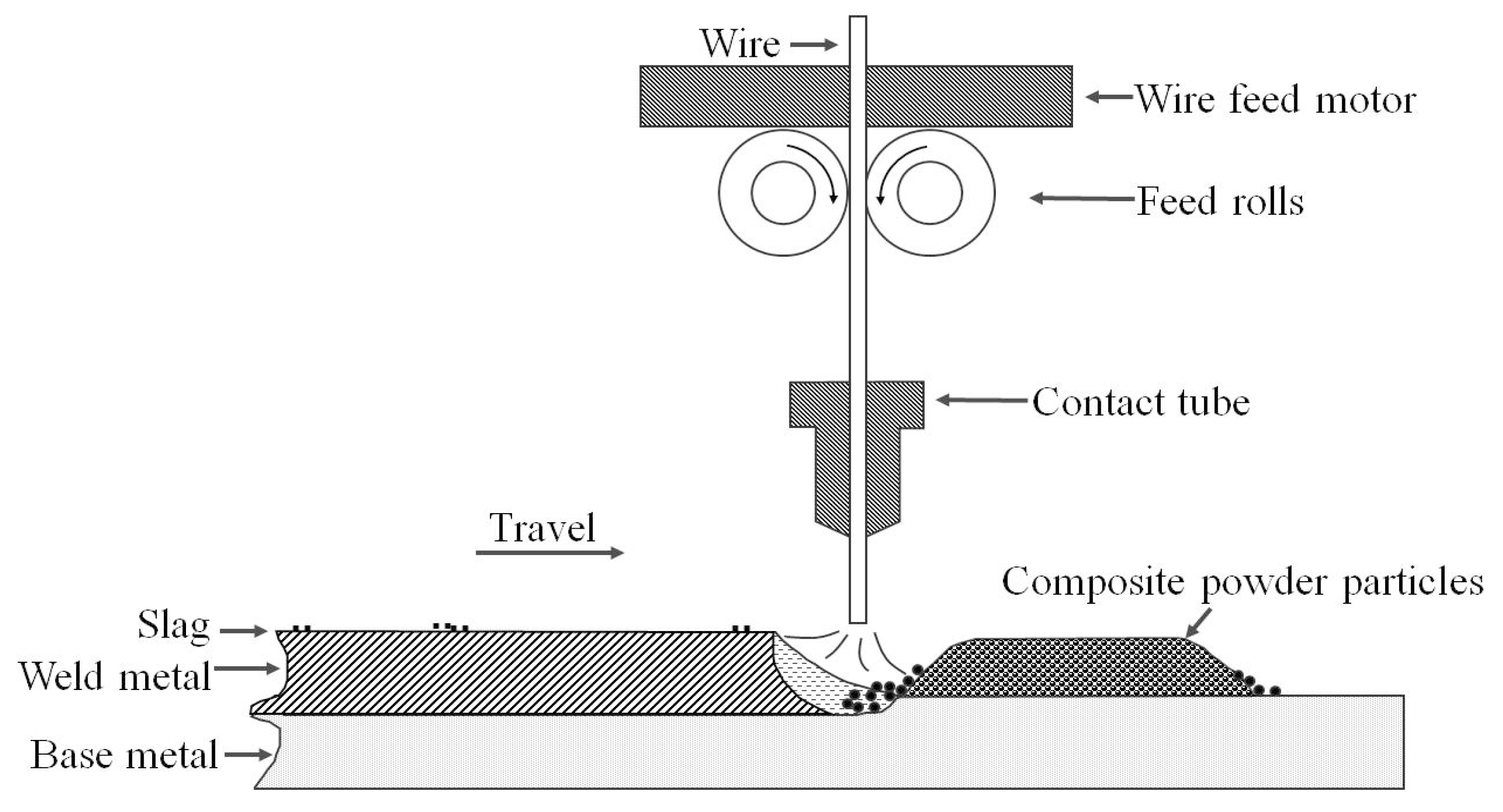
2.2. Welding Procedure
2.3. Microstructure of Hardfacing Alloys
2.4. Abrasive Wear Test
3. Results and Discussion
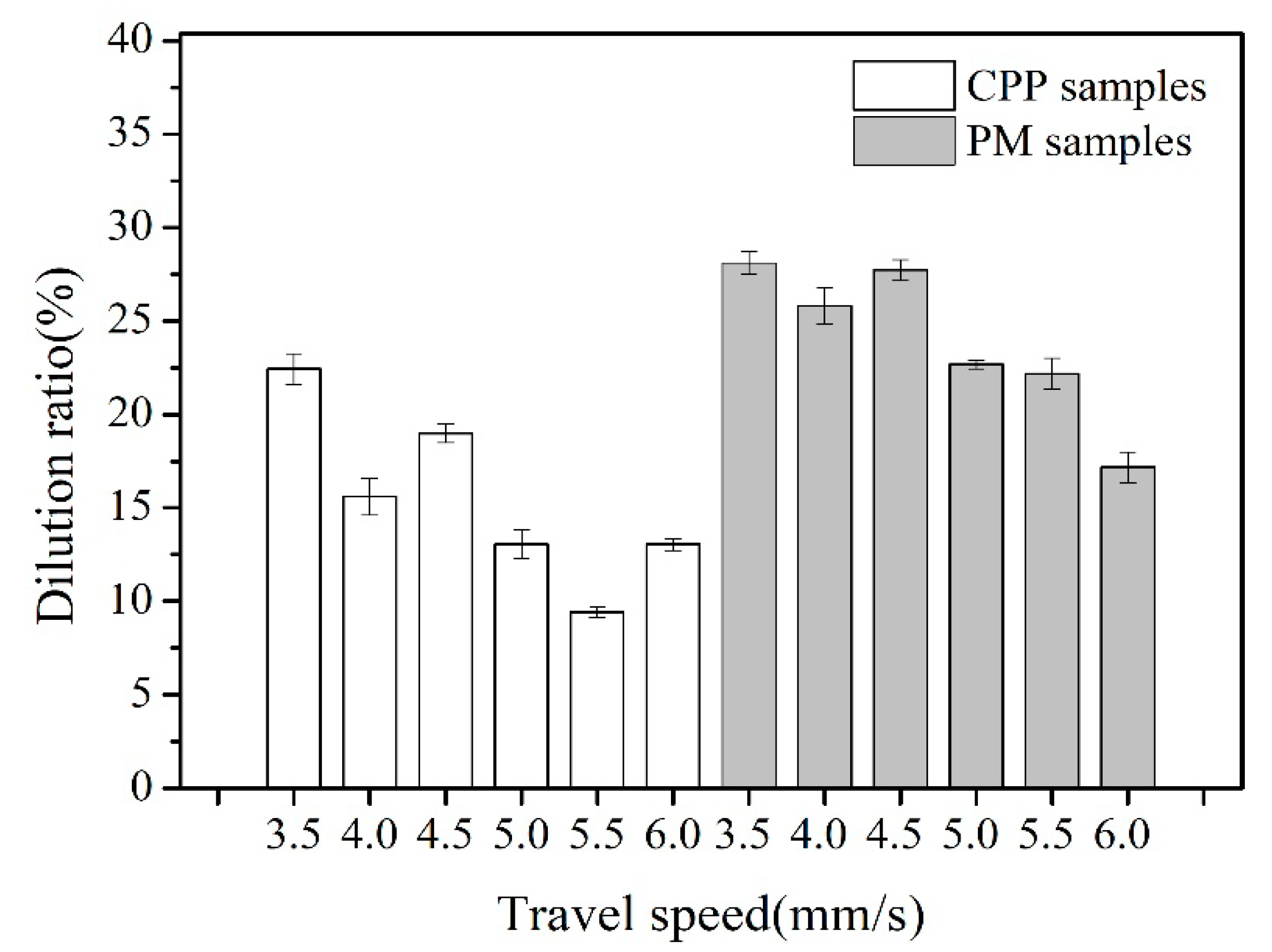
3.1. The Dilution Ratio of Base Metals
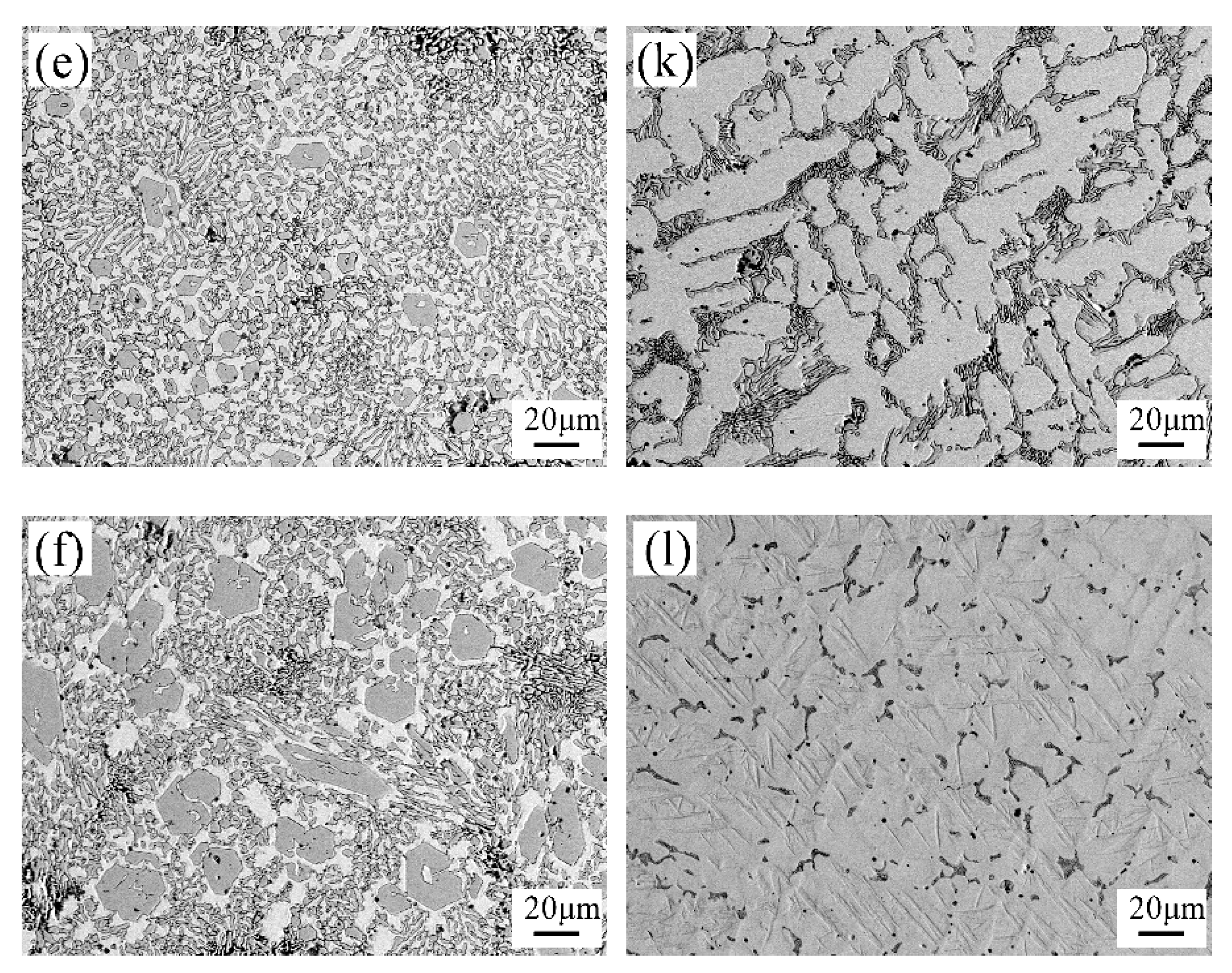
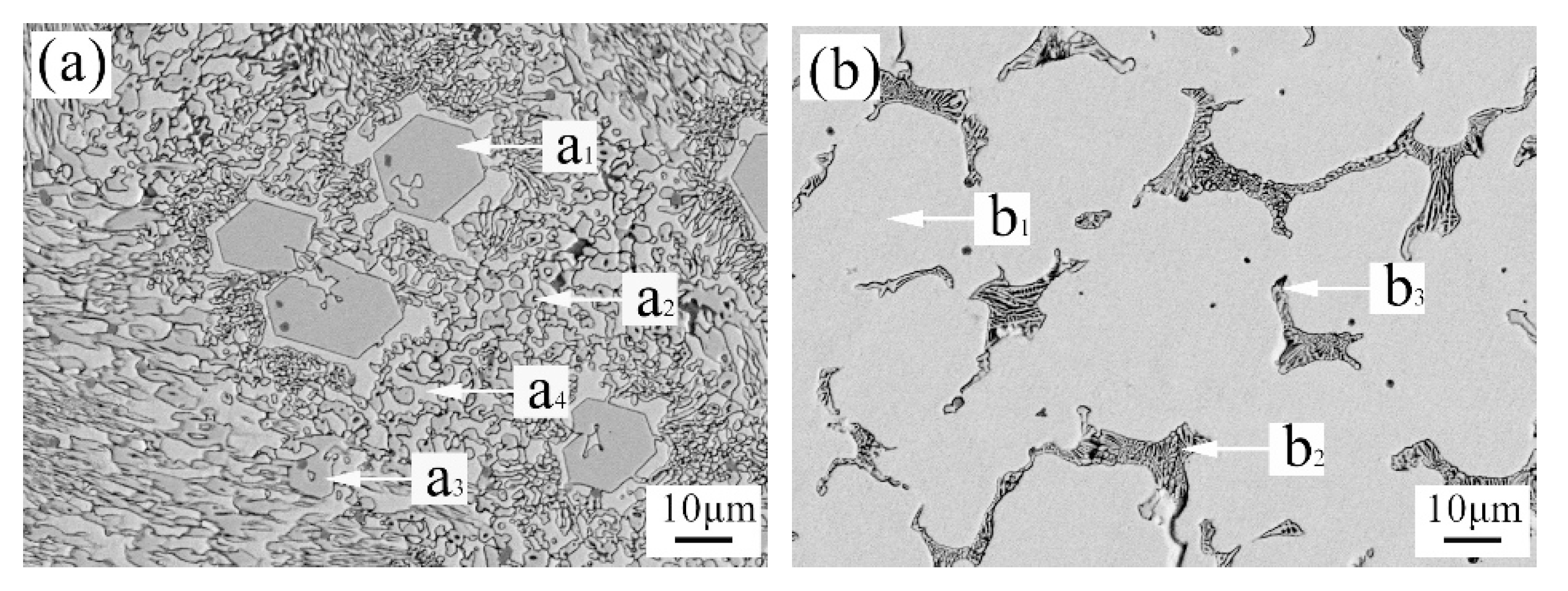
3.2. The Microstructure of Hardfacing Alloys
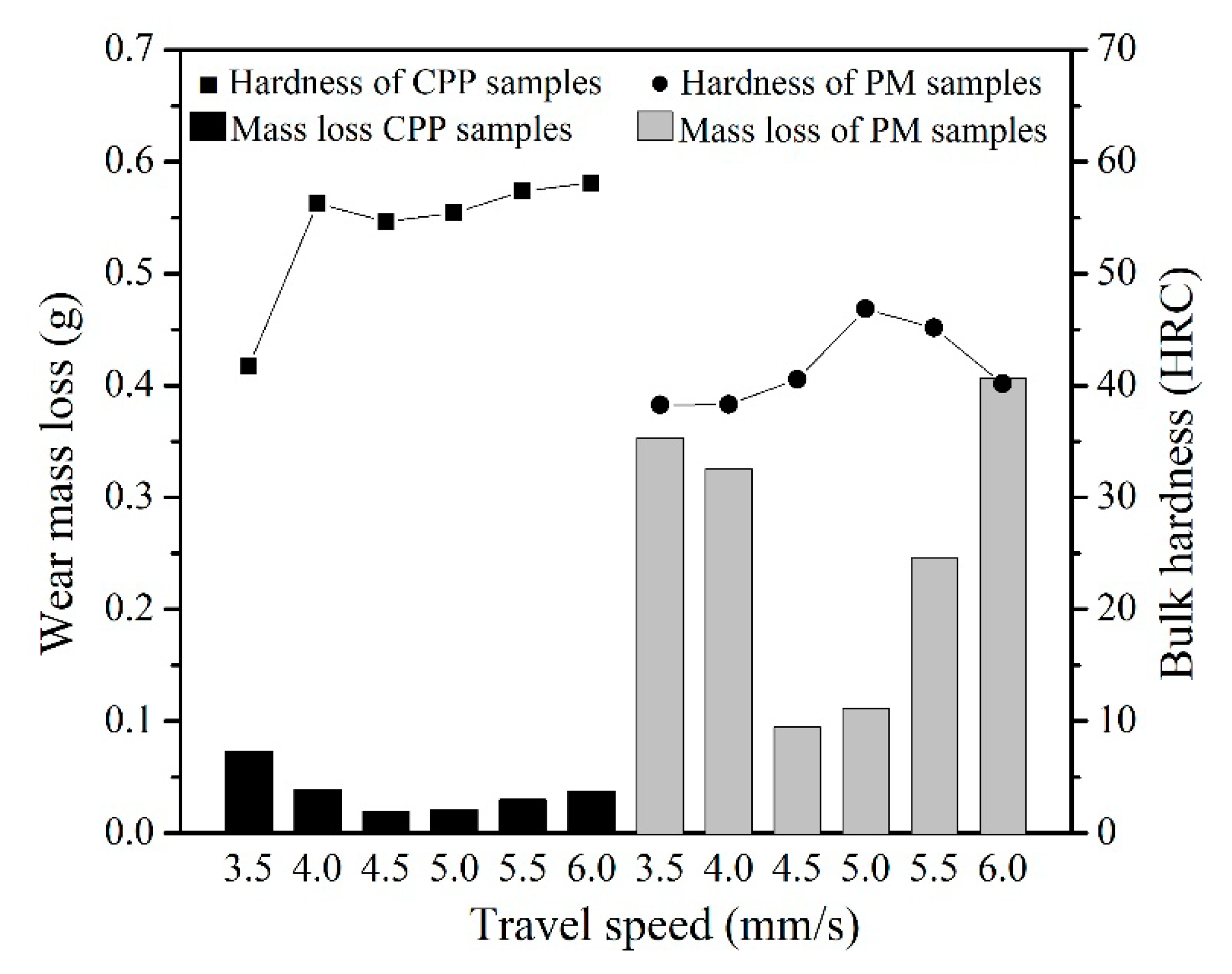
3.3. Abrasion Resistance of Hardfacing Alloys
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Correa, E.O.; Alcantara, N.G.; Tecco, D.G.; Kumar, R.V. The relationship between the microstructure and abrasive resistance of a hardfacing alloy in the Fe-Cr-C-Nb-V system. Metall. Mater. Trans. A 2007, 38, 1671–1780. [Google Scholar] [CrossRef]
- Wang, X.H.; Han, F.; Qu, S.Y.; Zou, Z.D. Microstructure of the Fe-based hardfacing layers reinforced by TiC-VC-Mo2C particles. Surf. Coat. Techol. 2008, 202, 1502–1509. [Google Scholar] [CrossRef]
- Rafi, H.K.; Ram, G.D.J.; Phanikumar, G.; Rao, K.P. Microstructure and Properties of Friction Surfaced Stainless Steel and Tool Steel Coatings. Mater. Sci. Forum 2010, 638–642, 864–869. [Google Scholar] [CrossRef]
- Srikarun, B.; Muangjunburee, P. The effect of iron-based hardfacing with chromium powder addition onto low carbon steel. Mater. Today Proc. 2018, 5, 9272–9280. [Google Scholar] [CrossRef]
- Morsy, M.; El-Kashif, E. The effect of microstructure on high-stress abrasion resistance of Fe-Cr-C hardfacing deposits. Weld. World 2014, 58, 491–497. [Google Scholar] [CrossRef]
- Sapate, S.G.; Ramarao, A.V. Erosive wear behaviour of weld hardfacing high chromium cast irons: Effect of erodent particles. Tribol. Int. 2006, 39, 206–212. [Google Scholar] [CrossRef]
- Dilawary, S.A.A.; Motallebzadeh, A.; Houdkova, S.; Medlin, R.; Haviar, S.; Lukac, F.; Afzal, M.; Cimenoglu, H. Modification of M2 Hardfacing: Effect of Molybdenum Alloying and Laser Surface Melting on Microstructure and Wear Performance. Wear 2018, 404, 111–121. [Google Scholar] [CrossRef]
- Sha, C.K.; Lin, J.C.; Tsai, H.L. The impact characteristics of Ti–6Al–4V plates hardfacing by laser alloying NiAl+ZrO2 powder. J. Mater. Process. Technol. 2003, 140, 197–202. [Google Scholar] [CrossRef]
- Zahiri, R.; Sundaramoorthy, R.; Lysz, P.; Subramanian, C. Hardfacing using ferro-alloy powder mixtures by submerged arc welding. Surf. Coat. Technol. 2014, 260, 220–229. [Google Scholar] [CrossRef]
- Liu, D.; Liu, R.; Wei, Y. Effects of titanium additive on microstructure and wear performance of iron-based slag-free self-shielded flux-cored wire. Surf. Coat. Technol. 2012, 207, 579–586. [Google Scholar] [CrossRef]
- Shen, S.; Oguocha, I.N.A.; Yannacopoulos, S. Effect of heat input on weld bead geometry of submerged arc welded ASTM A709 Grade 50 steel joints. J. Mater. Process. Technol. 2012, 212, 286–294. [Google Scholar] [CrossRef]
- Yuksel, N.; Sahin, S. Wear behavior–hardness–microstructure relation of Fe–Cr–C and Fe–Cr–C–B based hardfacing alloys. Mater. Des. 2014, 58, 491–498. [Google Scholar] [CrossRef]
- Zhang, A.F.; Xing, J.D.; Fang, L.; Su, J.Y. Inter-phase corrosion of chromium white cast irons in dynamic state. Wear 2004, 257, 198–204. [Google Scholar] [CrossRef]
- Berns, H. Comparison of wear resistant MMC and white cast iron. Wear 2003, 254, 47–54. [Google Scholar] [CrossRef]
- Xing, J.D.; Gao, Y.M.; Wang, E.Z.; Bao, C.G. Effect of phase stability on the wear resistance of white cast iron at 800 C. Wear 2002, 252, 755–760. [Google Scholar] [CrossRef]
- Aso, S.; Goto, S.; Komatsu, Y.; Hartono, W. Sliding wear of graphite crystallized chromium white cast iron. Wear 2001, 250, 511–517. [Google Scholar] [CrossRef]
- Banovic, S.W.; DuPont, J.N.; Marder, A.R. Dilution control in gas-tungsten-arc welds involving superaustenitic stainless steels and nickel-based alloys. Metall. Mater. Trans. B 2001, 32, 1171–1176. [Google Scholar] [CrossRef]
- Pawar, S.; Mukhopadhyay, G. Metallurgical and Tribological Evaluation of Fe-Cr-C Hardfacing Alloys. J. Fail. Anal. Prev. 2018, 18, 868–876. [Google Scholar] [CrossRef]
- Badisch, E.; Mitterer, C. Abrasive wear of high speed steels: Influence of abrasive particles and primary carbides on wear resistance. Tribol. Int. 2003, 36, 765–770. [Google Scholar] [CrossRef]
- Bergman, F.; Hedenqvist, P.; Hogmark, S. The influence of primary carbides and test parameters on abrasive and erosive wear of selected PM high speed steels. Tribol. Int. 1997, 30, 183–191. [Google Scholar] [CrossRef]
- Jiang, M.; Li, Z.X.; Wang, Y.J.; Shi, Y.W.; Jiang, J.M.; Li, X.B. Effect of vanadium on microstructures and properties of Fe-Cr-C self-shielded metal cored hardfacing alloys. Sci. Technol. Weld. Join. 2008, 13, 114–117. [Google Scholar] [CrossRef]
- Ren, S.; Zhu, J. The effect of substitutional alloying elements on the impact wear rate of medium carbon steels related to delamination and quasi-nanometer wear mechanisms. Mater. Sci. Eng. A 2011, 528, 7020–7023. [Google Scholar] [CrossRef]
- Ren, S.; Zhu, J. Wear mechanisms and hardness thresholds under repeated impact contact loading. Sci. China Technol. Sci. 2017, 61, 124–128. [Google Scholar] [CrossRef]
- Oo, H.Z.; Muangjunburee, P. Wear behaviour of hardfacing on 3.5% chromium cast steel by submerged arc welding. Mater. Today Proc. 2018, 5, 9281–9289. [Google Scholar] [CrossRef]
- Zhg, M.; Li, M.; Wang, J. Study on Composition, Microstructure and Wear Behavior of Fe-B-C Wear-Resistant Surfacing Alloys. J. Mater. Eng. Perform. 2017, 26, 6182–6192. [Google Scholar] [CrossRef]
- Buchely, M.F.; Gutierrez, J.C.; Leon, L.M.; Toro, A. The effect of microstructure on abrasive wear of hardfacing alloys. Wear 2005, 259, 52–61. [Google Scholar] [CrossRef]
- Gong, J.X.; Tang, T.S.; Xiao, Y.F. Effect of WC/W2C on the microstructure and abrasion resistance of high-boron hardfacing alloys. Acta Metall. Sinica (Engl. Lett.) 2010, 23, 439–445. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, T.K. Wear behaviour of Hardfacing Deposits on Cast Iron. Wear 2003, 255, 417–425. [Google Scholar] [CrossRef]
- Liu, Y.F.; Xia, Z.Y.; Han, J.M. Microstructure and wear behavior of (Cr,Fe)7C3 reinforced composite coating produced by plasma transferred arc weld-surfacing process. Surf. Coat. Technol. 2006, 201, 863–867. [Google Scholar] [CrossRef]
- Filipovic, M.; Romhanji, E.; Kamberovic, Z. Chemical Composition and Morphology of M7C3 Eutectic Carbide in High Chromium White Cast Iron Alloyed with Vanadium. ISIJ Int. 2012, 52, 2200–2204. [Google Scholar] [CrossRef]






| Materials | C | Si | Mn | S | P | Fe |
|---|---|---|---|---|---|---|
| H08A | 0.10 | 0.12 | 0.22 | ≤0.02 | ≤0.02 | Balance |
| Parameters | Current(A) | Voltage(V) | Wire Extension(mm) | Wire Feed Rate (mm/s) |
|---|---|---|---|---|
| 410 | 27 | 30 | 45 |
| Samples | Composite Powder Particles | Powder Mixture | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Travel speed (mm/s) | 1 # | 2 # | 3 # | 4 # | 5 # | 6 # | 1 # | 2 # | 3 # | 4 # | 5 # | 6 # |
| 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | 3.5 | 4.0 | 4.5 | 5.0 | 5.5 | 6.0 | |
| Alloys | C | Cr | Ti | V | Fe |
|---|---|---|---|---|---|
| Composite powder particles | 2.3–2.9 | 9.0–13.9 | 0.1–0.3 | 1.4–1.9 | Balance |
| Powder mixture | 1.4–2.7 | 2.7–9.1 | 0–0.1 | 0.3–1.4 | Balance |
| Point | Atom Fraction (%) | abs. Error (%) [1 Sigma] | ||||||
|---|---|---|---|---|---|---|---|---|
| a1 | a2 | a3 | a4 | b1 | b2 | b3 | ||
| C | 32.93 | 34.12 | 33.68 | 17.13 | 13.29 | 40.69 | 17.82 | 19.71 |
| Ti | -- | 1.19 | -- | -- | -- | -- | -- | 0.17 |
| V | 3.70 | 2.25 | 3.59 | 3.30 | 0.42 | 4.02 | 0.68 | 0.92 |
| Cr | 33.48 | 21.06 | 29.83 | 31.33 | 6.46 | 21.96 | 7.70 | 0.22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, H.; Gong, J.; Cheng, S.; Liu, C.; Huang, H. Effects of Travel Speed on the Microstructure and Abrasion Resistance of Hardfacing Alloys Deposited with Composite Powder Particles and Solid Wire. Metals 2020, 10, 740. https://doi.org/10.3390/met10060740
Yao H, Gong J, Cheng S, Liu C, Huang H. Effects of Travel Speed on the Microstructure and Abrasion Resistance of Hardfacing Alloys Deposited with Composite Powder Particles and Solid Wire. Metals. 2020; 10(6):740. https://doi.org/10.3390/met10060740
Chicago/Turabian StyleYao, Huiwen, Jianxun Gong, Shiyao Cheng, Chao Liu, and Hongjiang Huang. 2020. "Effects of Travel Speed on the Microstructure and Abrasion Resistance of Hardfacing Alloys Deposited with Composite Powder Particles and Solid Wire" Metals 10, no. 6: 740. https://doi.org/10.3390/met10060740
APA StyleYao, H., Gong, J., Cheng, S., Liu, C., & Huang, H. (2020). Effects of Travel Speed on the Microstructure and Abrasion Resistance of Hardfacing Alloys Deposited with Composite Powder Particles and Solid Wire. Metals, 10(6), 740. https://doi.org/10.3390/met10060740




