Solvent Extraction of Sc(III) by D2EHPA/TBP from the Leaching Solution of Vanadium Slag
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
2.2. General Experimental Procedure
3. Results and Discussion
3.1. Thermodynamic Analysis and Discussions
3.1.1. Thermodynamic Data and Equilibrium Calculation
3.1.2. Concentration Distribution of Ions Containing Scandium and Sulfur in the Sc-S-H2O System
3.2. Solvent Extraction of Sc(III) by Using D2EHPA and TBP
3.2.1. Effect of Extractant D2EHPA Concentration on the Sc Extraction
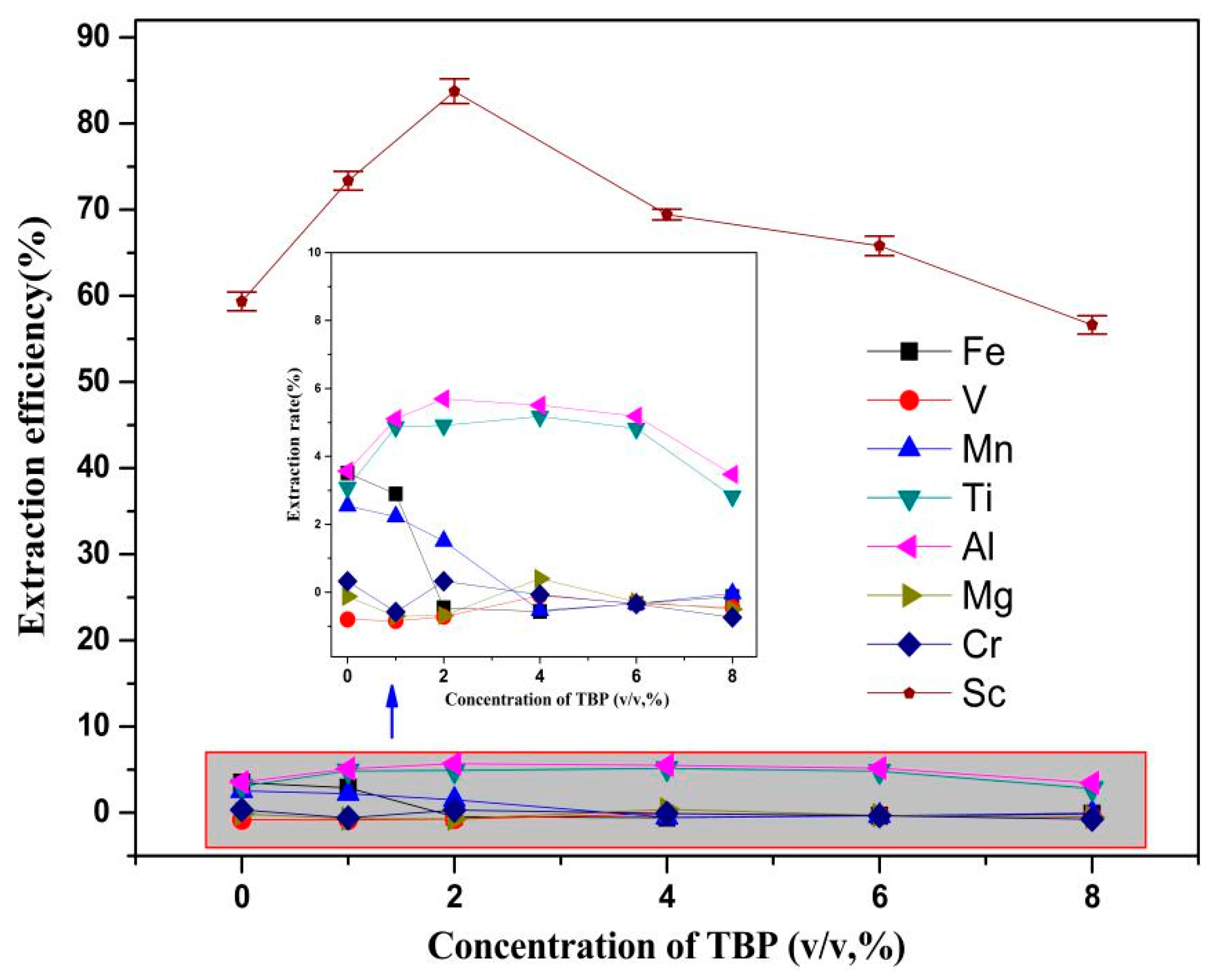
3.2.2. Effect of Modifier TBP Addition on the Sc Extraction
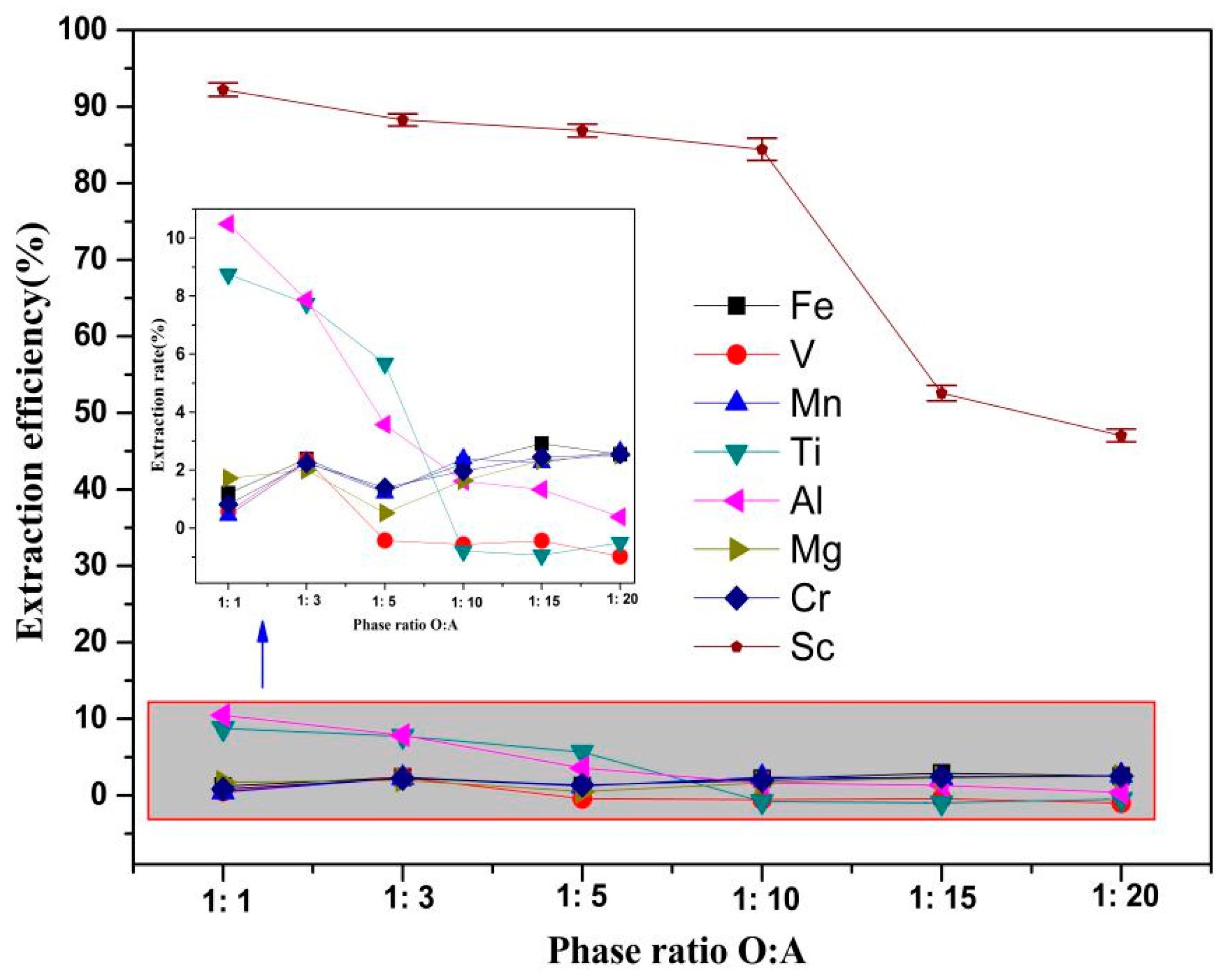
3.2.3. Effect of the O/A Phase Ratio on the Sc Extraction
3.2.4. Effect of the Stirring Speed on the Sc Extraction
3.3. Scrubbing of Co-extracted Elements and Stripping of Sc
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, S.; Li, S. Review of the extractive metallurgy of scandium in China (1978–1991). Hydrometallurgy 1996, 42, 337–343. [Google Scholar]
- Zhao, Y.; Zhang, Y.; Xing, W.; Xu, N. Treatment of titanium white waste acid using ceramic microfiltration membrane. Chem. Eng. J. 2005, 111, 31–38. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L.; Song, Y. Titanium white sulfuric acid concentration by direct contact membrane distillation. Chem. Eng. J. 2016, 285, 101–111. [Google Scholar] [CrossRef]
- Xu, T.; Yang, W. Sulfuric acid recovery from titanium white (pigment) waste liquor using diffusion dialysis with a new series of anion exchange membranes—Static runs. J. Membr. Sci. 2001, 183, 193–200. [Google Scholar]
- Li, L. Research Progress on Comprehensive Recycle of Waste Acid in Titanium Pigment Production at Home and Abroad. Hydrometall. China 2010, 29, 150–155. (In Chinese) [Google Scholar]
- Xu, H.; Fu, M. Research process in comprehensive utilization of spent acid and waste water in titanium dioxide production. Multipurp. Util. Miner. Resour. 2006, 4, 34–37. (In Chinese) [Google Scholar]
- Moskalyk, R.M.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Karasgania, A.A.; Rashchia, F.; Mostoufi, N.; Vahidi, E. Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy 2010, 102, 14–21. [Google Scholar] [CrossRef]
- Mirazimi, S.M.; Rashchi, F.; Saba, M. A new approach for direct leaching of vanadium from LD converter slag. Chem. Eng. Res. Des. 2015, 94, 131–140. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, L.; Li, G.; Wang, X.; Xiang, X. Leaching of vanadium from stone coal with sulfuric acid. Rare Met. 2009, 28, 1–4. [Google Scholar] [CrossRef]
- Xiang, J.; Huang, Q.; Lv, X. Extraction of vanadium from converter slag by two-step sulfuric acid leaching process. J. Clean. Prod. 2018, 170, 1089–1101. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Zhang, Y.; Lv, G. Pressure leaching of converter vanadium slag with waste titanium dioxide. Rare Met. 2016, 35, 576–580. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Huang, J.; Liu, T. Mechanisms of aid-leaching reagent calcium fluoride in the extracting vanadium processes from stone coal. Rare Met. 2013, 32, 57–62. [Google Scholar] [CrossRef]
- Zhou, X.; Wei, C.; Xia, W.; Li, M.; Li, C.; Deng, Z. Dissolution kinetics and thermodynamic analysis of vanadium trioxide during pressure oxidation. Rare Met. 2012, 31, 296–302. [Google Scholar] [CrossRef]
- Zhang, G.; Luo, D.; Deng, C.; Lv, L.; Liang, B.; Li, C. Simultaneous extraction of vanadium and titanium from vanadium slag using ammonium sulfate roasting-leaching process. J. Alloys Compd. 2018, 742, 504–511. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Lv, G.; Zhang, Y. Effects of Microwave Roasting on the Kinetics of Extracting Vanadium from Vanadium Slag. JOM 2016, 68, 577–583. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Lv, G.; Zhang, Y. Extraction of Vanadium from LD Converter Slag by Pressure Leaching Process with Titanium White Waste Acid. Rare Met. Mater. Eng. 2015, 44, 1894–1898. [Google Scholar]
- Zhang, T.; Lv, G.; Liu, Y.; Zhang, G. A Comprehensive Utilization Method of Titanium White Waste Acid. CN Patent 104178632 B, 22 June 2016. [Google Scholar]
- Wang, W.; Cheng, C. Separation and purification of scandium by solvent extraction and related technologies: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1237–1246. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.K.; Cheng, C. Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA. Sep. Purif. Technol. 2013, 108, 96–102. [Google Scholar] [CrossRef]
- Wang, W.; Pranolo, Y.K.; Cheng, C. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- Liu, C.; Chen, L.; Chen, J. Application of P507 and isooctanol extraction system in recovery of scandium from simulated red mud leach solution. J. Rare Earth 2019, 37, 1002–1008. [Google Scholar] [CrossRef]
- Nie, H.; Wang, Y.; Wang, Y. Recovery of scandium from leaching solutions of tungsten residue using solvent extraction with Cyanex 572. Hydrometallurgy 2018, 175, 117–123. [Google Scholar] [CrossRef]
- Dasa, S.; Beheraa, S.; Murmu, B. Extraction of scandium(III) from acidic solutions using organo-phosphoric acid reagents: A comparative study. Sep. Purif. Technol. 2018, 202, 248–258. [Google Scholar] [CrossRef]
- Korovin, V.; Shestak, Y.; Pogorelov, Y. Scandium extraction by neutral organo-phosphorus compounds supported on a porous carrier. Hydrometallurgy 1999, 52, 1–8. [Google Scholar] [CrossRef]
- Wu, D.; Niu, C.; Li, D. Solvent extraction of scandium(III), yttrium(III), lanthanum(III) and gadolinium(III) using Cyanex 302 in heptane from hydrochloric acid solutions. J. Alloys Compd. 2004, 374, 442–446. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Tang, S. Selective recovery of vanadium and scandium by ion exchange with D201 and solvent extraction using P507 from hydrochloric acid leaching solution of red mud. Chemosphere 2017, 175, 365–372. [Google Scholar] [CrossRef]
- Ochsentihn, M.; Lyberopulu, T.; Parissakis, G. Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal. Chim. Acta 1995, 315, 231–237. [Google Scholar]
- Yang, X.; Gu, Z.; Wang, D. Extraction and separation of scandium from rare earths by electrostatic pseudo liquid membrane. J. Membr. Sci. 1995, 106, 131–145. [Google Scholar] [CrossRef]
- Li, H.; Tong, Z.; Chen, Z. Extraction technique of the scandium from titanium white waste acid. Inorgan. Chem. Ind. 2006, 38, 132–137. (In Chinese) [Google Scholar]
- Li, Y.; Chen, J. Improvement of Sc Recovery from the Ti White Hydrolyzed Solution. Rare Met. Cem. Carbides 1997, 128, 1–5. (In Chinese) [Google Scholar]
- Feng, Y.; Wang, J.; Wang, H. Scandium Extraction from Titanium Dioxide Waste Acid Produced by Sulfuric Acid Method. Rare Earth 1997, 2, 46–49. (In Chinese) [Google Scholar]
- Man, L.; Fan, Y.; Huang, J. Separation of Scandium, Titanium from Waste Acidic Solution by Solvent Extraction. Hydrometall. China 2016, 147, 231–235. (In Chinese) [Google Scholar]
- Speight, J.G. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Zhou, X.; Wei, C.; Li, M. Thermodynamics of vanadium–sulfur–water systems at 298 K. Hydrometallurgy 2011, 106, 104–112. [Google Scholar] [CrossRef]
- Shao, K.; Li, Q.; Zhou, Q. Thermodynamic analysis for species of molybdenum and phosphorus in Ni-Mo ore acidic leaching solution. Chin. J. Nonferrous Met. 2017, 27, 1513–1519. [Google Scholar]
- Larson, W. Thermochemistry of Vanadium(5+) in Aqueous Solutions. J. Chem. Eng. Data 1996, 40, 1276–1280. [Google Scholar] [CrossRef]
- Xiao, C.; Xiao, L.; Gao, C. Thermodynamic study on removal of magnesium from lithium chloride solutions using phosphate precipitation method. Sep. Purif. Technol. 2015, 156, 582–587. [Google Scholar] [CrossRef]
- He, L.; Xu, W.; Song, Y.; Liu, X.; Zhao, Z. Selective removal of magnesium from a lithium-concentrated anolyte by magnesium ammonium phosphate precipitation. Sep. Purif. Technol. 2017, 187, 214–220. [Google Scholar] [CrossRef]
- Xiao, C.; Zeng, L.; Wei, J.; Xiao, L.; Zhang, G. Thermodynamic analysis for the separation of tungsten and aluminium in alkaline medium using solvent extraction. Hydrometallurgy 2017, 174, 91–96. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Lv, G. Thermodynamic study on the V(V)-P(V)-H2O system in acidic leaching solution of vanadium-bearing converter slag. Sep. Purif. Technol. 2019, 218, 164–172. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, Y.; Li, H. A New Method for Metallurgical Thermodynamic Equilibrium Calculation by EXCEL. Rare Met. Cem. Carbides 2005, 33, 48–53. (In Chinese) [Google Scholar]
- Zhao, Z.; Cao, C.; Li, H. Thermodynamics on soda decomposition of scheelite. Chin. J. Nonferrous Met. 2008, 2, 356–361. [Google Scholar]
- Zhang, W.; Zhao, Z. Thermodynamics of W and Mo sulfidation by using new sulfiding agent P2S5. Chin. J. Nonferrous Met. 2014, 5, 1375–1383. [Google Scholar]
- Zhao, Z.; Liu, X. Thermodynamic analysis of Li-Fe-P-H2O system. Chin. J. Nonferrous Met. 2006, 16, 1257–1263. [Google Scholar]
- Deng, Z.; Wei, C.; Li, M.; Fan, G.; Ge, H. Technology of Extracting Vanadium and Removing Iron from Stone-Coal Oxygen Pressure Acid-Leaching Solution. Chin. J. Rare Met. 2009, 33, 290–294. [Google Scholar]
- Zhang, Q. Separation Science and Engineering of Metallurgy; Science Press: Beijing, China, 2004. [Google Scholar]
- Ma, Y.; Wang, X.; Wang, M.; Jiang, C. Separation of V(IV) and Fe(III) from the acid leach solution of stone coal by D2EHPA/TBP. Hydrometallurgy 2015, 153, 38–45. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, T.; Lv, G.; Zhou, W. Extraction Separation of Sc(III) and Fe(III) from a Strongly Acidic and Highly Concentrated Ferric Solution by D2EHPA/TBP. JOM 2018, 70, 2837–2845. [Google Scholar] [CrossRef]
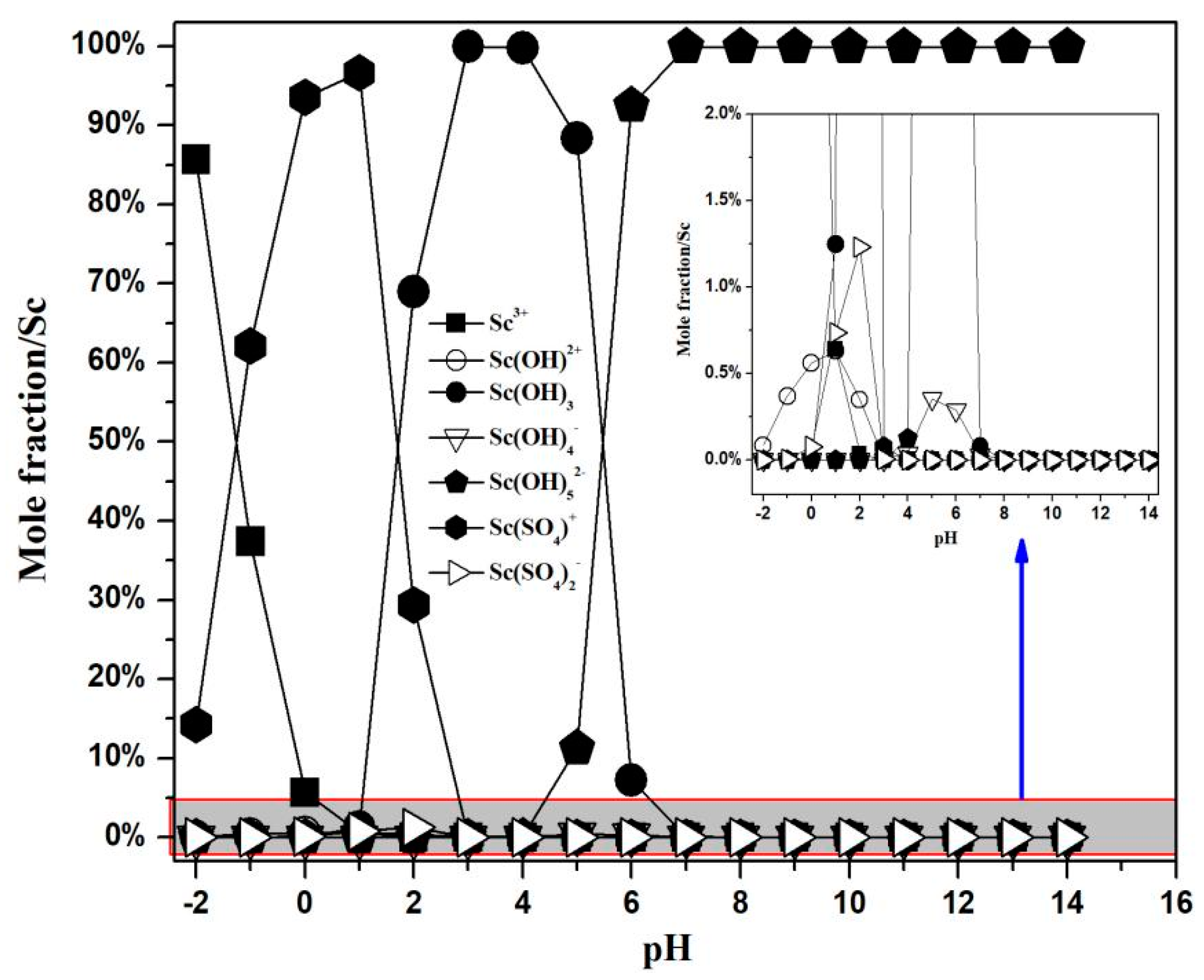
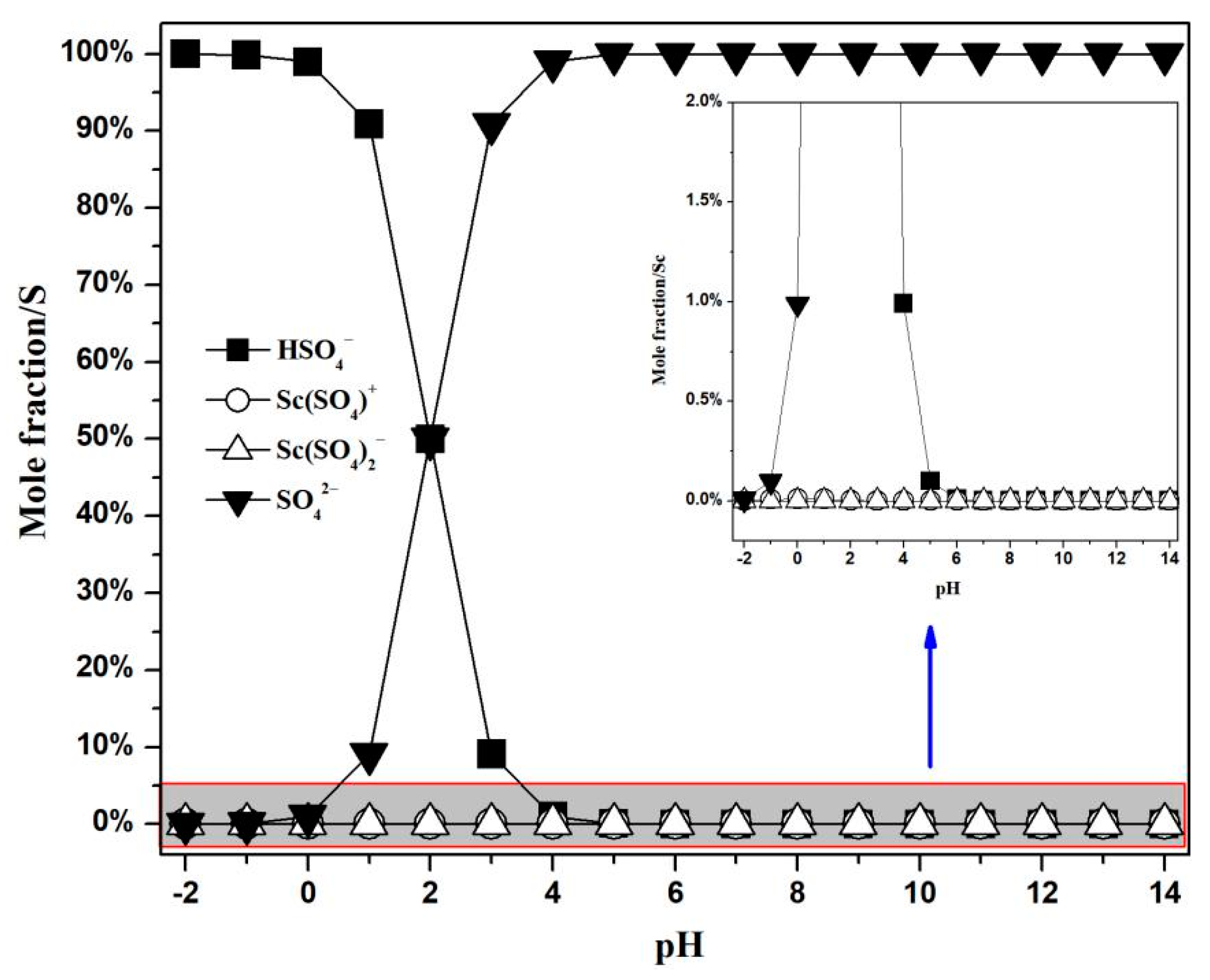



| No. | Equilibrium Reaction | lgK [34] |
|---|---|---|
| 1 | HSO4−= H+ + SO42− | 2.00 |
| 2 | Sc3+ + OH− = Sc(OH)2+ | 2.40 |
| 3 | Sc3+ + 4OH− = Sc(OH)4− | 3.28 |
| 4 | Sc3+ + 5OH− = Sc(OH)52− | 3.13 |
| 5 | Sc3+ + SO42− = Sc(SO4)+ | 0.62 |
| 6 | Sc3+ + 2SO42−= Sc(SO4)2− | 0.94 |
| 7 | Sc3+ + 3OH− = Sc(OH)3 | 7.33 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Zhang, T.-a.; Zhang, W.; Lv, G. Solvent Extraction of Sc(III) by D2EHPA/TBP from the Leaching Solution of Vanadium Slag. Metals 2020, 10, 790. https://doi.org/10.3390/met10060790
Cao X, Zhang T-a, Zhang W, Lv G. Solvent Extraction of Sc(III) by D2EHPA/TBP from the Leaching Solution of Vanadium Slag. Metals. 2020; 10(6):790. https://doi.org/10.3390/met10060790
Chicago/Turabian StyleCao, Xuejiao, Ting-an Zhang, Weiguang Zhang, and Guozhi Lv. 2020. "Solvent Extraction of Sc(III) by D2EHPA/TBP from the Leaching Solution of Vanadium Slag" Metals 10, no. 6: 790. https://doi.org/10.3390/met10060790
APA StyleCao, X., Zhang, T.-a., Zhang, W., & Lv, G. (2020). Solvent Extraction of Sc(III) by D2EHPA/TBP from the Leaching Solution of Vanadium Slag. Metals, 10(6), 790. https://doi.org/10.3390/met10060790




