Oxidized and Non-Oxidized Multiwalled Carbon Nanotubes as Materials for Adsorption of Lanthanum(III) Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. La(III) Uptake by Non-Oxidized Multiwalled Carbon Nanotubes (MWCNTs)
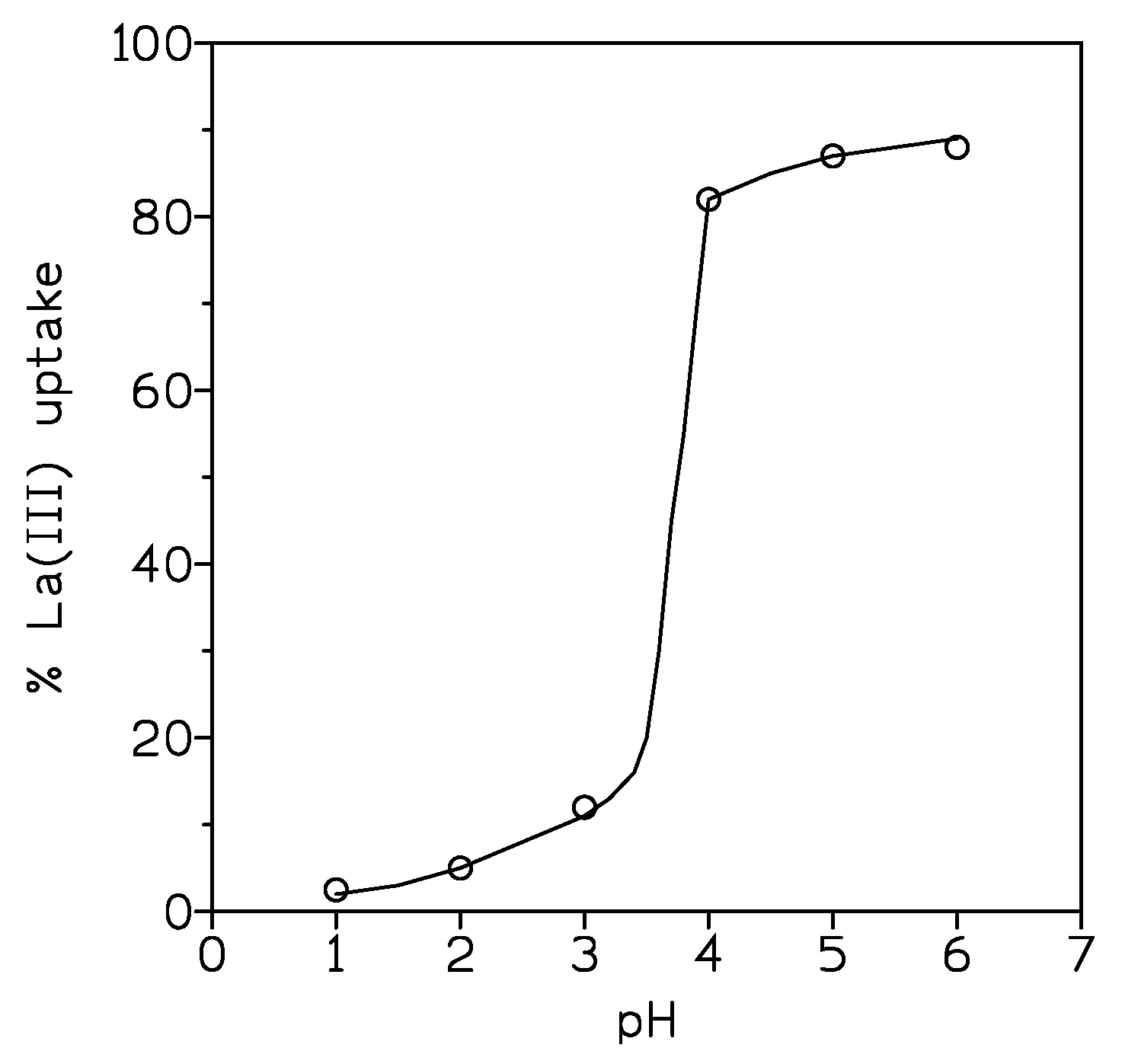
3.1.1. Influence of the Aqueous pH
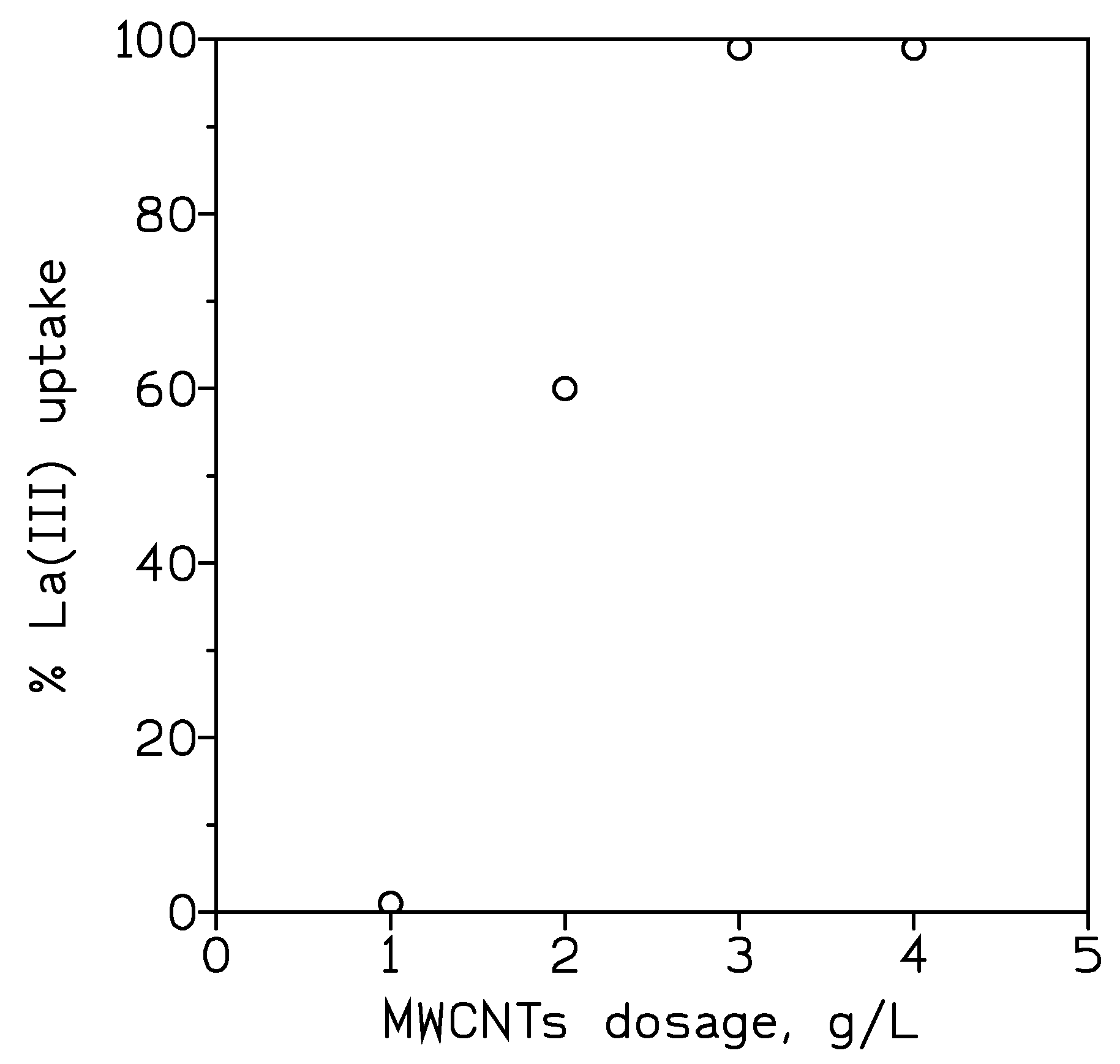
3.1.2. Effect of Adsorbent Dosage
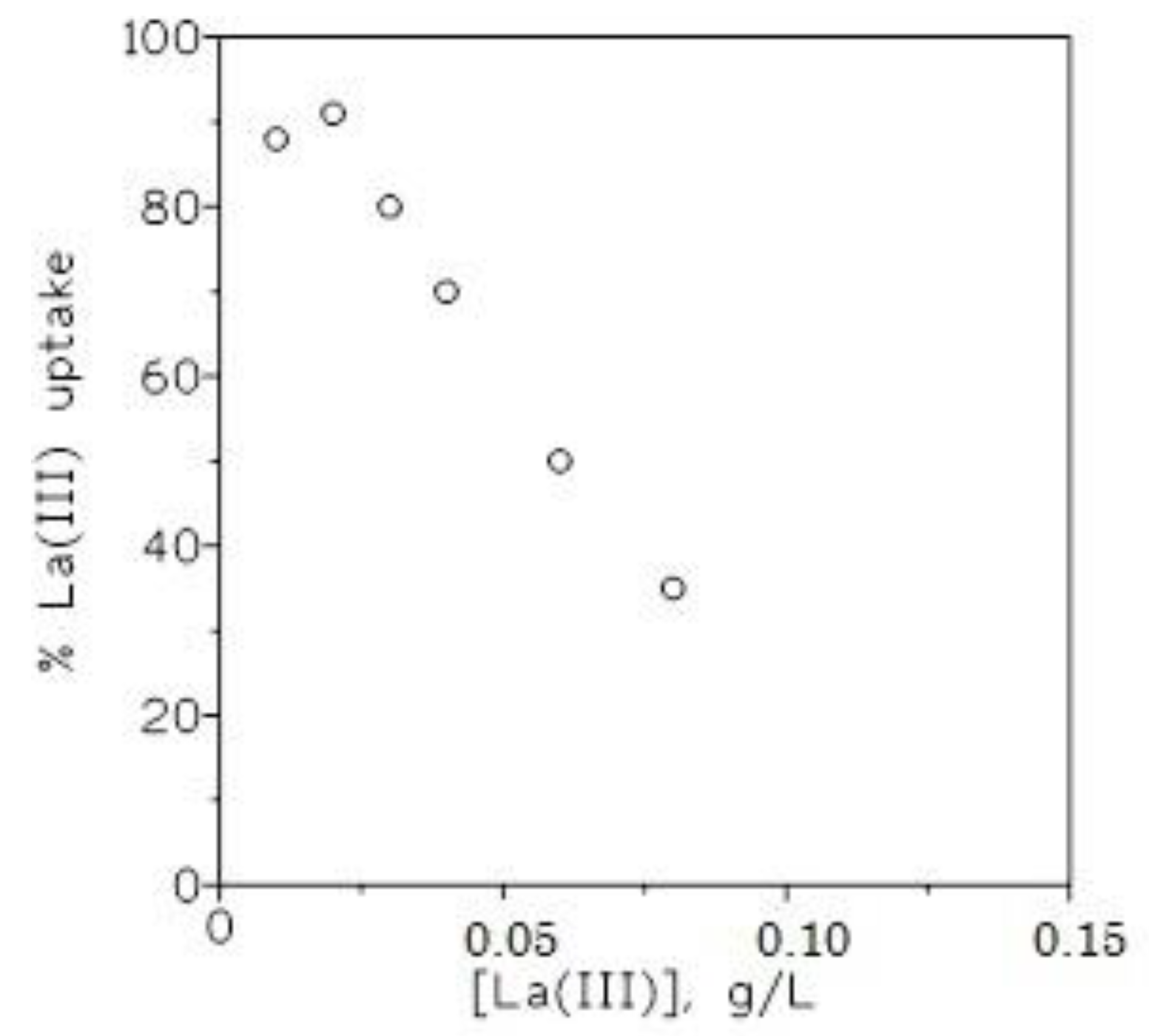
3.1.3. Effect of Metal Concentration
3.2. La(III) Uptake by Oxidized Multiwalled Carbon Nanotubes (ox-MWCNTs)
3.2.1. Effect of Stirring Speed
3.2.2. Effect of the Aqueous pH
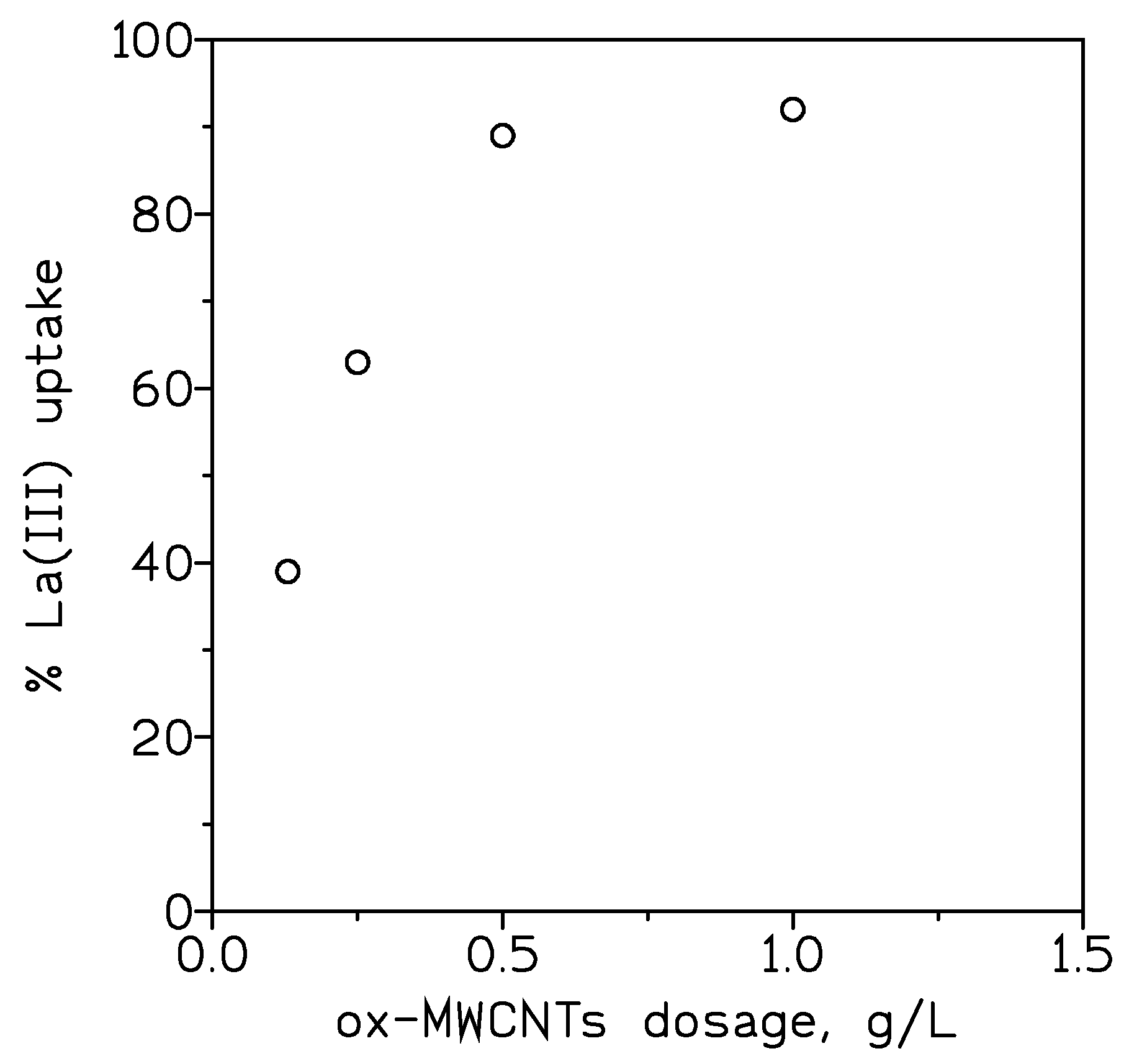
3.2.3. Effect of ox-MWCNTs Dosage
3.2.4. Effect of Initial Lanthanum Concentration
3.3. Elution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klinger, J.M. A historical geography of rare earth elements: From discovery to the atomic age. Extr. Ind. Soc. 2015, 2, 572–580. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Zhao, Y.; Zhang, C.; Wei, Y. Rare Earth Elements supply vs. clean energy technologies: new problems to be solve. Gospod. Surowcami Miner. 2016, 32, 29–44. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, K.M.; Wall, F.; Merriman, D. The Rare Earth Elements: Demand, Global Resources, and Challenges for Resourcing Future Generations. Nat. Resour. Res. 2018, 27, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Jacinto, J.; Henriques, B.; Duarte, A.C.; Vale, C.; Pereira, E. Removal and recovery of Critical Rare Elements from contaminated waters by living Gracilaria gracilis. J. Hazard. Mater. 2018, 344, 531–538. [Google Scholar] [CrossRef] [PubMed]
- EU Report on Critical Raw Materials and the Circular Economy - Publications Office of the EU. Available online: http://publications.europa.eu/resource/cellar/d1be1b43-e18f-11e8-b690-01aa75ed71a1.0001.01/DOC_1 (accessed on 15 May 2020).
- Omodara, L.; Pitkäaho, S.; Turpeinen, E.-M.; Saavalainen, P.; Oravisjärvi, K.; Keiski, R.L. Recycling and substitution of light rare earth elements, cerium, lanthanum, neodymium, and praseodymium from end-of-life applications—A review. J. Clean. Prod. 2019, 236, 117573. [Google Scholar] [CrossRef]
- Jowitt, S.M.; Werner, T.T.; Weng, Z.; Mudd, G.M. Recycling of the rare earth elements. Curr. Opin. Green Sustain. Chem. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Hubicki, Z.; Fila, D. Recovery of rare earth elements from acidic solutions using macroporous ion exchangers. Sep. Sci. Technol. 2019, 54, 2059–2076. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: a critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.C.; Forsberg, K.M. Separation of valuable elements from NiMH battery leach liquor via antisolvent precipitation. Sep. Purif. Technol. 2020, 234, 115812. [Google Scholar] [CrossRef]
- Fila, D.; Hubicki, Z.; Kołodyńska, D. Recovery of metals from waste nickel-metal hydride batteries using multifunctional Diphonix resin. Adsorption 2019, 25, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Innocenzi, V.; Ippolito, N.M.; De Michelis, I.; Prisciandaro, M.; Medici, F.; Vegliò, F. A review of the processes and lab-scale techniques for the treatment of spent rechargeable NiMH batteries. J. Power Sources 2017, 362, 202–218. [Google Scholar] [CrossRef]
- Ueberschaar, M.; Geiping, J.; Zamzow, M.; Flamme, S.; Rotter, V.S. Assessment of element-specific recycling efficiency in WEEE pre-processing. Resour. Conserv. Recycl. 2017, 124, 25–41. [Google Scholar] [CrossRef] [Green Version]
- García-Díaz, I.; López, F.; Alguacil, F. Carbon Nanofibers: A New Adsorbent for Copper Removal from Wastewater. Metals (Basel) 2018, 8, 914. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Tangparitkul, S.; Hendry, B.; Harper, J.; Kim, Y.K.; Hunter, T.N.; Lee, J.W.; Harbottle, D. Selective separation of cesium contaminated clays from pristine clays by flotation. Chem. Eng. J. 2019, 355, 797–804. [Google Scholar] [CrossRef]
- Danyliuk, N.; Tomaszewska, J.; Tatarchuk, T. Halloysite nanotubes and halloysite-based composites for environmental and biomedical applications. J. Mol. Liq. 2020, 309, 113077. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef]
- Nuithitikul, K.; Phromrak, R.; Saengngoen, W. Utilization of chemically treated cashew-nut shell as potential adsorbent for removal of Pb(II) ions from aqueous solution. Sci. Rep. 2020, 10, 3343. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Manna, K.; Srivastava, S.K.; Gupta, A.K.; Yadav, M.K. Hollow Polyaniline Microsphere/Fe3O4 Nanocomposite as an Effective Adsorbent for Removal of Arsenic from Water. Sci. Rep. 2020, 10, 4982. [Google Scholar] [CrossRef] [Green Version]
- Lapo, B.; Bou, J.J.; Hoyo, J.; Carrillo, M.; Peña, K.; Tzanov, T.; Sastre, A.M. A potential lignocellulosic biomass based on banana waste for critical rare earths recovery from aqueous solutions. Environ. Pollut. 2020, 264, 114409. [Google Scholar] [CrossRef]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: a review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Alcaraz, L.; Escudero, M.E.; Alguacil, F.J.; Llorente, I.; Urbieta, A.; Fernández, P.; López, F.A. Dysprosium Removal from Water Using Active Carbons Obtained from Spent Coffee Ground. Nanomaterials 2019, 9, 1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, F.; Astruc, D. Nanomaterials for removal of toxic elements from water. Coord. Chem. Rev. 2018, 356, 147–164. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, H. Recent advances of 3D graphene-based adsorbents for sample preparation of water pollutants: A review. Chem. Eng. J. 2020, 393, 124691. [Google Scholar] [CrossRef]
- Vilardi, G.; Mpouras, T.; Dermatas, D.; Verdone, N.; Polydera, A.; Di Palma, L. Nanomaterials application for heavy metals recovery from polluted water: The combination of nano zero-valent iron and carbon nanotubes. Competitive adsorption non-linear modeling. Chemosphere 2018, 201, 716–729. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F.A. On the Active Adsorption of Chromium(III) from Alkaline Solutions Using Multiwalled Carbon Nanotubes. Appl. Sci. 2019, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Alguacil, F. Adsorption of Gold(I) and Gold(III) Using Multiwalled Carbon Nanotubes. Appl. Sci. 2018, 8, 2264. [Google Scholar] [CrossRef] [Green Version]
- Alguacil, F.J.; Lopez, F.A.; Garcia-Diaz, I. Extracting metals from aqueous solutions using Ti-based nanostructures: A review. Desalin. Water Treat. 2016, 57, 17603–17615. [Google Scholar] [CrossRef]
- Crane, R.A.; Sapsford, D.J. Sorption and fractionation of rare earth element ions onto nanoscale zerovalent iron particles. Chem. Eng. J. 2018, 345, 126–137. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, Z.; Wei, Z.; Spinney, R.; Sillanpää, M.; Tang, J.; Tam, M.; Xiao, R. Polyethylenimine-modified chitosan materials for the recovery of La(III) from leachates of bauxite residue. Chem. Eng. J. 2020, 388, 124307. [Google Scholar] [CrossRef]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Sillanpää, M. Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: A critical review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef]
- Oral, A.E.; Aytas, S.; Yusan, S.; Sert, S.; Gok, C.; Elmastas Gultekin, O. Preparation and Characterization of a Graphene-Based Magnetic Nanocomposite for the Adsorption of Lanthanum Ions from Aqueous Solution. Anal. Lett. 2020, 53, 1812–1833. [Google Scholar] [CrossRef]
- Liao, Q.; Zou, D.; Pan, W.; Linghu, W.; Shen, R.; Li, X.; Asiri, A.M.; Alamry, K.A.; Sheng, G.; Zhan, L.; et al. Highly efficient capture of Eu(III), La(III), Nd(III), Th(IV) from aqueous solutions using g-C3N4 nanosheets. J. Mol. Liq. 2018, 252, 351–361. [Google Scholar] [CrossRef]
- Cardoso, C.E.D.; Almeida, J.C.; Lopes, C.B.; Trindade, T.; Vale, C.; Pereira, E. Recovery of Rare Earth Elements by Carbon-Based Nanomaterials—A Review. Nanomaterials 2019, 9, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayazit, G.; Tartan, B.E.; Güi, Ü. Biosorption, isotherm and kinetic properties of common textile dye by phormidium animale. Glob. NEST J. 2019. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Manzoor, U. A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng. 2014, 2014, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of Apparent Pseudo-Second-Order Adsorption Kinetics onto Cellulosic Materials: A Review. Bioresources 2019, 14, 7582–7626. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H. Über die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ippolito, N.M.; Maffei, G.; Medici, F.; Piga, L. Adsorption and regeneration of fluoride ion on a high alumina content bauxite. Chem. Eng. Trans. 2016, 47, 217–222. [Google Scholar] [CrossRef]
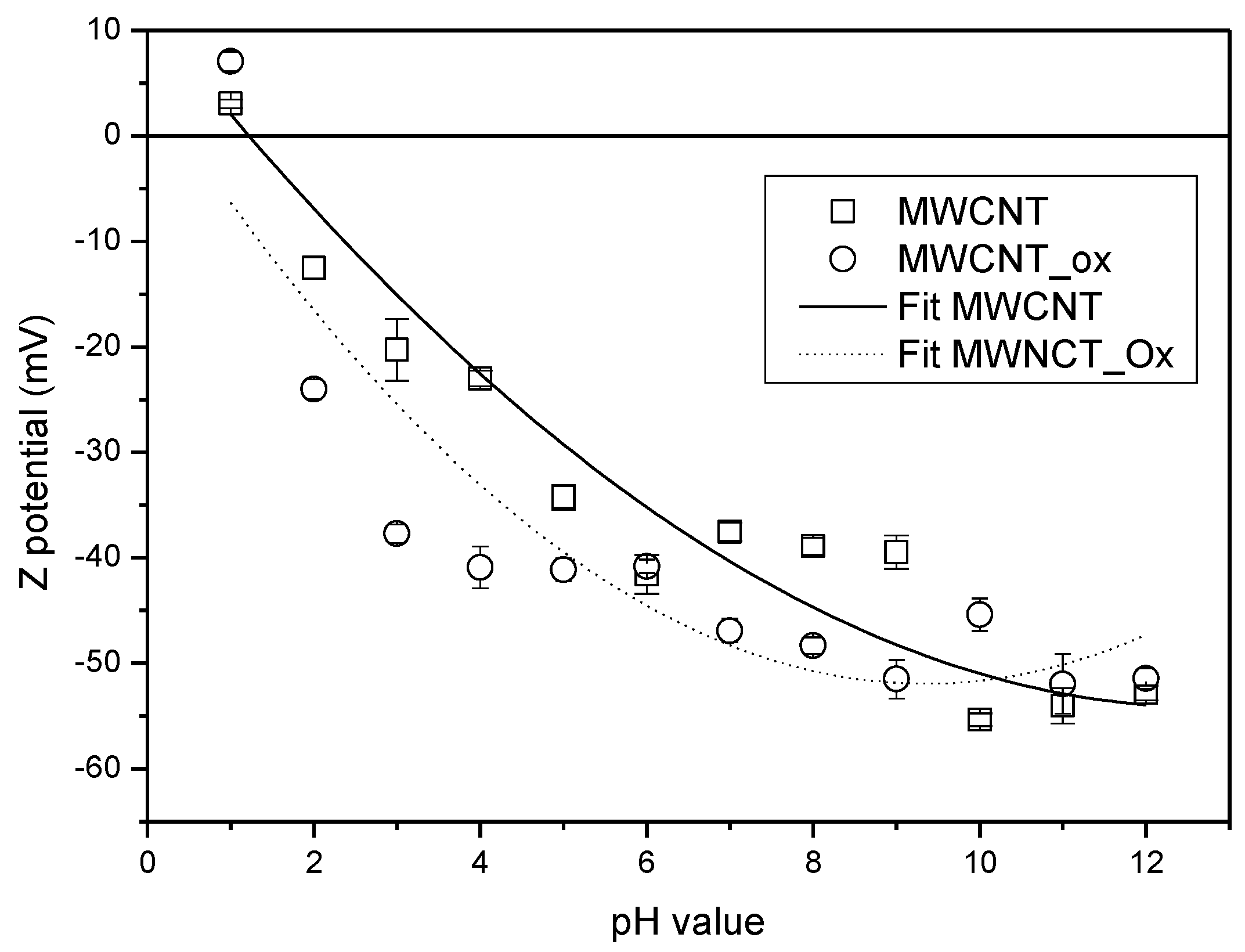
| Adsorbent | Purity (%) | a Functionalization Grade (%) | BET (m2/g) | Average Diameter (nm) | Density | Length (µm) | Method of Process |
|---|---|---|---|---|---|---|---|
| MWCNT | >98% | - | 263 | 6-9 | 2.1 | 5 | CVD |
| ox-MWCNT | >80% | >8 | 307 | 9.5 | - | 1.5 | CVD |
| pH | % La(III) Uptake |
|---|---|
| 1 | 15 |
| 2 | 32 |
| 3 | 61 |
| 4 | 80 |
| 6 | 98 |
| [La]0, mg/L | % La(III) Uptake | [La]c,e, mg/g |
|---|---|---|
| 10 | 98 | 3.3 |
| 20 | 75 | 5.0 |
| 40 | 46 | 6.1 |
| 80 | 20 | 5.3 |
| Stirring Speed (min−1) | % La(III) Uptake |
|---|---|
| 250 | 70 |
| 350 | 90 |
| 500 | 99 |
| 750 | 97 |
| 1000 | 94 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alguacil, F.J.; García-Díaz, I.; Escudero Baquero, E.; Rodríguez Largo, O.; López, F.A. Oxidized and Non-Oxidized Multiwalled Carbon Nanotubes as Materials for Adsorption of Lanthanum(III) Aqueous Solutions. Metals 2020, 10, 765. https://doi.org/10.3390/met10060765
Alguacil FJ, García-Díaz I, Escudero Baquero E, Rodríguez Largo O, López FA. Oxidized and Non-Oxidized Multiwalled Carbon Nanotubes as Materials for Adsorption of Lanthanum(III) Aqueous Solutions. Metals. 2020; 10(6):765. https://doi.org/10.3390/met10060765
Chicago/Turabian StyleAlguacil, Francisco J., Irene García-Díaz, Esther Escudero Baquero, Olga Rodríguez Largo, and Félix A. López. 2020. "Oxidized and Non-Oxidized Multiwalled Carbon Nanotubes as Materials for Adsorption of Lanthanum(III) Aqueous Solutions" Metals 10, no. 6: 765. https://doi.org/10.3390/met10060765






