Effect of Phase Distribution on the Antibacterial Property and Cytotoxicity of Ti-5Al-2.5Cu Alloy after Heat Treatment at Various Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Microstructure Characterization
2.3. Antibacterial Test
2.4. Culture of Cell
2.5. Cell Viability Assay
3. Results
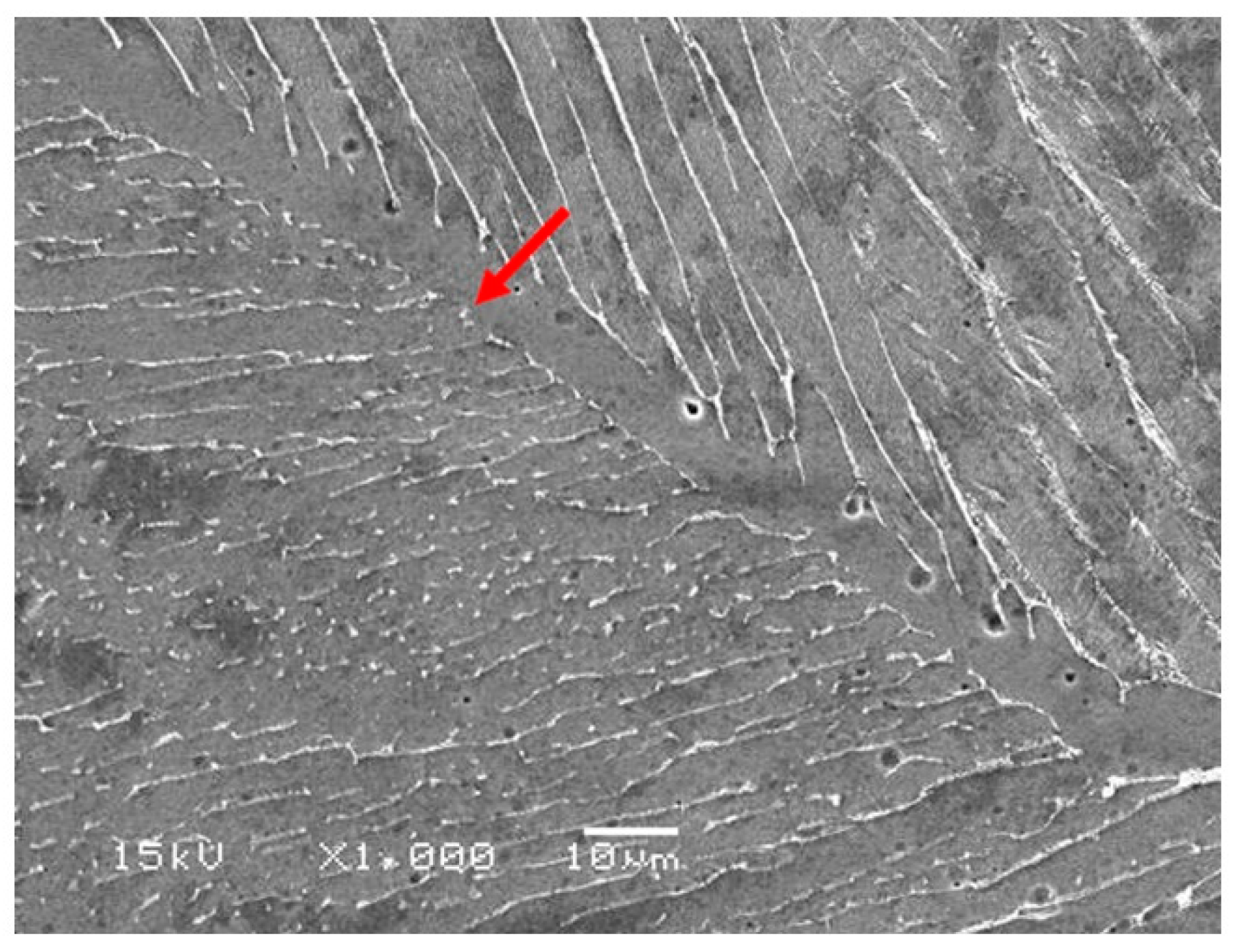
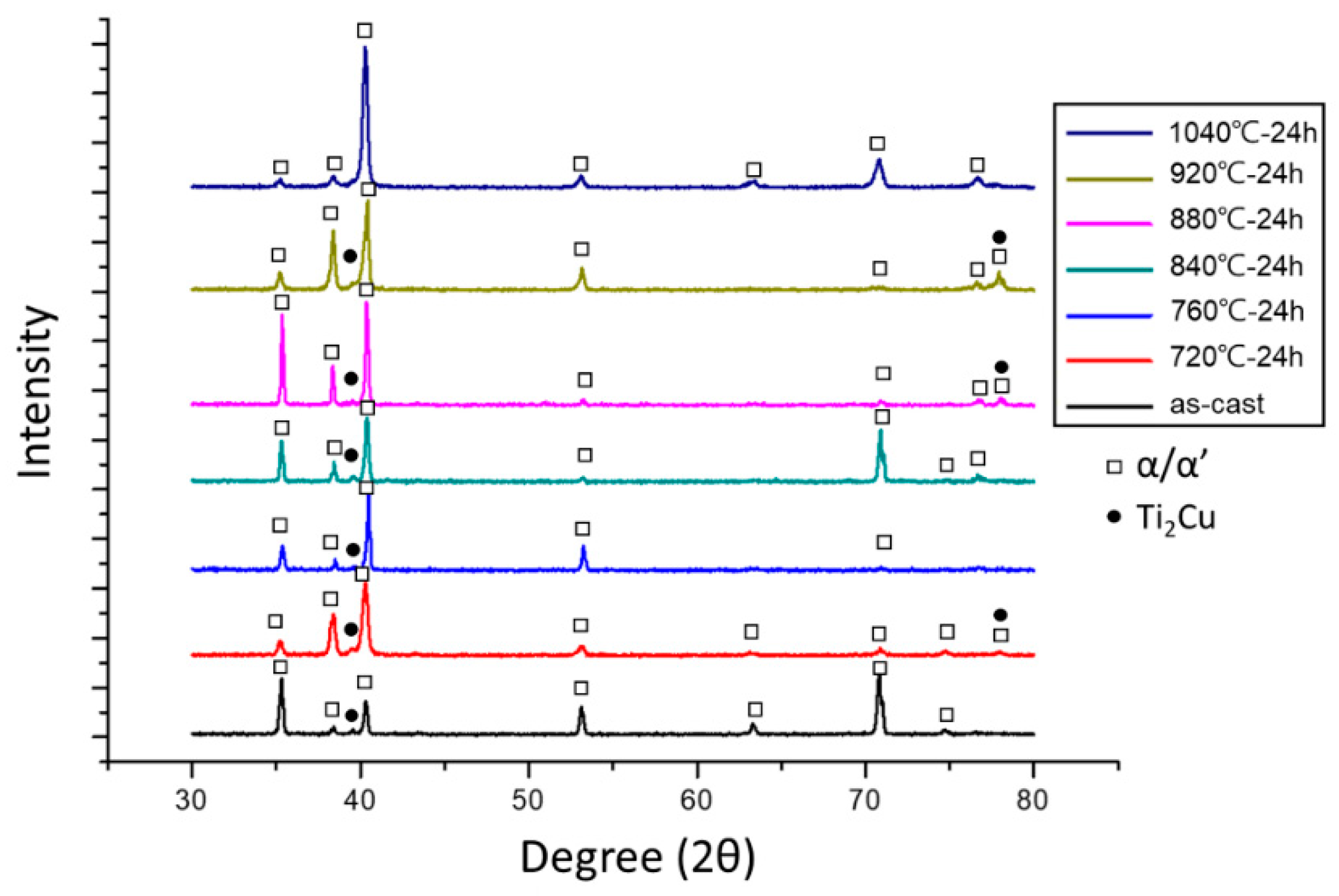
3.1. Microstructural Observations
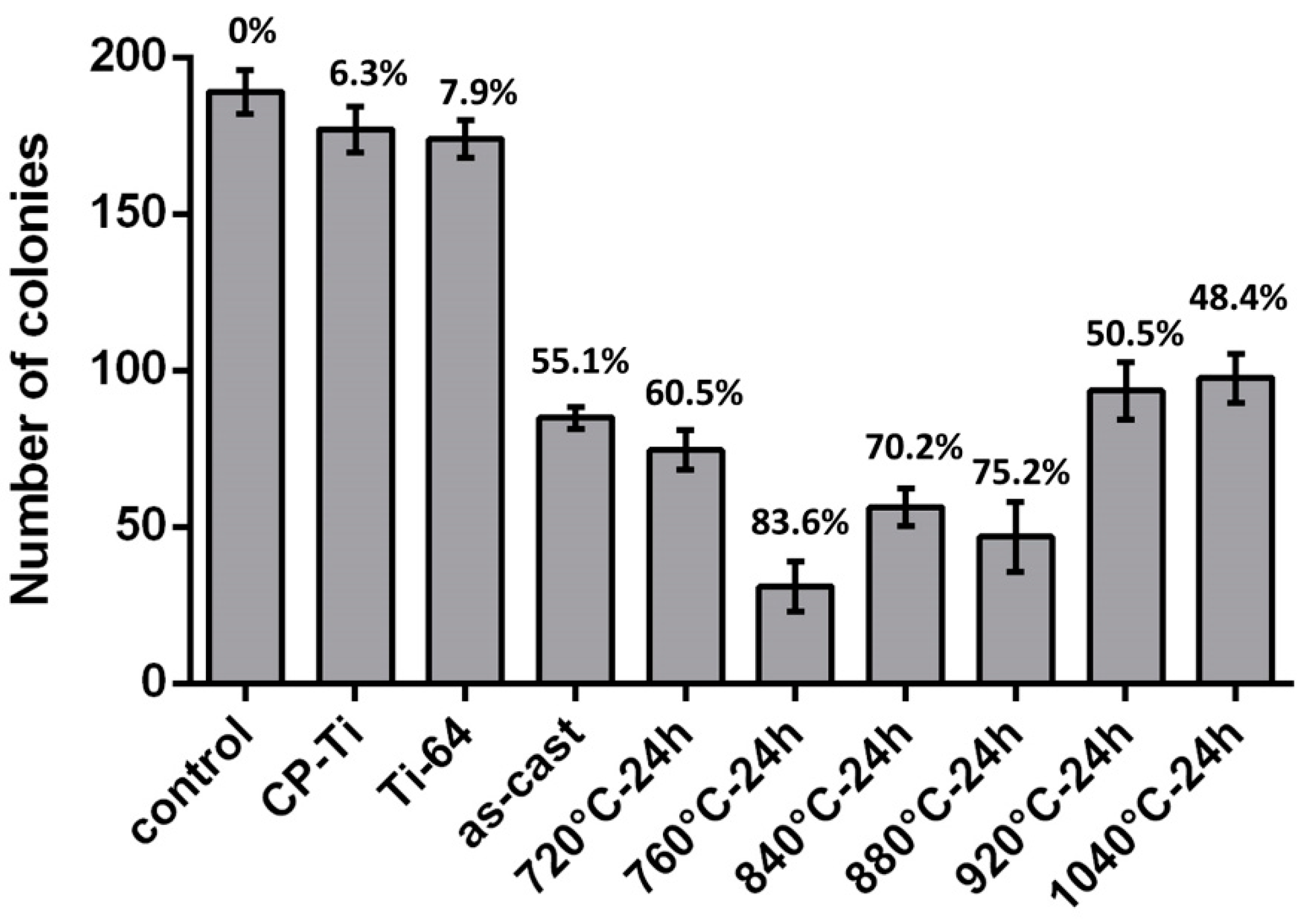
3.2. Antibacterial Property
3.3. Cytotoxicity Evaluation
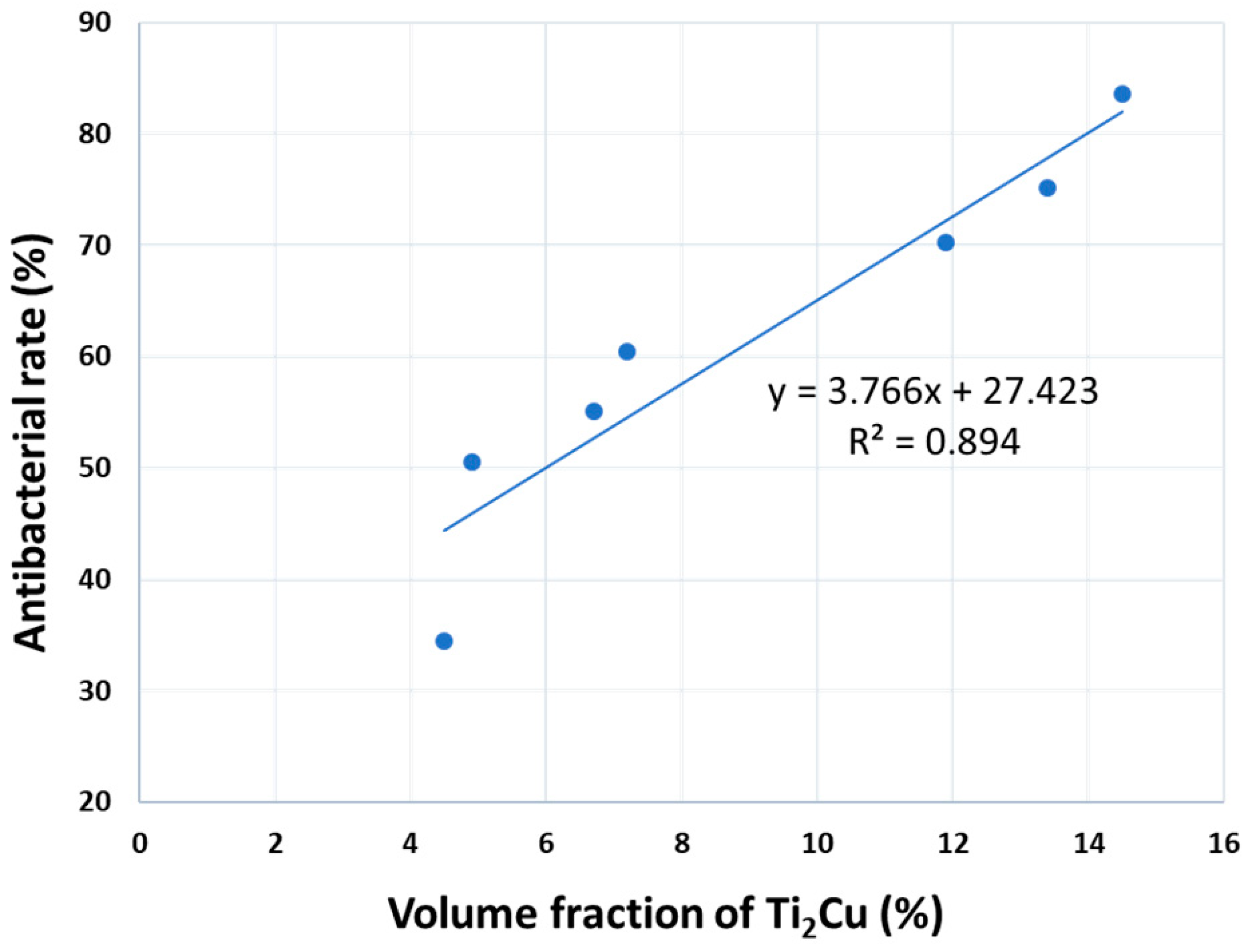
4. Discussion
5. Conclusions
- The microstructure of the present Ti-5Al-2.5Cu alloy was essentially an acicular α’ martensite. Some Ti2Cu-phase precipitates were also observed, which improved the antibacterial property of the alloy.
- During various heat treatments, a high volume fraction of the Ti2Cu phase was obtained after annealing at 760–880 °C. The addition of 5.0 wt% Al increased the temperature at which the Ti2Cu-phase precipitates occurred.
- The antibacterial property was linearly related to the ratio of precipitates and was accumulated using a basic antibacterial value. The optimal heat treatment for the Ti-5Al-2.5Cu alloy was annealing at 760 °C for 24 h, at which it exhibited promising antibacterial properties.
- The Ti-5Al-2.5Cu alloy heat treated under different conditions exhibited promising non-cytotoxicity when cultured with MG-63 cells for 1, 4, and 7 days.
Author Contributions
Funding
Conflicts of Interest
References
- Ou, K.-L.; Weng, C.-C.; Lin, Y.-H.; Huang, M.-S. A promising of alloying modified beta-type titanium-niobium implant for biomedical applications: Microstructural characteristics, in vitro biocompatibility and antibacterial performance. J. Alloys Compd. 2017, 697, 231–238. [Google Scholar] [CrossRef]
- Zhang, E.; Zheng, L.; Liu, J.; Bai, B.; Liu, C. Influence of cu content on the cell biocompatibility of Ti-Cu sintered alloys. Mater. Sci. Eng. C 2015, 46, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Sun, Q.Y.; Xiao, L.; Sun, J.; Zhang, L.C.; Guo, X.D.; Li, X.D. Age hardening and its modeling of Ti–2.5cualloy. Mater. Sci. Eng. A 2013, 568, 118–122. [Google Scholar] [CrossRef]
- Elias, C.N.; Fernandes, D.J.; Souza, F.M.; Monteiro, E.S.; Biasi, R.S. Mechanical and clinical properties of titanium and titanium-based alloys (Ti g2, Ti g4 cold worked nanostructured and Ti g5) for biomedical applications. J. Mater. Res. Technol. 2019, 8, 1060–1069. [Google Scholar] [CrossRef]
- Elias, C.; Lima, J.; Valiev, R. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Lee, S.H.; Todai, M.; Tane, M.; Hagihara, K.; Nakajima, H.; Nakano, T. Biocompatible low young’s modulus achieved by strong crystallographic elastic anisotropy in Ti–15Mo–5Zr–3Al alloy single crystal. J. Mech. Behav. Biomed. Mater. 2012, 14, 48–54. [Google Scholar] [CrossRef]
- Bordji, K.; Jouzeau, J.Y.; Mainard, D.; Payan, E.; Netter, P.; Rie, K.T.; Stucky, T.; Hage-Ali, M. Cytocompatibility of Ti-6Al-4V and Ti-5Al-2.5Fe alloys according to three surface treatments, using human fibroblasts and osteoblasts. Biomaterials 1996, 17, 929–940. [Google Scholar] [CrossRef]
- Bolzoni, L.; Raynova, S.; Yang, F. Strengthening mechanisms of Ti via Al addition. J. Alloys Compd. 2020, 820, 153447. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A 2002, 33, 477. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, L.; Liu, R.; Yang, K.; Zhang, Y.; Liao, Z.; Liu, W.; Qi, M.; Misra, R.D.K. Effect of heat treatment on cu distribution, antibacterial performance and cytotoxicity of Ti–6Al–4V–5Cu alloy. J. Mater. Sci. Technol. 2015, 31, 723–732. [Google Scholar] [CrossRef]
- Jin, X.; Gao, L.; Liu, E.; Yu, F.; Shu, X.; Wang, H. Microstructure, corrosion and tribological and antibacterial properties of Ti-Cu coated stainless steel. J. Mech. Behav. Biomed. Mater. 2015, 50, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojcieszak, D.; Kaczmarek, D.; Antosiak, A.; Mazur, M.; Rybak, Z.; Rusak, A.; Osekowska, M.; Poniedzialek, A.; Gamian, A.; Szponar, B. Influence of Cu-Ti thin film surface properties on antimicrobial activity and viability of living cells. Mater. Sci. Eng. C 2015, 56, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-H.; Chen, K.-K.; Chao, C.-Y.; Chang, Y.-H.; Du, J.-K. Effect of Ti2Cu precipitation on antibacterial property of Ti-5Cu alloy. Mater. Sci. Eng. C 2020, 108, 110433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Wang, X.; Chen, M.; Hou, B. Effect of the existing form of cu element on the mechanical properties, bio-corrosion and antibacterial properties of Ti-Cu alloys for biomedical application. Mater. Sci. Eng. C 2016, 69, 1210–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, F.; Liu, C.; Wang, H.; Ren, B.; Yang, K.; Zhang, E. Effect of cu content on the antibacterial activity of titanium-copper sintered alloys. Mater. Sci. Eng. C 2014, 35, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, R.; Ullah, I.; Zhang, S.; Sun, Z.; Ren, L.; Yang, K. Rough surface of copper-bearing titanium alloy with multifunctions of osteogenic ability and antibacterial activity. J. Mater. Sci. Technol. 2020, 48, 130–139. [Google Scholar] [CrossRef]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium-copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Souza, S.A.; Afonso, C.R.M.; Ferrandini, P.L.; Coelho, A.A.; Caram, R. Effect of cooling rate on Ti–Cu eutectoid alloy microstructure. Mater. Sci. Eng. C 2009, 29, 1023–1028. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, J.F.; Zhao, Y.; Sun, J. Effect of semi-solid forging temperature on microstructure and mechanical properties of Ti14 alloy. J. Alloys Compd. 2009, 487, 314–320. [Google Scholar] [CrossRef]
- Yao, X.; Sun, Q.Y.; Xiao, L.; Sun, J. Effect of Ti2Cu precipitates on mechanical behavior of Ti–2.5cu alloy subjected to different heat treatments. J. Alloys Compd. 2009, 484, 196–202. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Yu, Z.T.; Zhu, R.H.; Gu, H.C. Mechanical behavior and deformation mechanisms of Ti–2.5cu alloy reinforced by nano-scale precipitates at 293 and 77 k. Mater. Sci. Eng. A 2004, 364, 159–165. [Google Scholar] [CrossRef]
- Gerdemann, S.J. Titanium process technologies. Adv. Mater. Processes 2001, 159, 41–43. [Google Scholar]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the clinical implications of anti-infective biomaterials and infection-resistant surfaces. Biomaterials 2013, 34, 8018–8029. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Hirsch, J.; Lekholm, U.; Thomsen, P. Differential diagnosis and treatment strategies for biologic complications and failing oral implants: A review of the literature. Int. J. Oral Maxillofac. Implants 1999, 14, 473–490. [Google Scholar]
- Ren, X.; van der Mei, H.C.; Ren, Y.; Busscher, H.J. Keratinocytes protect soft-tissue integration of dental implant materials against bacterial challenges in a 3D-tissue infection model. Acta Biomater. 2019, 96, 237–246. [Google Scholar] [CrossRef]
- Heublein, B.; Rohde, R.; Kaese, V.; Niemeyer, M.; Hartung, W.; Haverich, A. Biocorrosion of magnesium alloys: A new principle in cardiovascular implant technology? Heart 2003, 89, 651. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.F.; Cremasco, A.; Contieri, R.J.; Lopes, E.S.N.; Afonso, C.R.M.; Caram, R. Hexagonal martensite decomposition and phase precipitation in Ti–Cu alloys. Mater. Des. 2011, 32, 4608–4613. [Google Scholar] [CrossRef]
- Kikuchi, M.; Takada, Y.; Kiyosue, S.; Yoda, M.; Woldu, M.; Cai, Z.; Okuno, O.; Okabe, T. Mechanical properties and microstructures of cast Ti-Cu alloys. Dent. Mater. 2003, 19, 174–181. [Google Scholar] [CrossRef]
- Ren, L.; Ma, Z.; Li, M.; Zhang, Y.; Liu, W.; Liao, Z.; Yang, K. Antibacterial properties of Ti–6Al–4V–xCu alloys. J. Mater. Sci. Technol. 2014, 30, 699–705. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; ul Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Environ. Sci. Technol. 1989, 18, 295–315. [Google Scholar] [CrossRef]





| Heat Treatment | Phase | Ti | Al * (Mean ± SD) | Cu * (Mean ± SD) |
|---|---|---|---|---|
| 720 °C, 24 h | Matrix | Bal. | 5.13 ± 0.23 | 2.29 ± 0.13 |
| Precipitate-1 (Ti2Cu phase) | Bal. | 3.99 ± 0.20 | 34.12 ± 0.27 | |
| 920 °C, 24 h | Matrix | Bal. | 5.42 ± 0.14 | 2.16 ± 0.19 |
| Precipitate-2 (Ti2Cu phase) | Bal. | 4.32 ± 0.17 | 23.33 ± 0.21 | |
| 1040 °C, 24 h | Matrix | Bal. | 5.35 ± 0.19 | 2.04 ± 0.15 |
| Precipitate-3 (Cu-rich phase) | Bal. | 4.87 ± 0.22 | 7.23 ± 0.17 |
| Specimens | Phase (%) | Experimental Antibacterial Rate | Inferential Antibacterial Rate | |
|---|---|---|---|---|
| α-Ti | Ti2Cu + Cu-rich | |||
| As-cast | Bal. | 6.7 | 55.1 | 52.7 |
| 720 °C, 24 h | Bal. | 7.2 | 60.5 | 54.5 |
| 760 °C, 24 h | Bal. | 14.5 | 83.6 | 82.0 |
| 840 °C, 24 h | Bal. | 11.9 | 70.2 | 72.2 |
| 880 °C, 24 h | Bal. | 13.4 | 75.2 | 77.9 |
| 920 °C, 24 h | Bal. | 4.9 | 50.5 | 45.9 |
| 1040 °C, 24 h | Bal. | 4.5 | 34.5 | 44.4 |
| Inferential antibacterial rate (IAR) was calculated using the linear regression equation: IAR = BAR + (Dp × Fp × 100%) | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Chao, C.-Y.; Chang, Y.-T.; Du, J.-K. Effect of Phase Distribution on the Antibacterial Property and Cytotoxicity of Ti-5Al-2.5Cu Alloy after Heat Treatment at Various Temperatures. Metals 2020, 10, 858. https://doi.org/10.3390/met10070858
Chang Y-H, Chao C-Y, Chang Y-T, Du J-K. Effect of Phase Distribution on the Antibacterial Property and Cytotoxicity of Ti-5Al-2.5Cu Alloy after Heat Treatment at Various Temperatures. Metals. 2020; 10(7):858. https://doi.org/10.3390/met10070858
Chicago/Turabian StyleChang, Yen-Hao, Chih-Yeh Chao, Yuan-Ting Chang, and Je-Kang Du. 2020. "Effect of Phase Distribution on the Antibacterial Property and Cytotoxicity of Ti-5Al-2.5Cu Alloy after Heat Treatment at Various Temperatures" Metals 10, no. 7: 858. https://doi.org/10.3390/met10070858




