Bioleaching of Heavy Metals from Municipal Solid Waste Incineration Fly Ash: Availability of Recoverable Sulfur Prills and Form Transformation of Heavy Metals
Abstract
:1. Introduction
2. Materials and Methods
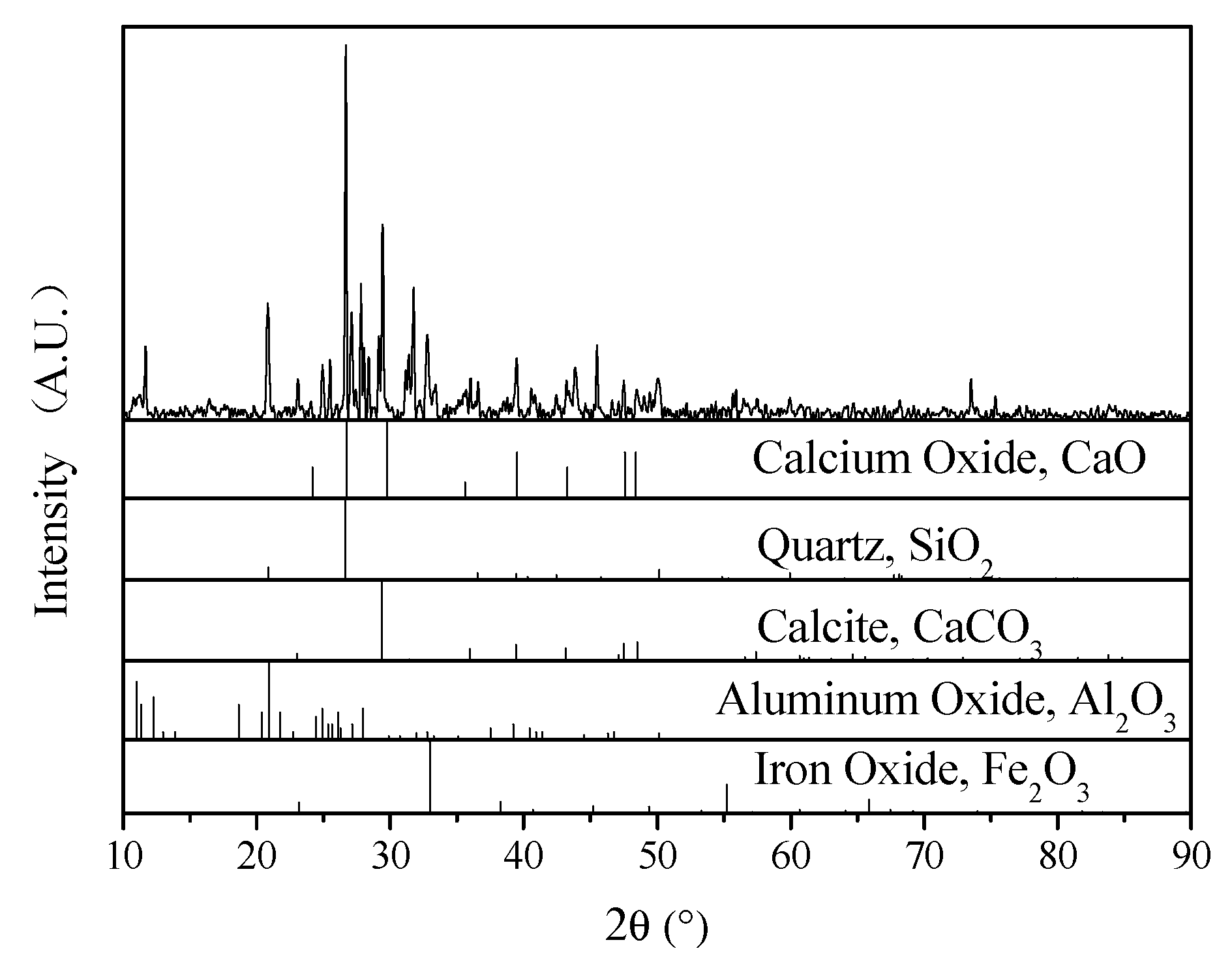
2.1. Municipal Solid Waste Incineration Fly Ash
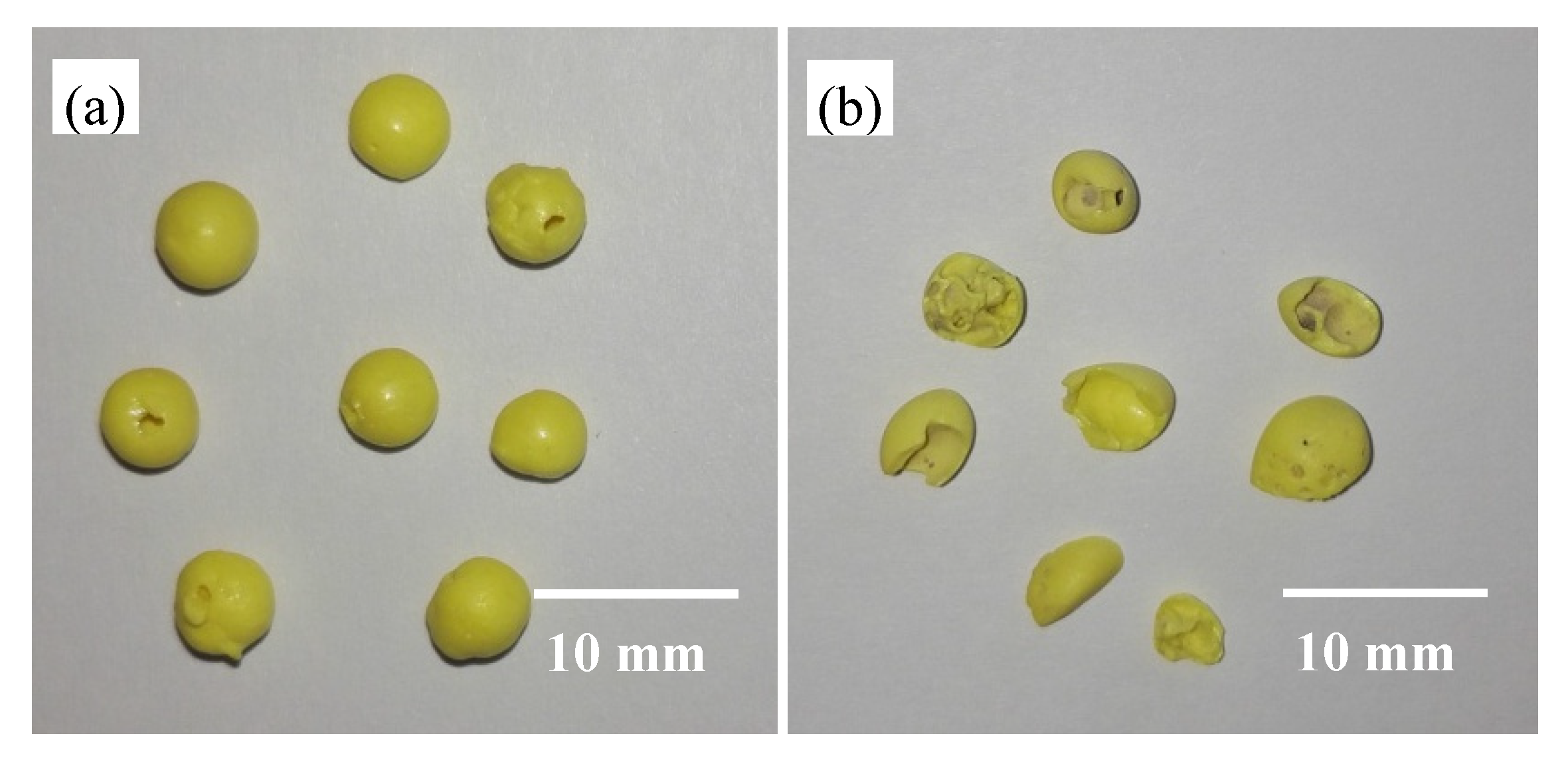
2.2. Preparation of the Recyclable Sulfur Prills
2.3. Sulfur Oxidizing Bacteria
2.4. Bioleaching Experiments
2.5. Analytical Methods
3. Results and Discussion
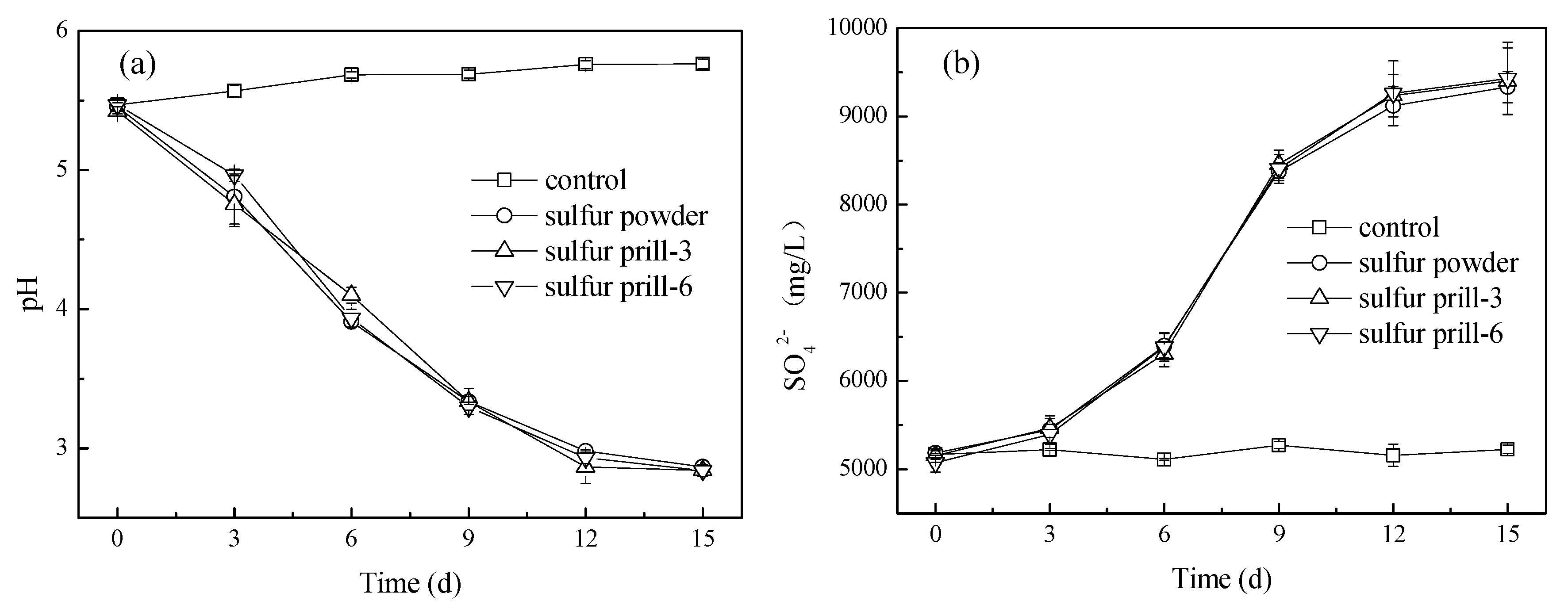
3.1. pH and Sulfate Variations in Bioleaching with Different Sulfur Forms
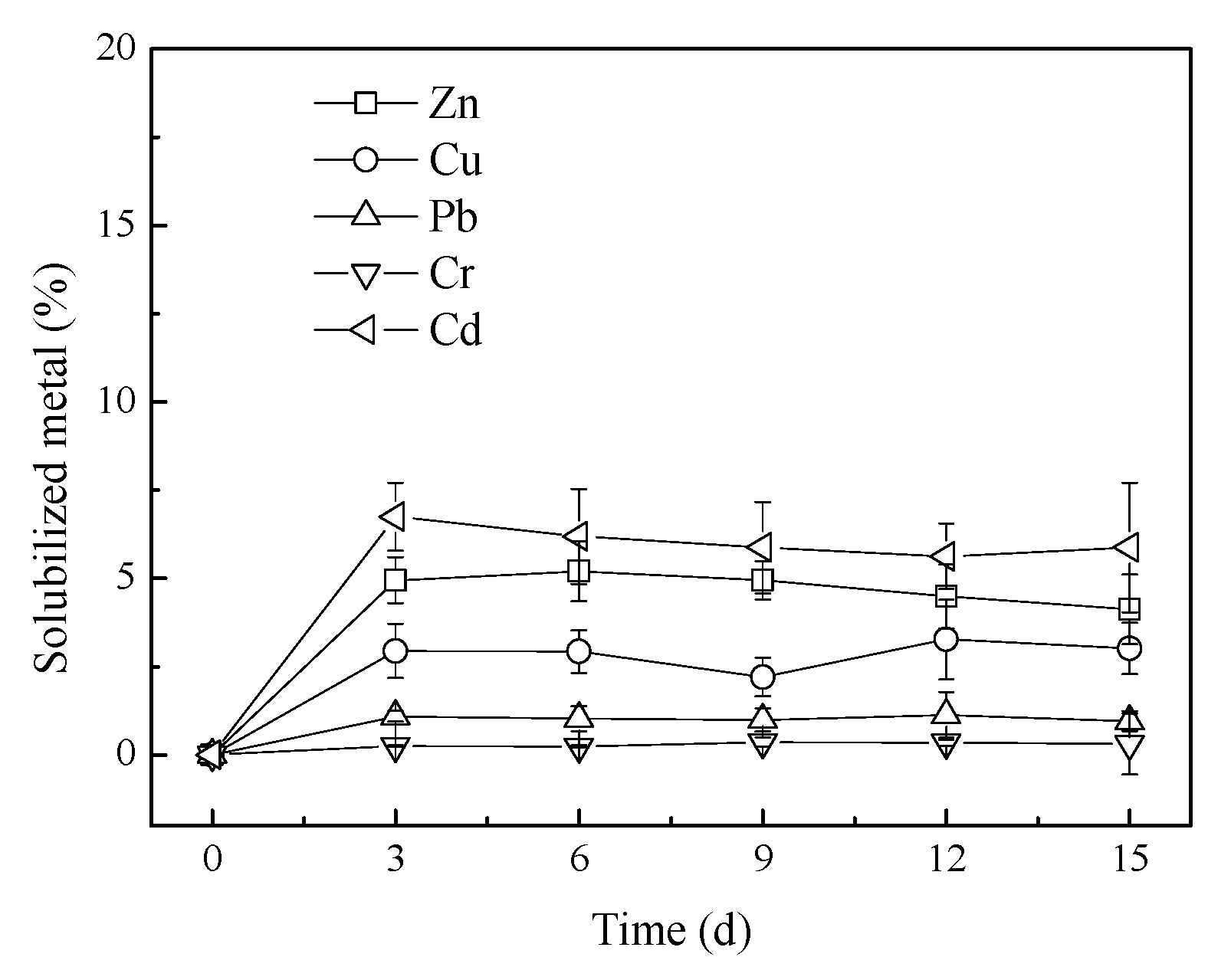
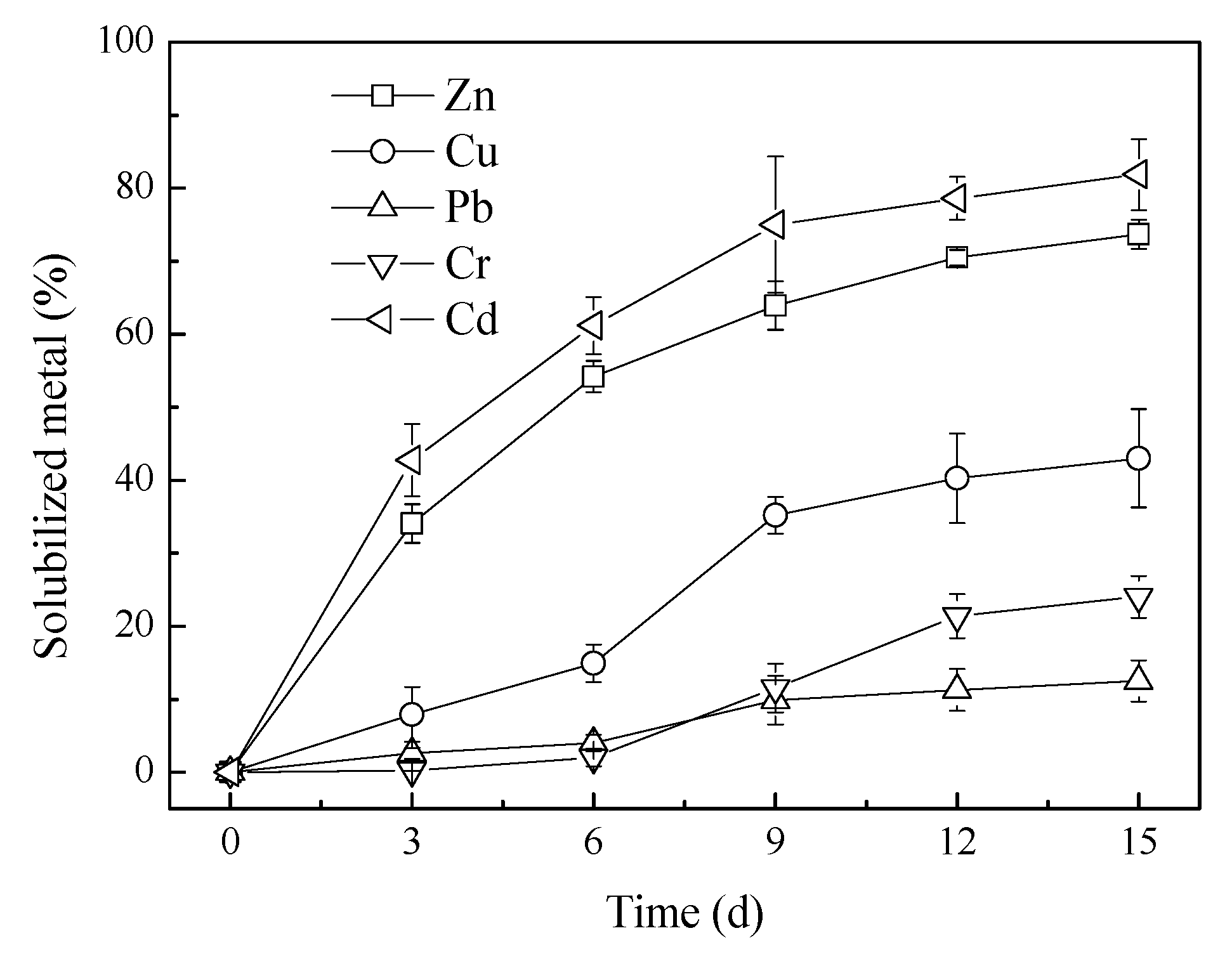
3.2. Heavy Metals Solubilization in Bioleaching
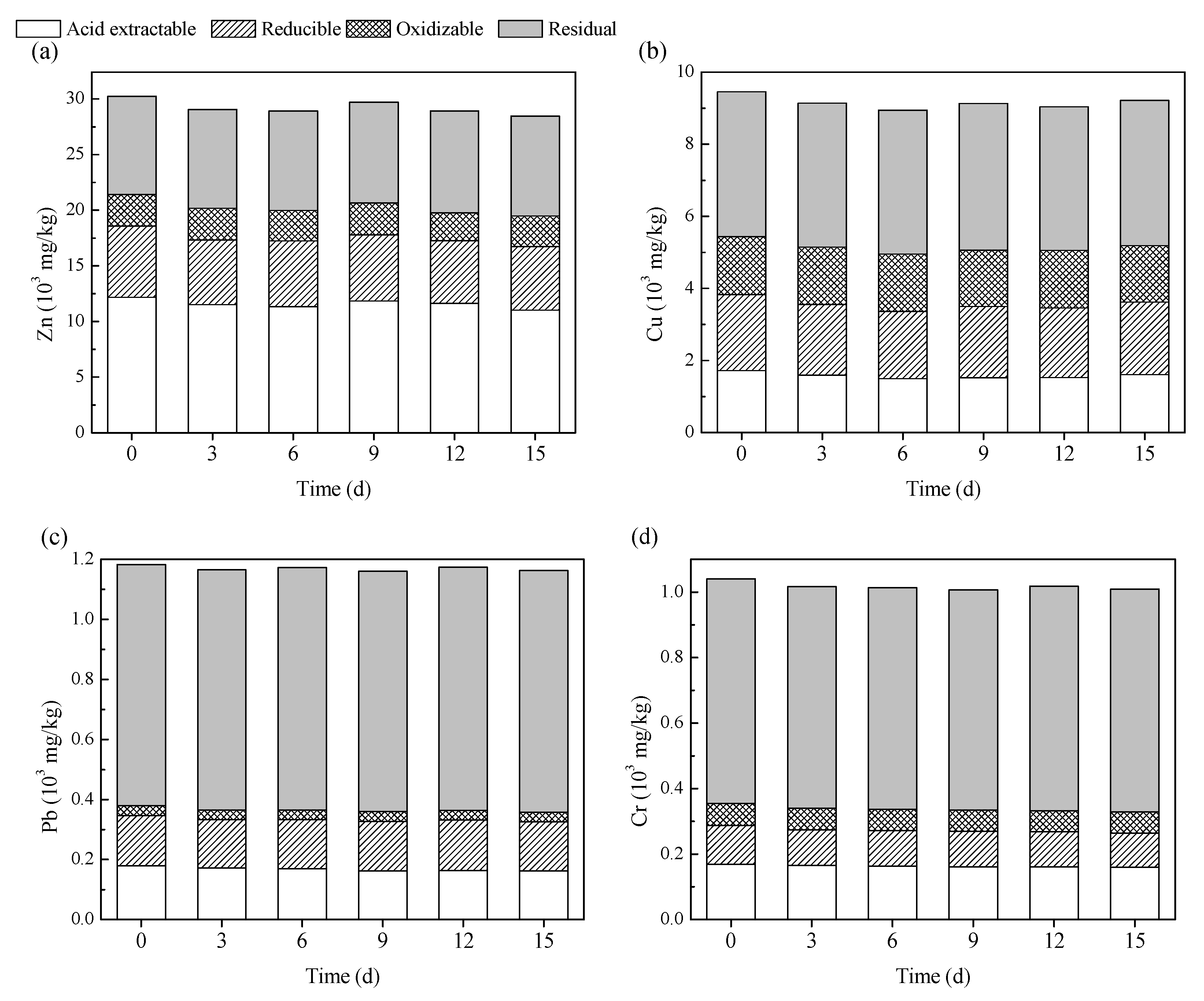
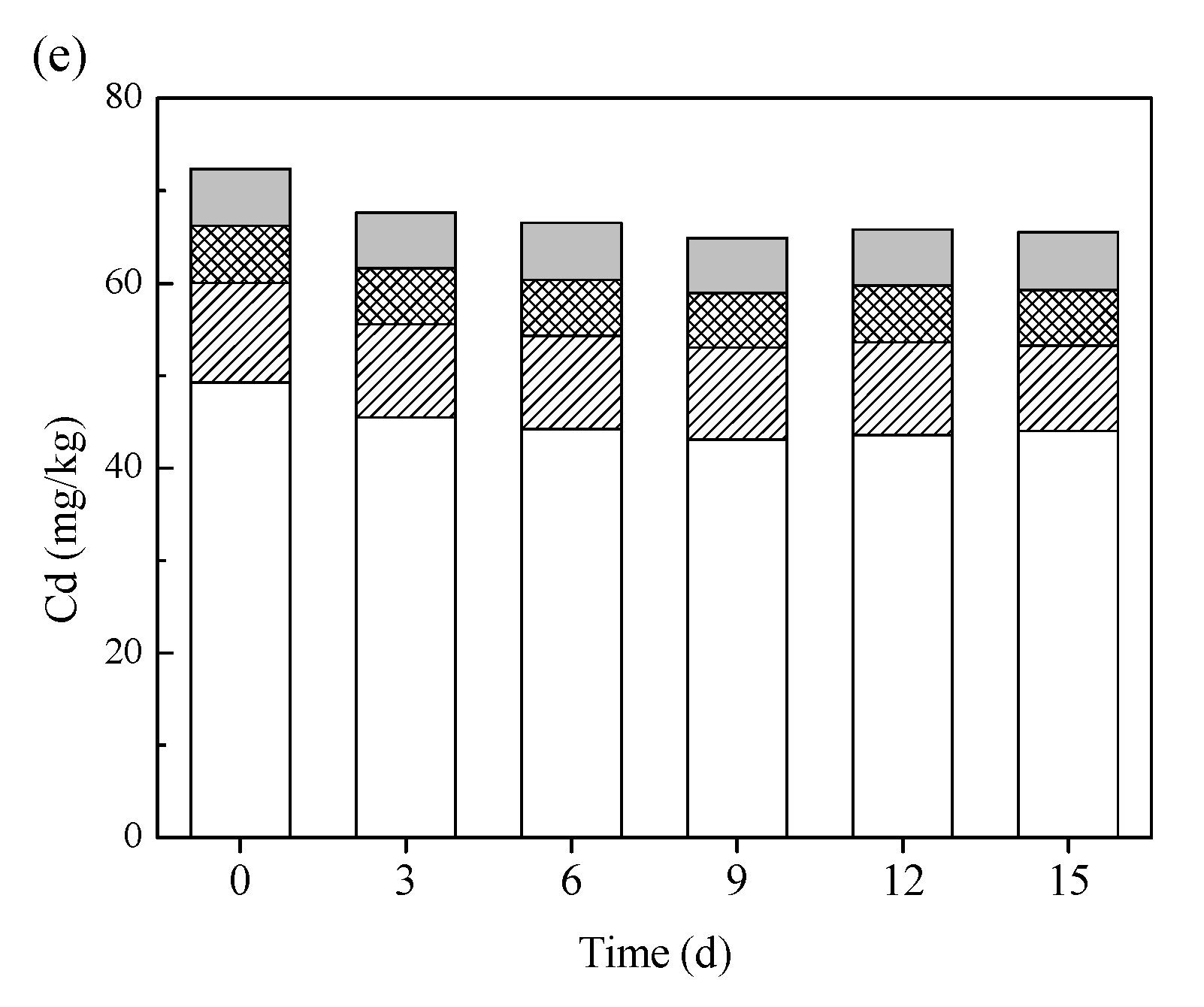
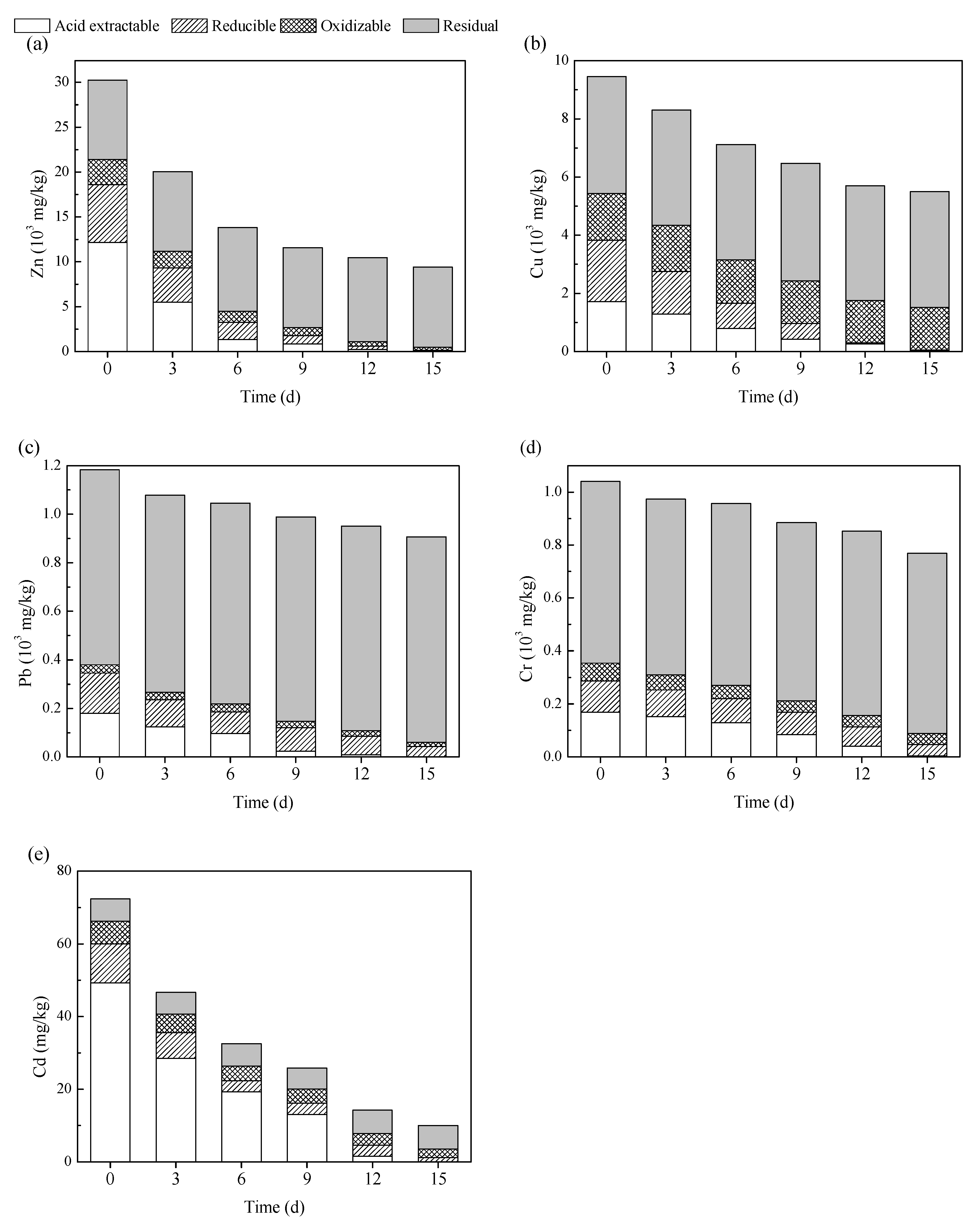
3.3. Chemical Speciation of Heavy Metals during Bioleaching
3.4. Availability of Recovered Sulfur Prills in Bioleaching
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Luo, H.; Cheng, Y.; He, D.; Yang, E. Review of leaching behavior of municipal solid waste incineration (MSWI) ash. Sci. Total Environ. 2019, 668, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Gomes, H.I.; Funari, V.; Ferrari, R. Bioleaching for resource recovery from low-grade wastes like fly and bottom ashes from municipal incinerators: A SWOT analysis. Sci. Total Environ. 2020, 715, 136945. [Google Scholar] [CrossRef]
- Wiles, C.C. Municipal solid waste combustion ash: State-of-the-knowledge. J. Hazard. Mater. 1996, 47, 325–344. [Google Scholar] [CrossRef]
- Yin, K.; Chan, W.P.; Dou, X.; Ren, F.; Chang, V.W.C. Cr, Cu, Hg and Ni release from incineration bottom ash during utilization in land reclamation—based on lab-scale batch and column leaching experiments and a modeling study. Chemosphere 2018, 197, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.H.K.; Ip, A.W.M.; Barford, J.; Mckay, G. Use of Incineration MSW Ash: A Review. Sustainability 2010, 2, 1943–1968. [Google Scholar] [CrossRef] [Green Version]
- Youcai, Z.; Lijie, S.; Guojian, L. Chemical stabilization of MSW incinerator fly ashes. J. Hazard. Mater. 2002, 95, 47–63. [Google Scholar] [CrossRef]
- Shi, H.S.; Kan, L.L. Characteristics of municipal solid wastes incineration (MSWI) fly ash-cement matrices and effect of mineral admixtures on composite system. Constr. Build. Mater. 2009, 23, 2160–2166. [Google Scholar] [CrossRef]
- Yang, R.; Liao, W.P.; Wu, P.H. Basic characteristics of leachate produced by various washing processes for MSWI ashes in Taiwan. J. Environ. Manag. 2012, 104, 67–76. [Google Scholar] [CrossRef]
- Benassi, L.; Dalipi, R.; Consigli, V.; Pasquali, M.; Bontempi, E. Integrated management of ash from industrial and domestic combustion: A new sustainable approach for reducing greenhouse gas emissions from energy conversion. Environ. Sci. Pollut. Res. 2017, 24, 14834–14846. [Google Scholar] [CrossRef]
- Rodella, N.; Pasquali, M.; Zacco, A.; Bilo, F.; Borgese, L.; Bontempi, N.; Tomasoni, G.; Depero, L.E.; Bontempi, E. Beyond waste: New sustainable fillers from fly ashes stabilization, obtained by low cost raw materials. Heliyon 2016, 2, e00163. [Google Scholar] [CrossRef] [Green Version]
- Rodella, N.; Bosio, A.; Dalipi, R.; Zacco, A.; Borgese, L.; Depero, L.E.; Bontempi, E. Waste silica sources as heavy metal stabilizers for municipal solid waste incineration fly ash. Arab. J. Chem. 2017, 10, S3676–S3681. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; Liu, X.; Zhang, R.; Deng, W.; Zhou, L. Removal of Contaminating Metals from Soil by Sulfur-Based Bioleaching and Biogenic Sulfide-Based Precipitation. Geomicrobiol. J. 2013, 30, 473–478. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, R.; Liu, X.; Zhou, L. Selective recovery of soil-borne metal contaminants through integrated solubilization by biogenic sulfuric acid and precipitation by biogenic sulfide. J. Hazard. Mater. 2012, 219, 119–126. [Google Scholar] [CrossRef]
- Fang, D.; Zhang, R.; Zhou, L.; Li, J. A combination of bioleaching and bioprecipitation for deep removal of contaminating metals from dredged sediment. J. Hazard. Mater. 2011, 192, 226–233. [Google Scholar] [CrossRef]
- Fang, D.; Zhao, L.; Zhou, L.X.; Shan, H.X. Effects of sulfur forms on heavy metals bioleaching from contaminated sediments. J.Environ. Sci. Health Part A 2009, 44, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Argumedo-Delira, R.; Gómez-Martínez, M.J.; Soto, B.J. Gold Bioleaching from printed circuit boards of mobile phones by Aspergillus niger in a culture without agitation and with glucose as a carbon Source. Metals 2019, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Li, Q.; Zhang, Y.; Yang, Y.B.; Xu, B.; Jiang, T. Formation process of the passivating products from Arsenopyrite bioleaching by Acidithiobacillus ferrooxidans in 9K culture medium. Metals 2019, 9, 1320. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Sethurajan, M.; Vossenberg, J.V.D.; Meers, E.; Hullebusch, E.D. Recovery of phosphorus from municipal wastewater treatment sludge through bioleaching using Acidithiobacillus thiooxidans. J. Environ. Manag. 2020, 270, 110818. [Google Scholar] [CrossRef]
- Zhang, R.C.; Qiu, H.L.; Wang, T.; Feng, F.; Li, M.T. Recovery of Cu and Zn from waste incineration fly ash through integrated sulfur bio-oxidation and bio-reduction. Chin. J. Environ. Eng. 2019, 13, 1220–1227. [Google Scholar]
- Li, S.; Tian, Z.; Liu, R.; Zhou, W.; Cheng, H.; Sun, J.; Zhao, K.; Wang, Y.; Zhou, H. Effective multi-metal removal from plant incineration ash via the combination of bioleaching and brine leaching. Rsc. Adv. 2020, 10, 1388–1399. [Google Scholar] [CrossRef] [Green Version]
- Fonti, V.; Dell’Anno, A.; Beolchini, F. Does bioleaching represent a biotechnological strategy for remediation of contaminated sediments? Sci. Total Environ. 2016, 563–564, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.C.; Gu, X.Y.; Wong, J.W.C. Comparison of bioleaching of heavy metals from sewage sludge using iron- and sulfur-oxidizing bacteria. Adv. Environ. Res. 2003, 7, 603–607. [Google Scholar] [CrossRef]
- Chen, S.; Cheng, Y. Effects of sulfur dosage and inoculum size on pilot-scale thermophilic bioleaching of heavy metals from sewage sludge. Chemosphere 2019, 234, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chiu, Y.C.; Chang, P.L.; Lin, J.G. Assessment of recoverable forms of sulfur particles used in bioleaching of contaminated sediments. Water Res. 2003, 37, 450–458. [Google Scholar] [CrossRef]
- Funari, V.; Makinen, J.; Salminen, J.; Braga, R.; Dinelli, E.; Revitzer, H. Metal removal from Municipal Solid Waste Incineration fly ash: A comparison between chemical leaching and bioleaching. Waste Manag. 2017, 60, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chang, M.; Hu, P.; Ni, J. Bioleaching of Fly Ash from Municipal Solid Waste Incinerator Using Sewage Sludge and Pig Manure as Culture Media. Environ. Sci. 2005, 26, 180–185. [Google Scholar]
- Chang, C.; Chen, S.; Klipkhayai, P.; Chiemchaisri, C. Bioleaching of heavy metals from harbor sediment using sulfur-oxidizing microflora acclimated from native sediment and exogenous soil. Environ. Sci. Pollut. Res. 2019, 26, 6818–6828. [Google Scholar] [CrossRef]
- Seidel, H.; Wennrich, R.; Hoffmann, P.; LöSer, C. Effect of different types of elemental sulfur on bioleaching of heavy metals from contaminated sediments. Chemosphere 2006, 62, 1444–1453. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, M.; Hsieh, L.; Cai, Y.; Shen, Y.; Wang, H.; Lin, Q.; Shen, C.; Hu, B.; Lou, L. Feasibility of bioleaching of heavy metals from sediment with indigenous bacteria using agricultural sulfur soil conditioners. Sci. Total Environ. 2020, 703, 134812. [Google Scholar] [CrossRef]
- Hyne, J.B. Microcrystalline structure and surface area of elemental sulphur as factors influencing its oxidation by Thiobacillus albertis. Can. J. Microbiol. 1985, 32, 237–242. [Google Scholar]
- Kolmert, Å.; Wikström, P.; Hallberg, K.B. A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J. Microbiol. Methods 2000, 41, 179–184. [Google Scholar] [CrossRef]
- Song, T.Y.; Cheng, P.; Xu, J.N.; Cheng, G.Z.; Wang, L. Inorganic Chemistry, 4th ed.; Higher Education Press: Beijing, China, 2019. [Google Scholar]
- Baran, A.; Tarnawski, M. Assessment of heavy metals mobility and toxicity in contaminated sediments by sequential extraction and a battery of bioassays. Ecotoxicology 2015, 24, 1279–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, Q.; Zhou, Y.; Tyrer, M.; Yu, Y. Stabilization of heavy metals in MSWI fly ash using silica fume. Waste Manag. 2014, 34, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Yaashikaa, P.R. Recent trends and challenges in bioleaching technologies. In Biovalorisation of Waste to Renewable Chemicals and Biofuels; Rathinam, N.K., Sani, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–388. [Google Scholar]
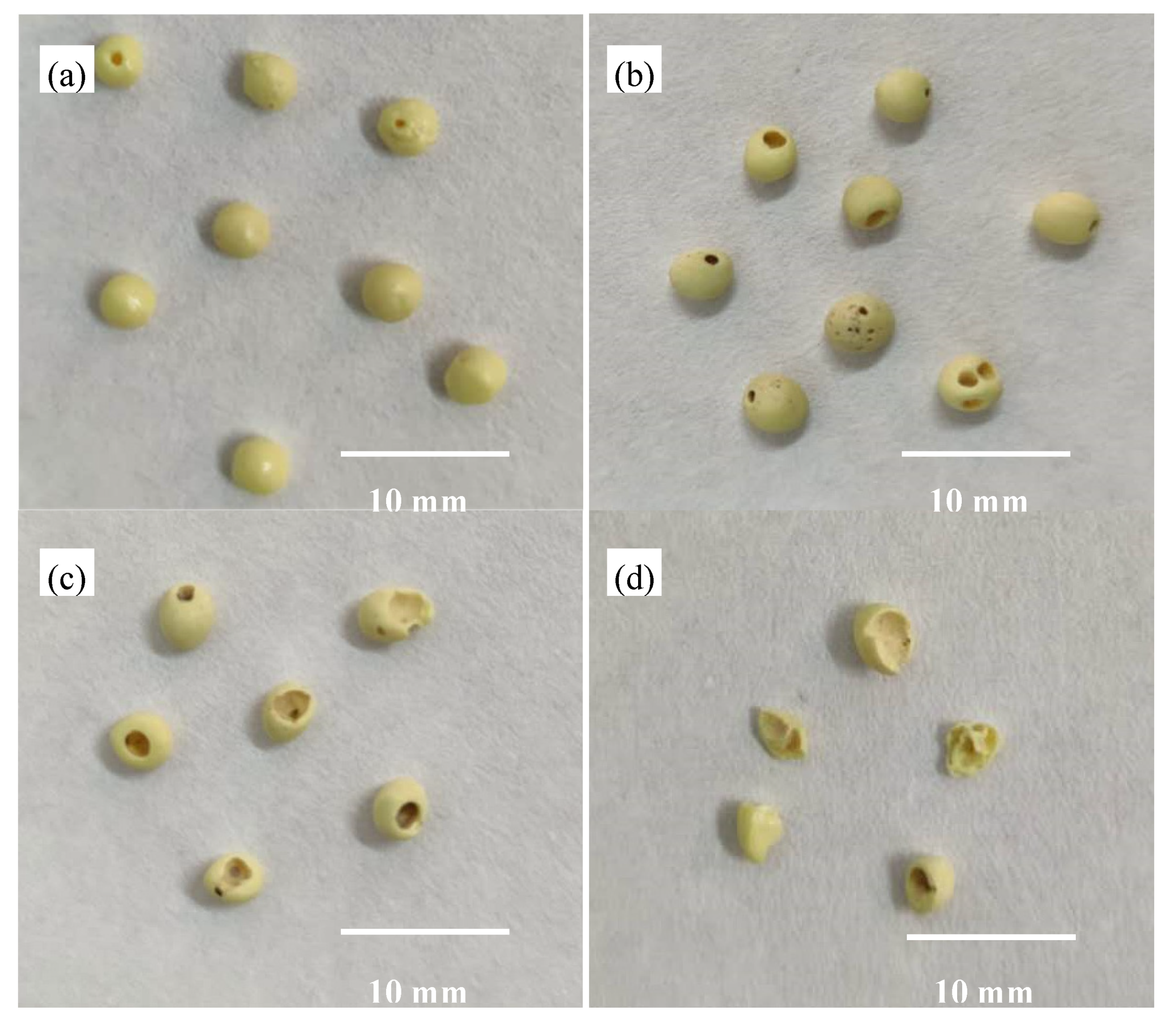
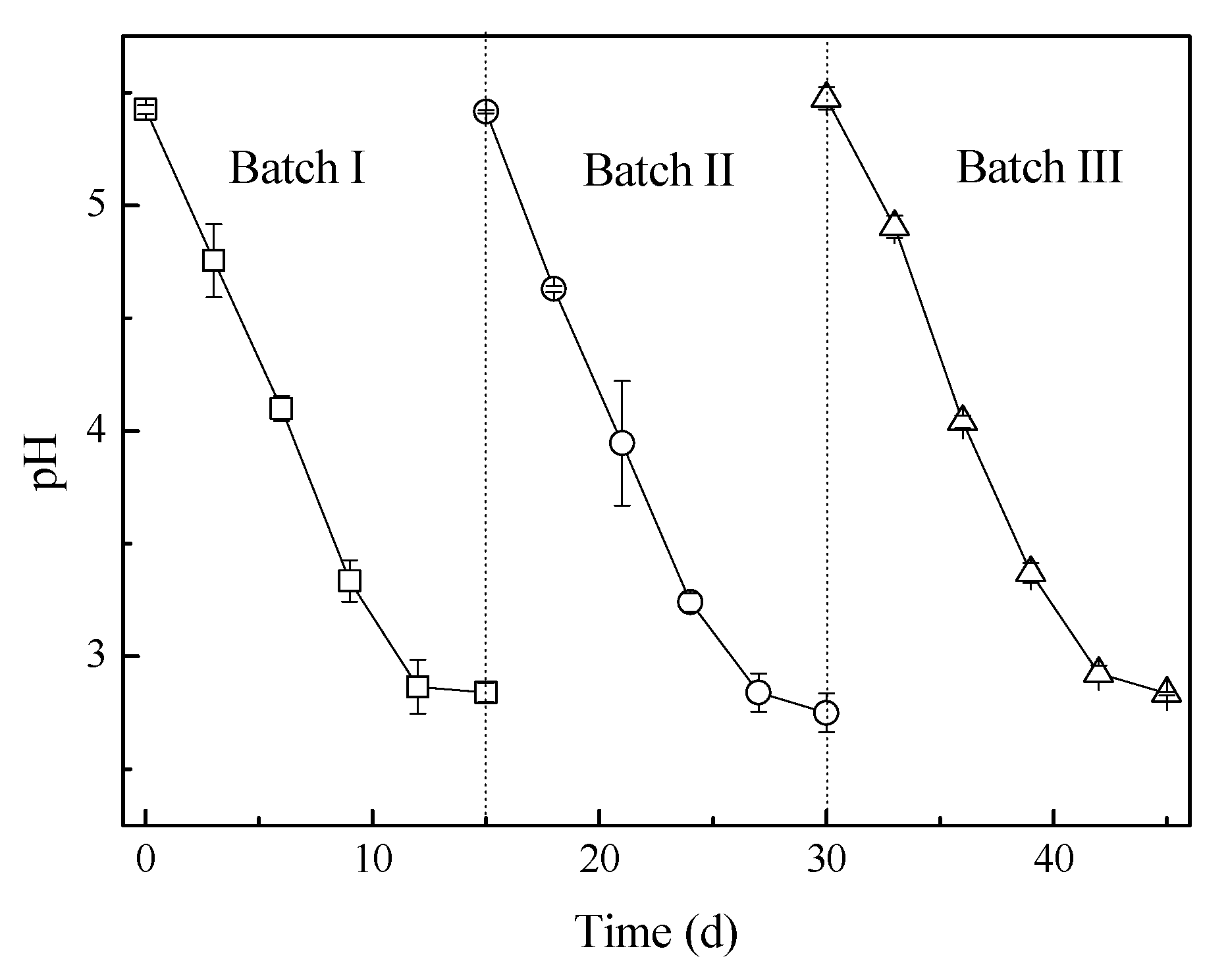
| Sulfur | Oxidation Ratio of S (%) | Recovery Rate of S (%) | Residual Rate of S (%) | Residue of S (g S/g fly ash) | |
|---|---|---|---|---|---|
| Sulfur prill-3 | Batch I | 28.3 ± 2.5 | 93.6 | 6.4 | 0.01 |
| Batch II | 28.5 ± 3.7 | 78.9 | 21.1 | 0.04 | |
| Batch III | 27.9 ± 3.5 | 61.6 | 38.4 | 0.07 | |
| Sulfur prill-6 | 27.7 ± 2.7 | 54.1 | 45.9 | 0.08 | |
| Sulfur powder | 27.6 ± 1.2 | 0 | 100 | 0.181 | |
| Heavy Metals | Raw Fly Ash | Bioleached Fly Ash | Threshold Limit 1 | Threshold Limit 2 |
|---|---|---|---|---|
| Zn | 68.4 ± 2.41 | 0.27 ± 0.09 | 100 | 75 |
| Cu | 3.17 ± 0.65 | 0.92 ± 0.14 | 40 | 75 |
| Pb | 0.79 ± 0.23 | 0.07 ± 0.02 | 0.25 | 5 |
| Cr | 0.18 ± 0.06 | 0.03 ± 0.01 | 4.5 | 12 |
| Cd | 0.53 ± 0.15 | 0.01 ± 0.01 | 0.15 | 0.5 |
| Batches | Removal Rate (%) | Extractable Concentration (mg/L) | |||||
|---|---|---|---|---|---|---|---|
| Zn | Cu | Pb | Cr | Cd | Pb | Cd | |
| Batch I | 73.7 ± 1.99 | 43.0 ± 6.73 | 12.5 ± 2.84 | 24.1 ± 2.87 | 81.9 ± 4.84 | 0.08 ± 0.04 | 0.02 ± 0.01 |
| Batch II | 74.6 ± 3.49 | 41.0 ± 4.82 | 12.7 ± 0.94 | 22.0 ± 3.31 | 80.0 ± 8.17 | 0.11 ± 0.04 | 0.01 ± 0.01 |
| Batch III | 72.0 ± 5.13 | 42.3 ± 1.42 | 11.1 ± 1.71 | 23.1 ± 0.98 | 79.1 ± 5.73 | 0.05 ± 0.01 | 0.03 ± 0.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wei, X.; Hao, Q.; Si, R. Bioleaching of Heavy Metals from Municipal Solid Waste Incineration Fly Ash: Availability of Recoverable Sulfur Prills and Form Transformation of Heavy Metals. Metals 2020, 10, 815. https://doi.org/10.3390/met10060815
Zhang R, Wei X, Hao Q, Si R. Bioleaching of Heavy Metals from Municipal Solid Waste Incineration Fly Ash: Availability of Recoverable Sulfur Prills and Form Transformation of Heavy Metals. Metals. 2020; 10(6):815. https://doi.org/10.3390/met10060815
Chicago/Turabian StyleZhang, Ruichang, Xuefeng Wei, Qiang Hao, and Ruofan Si. 2020. "Bioleaching of Heavy Metals from Municipal Solid Waste Incineration Fly Ash: Availability of Recoverable Sulfur Prills and Form Transformation of Heavy Metals" Metals 10, no. 6: 815. https://doi.org/10.3390/met10060815





