Intermetallic/Ceramic Composites Synthesized from Al–Ni–Ti Combustion with B4C Addition
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
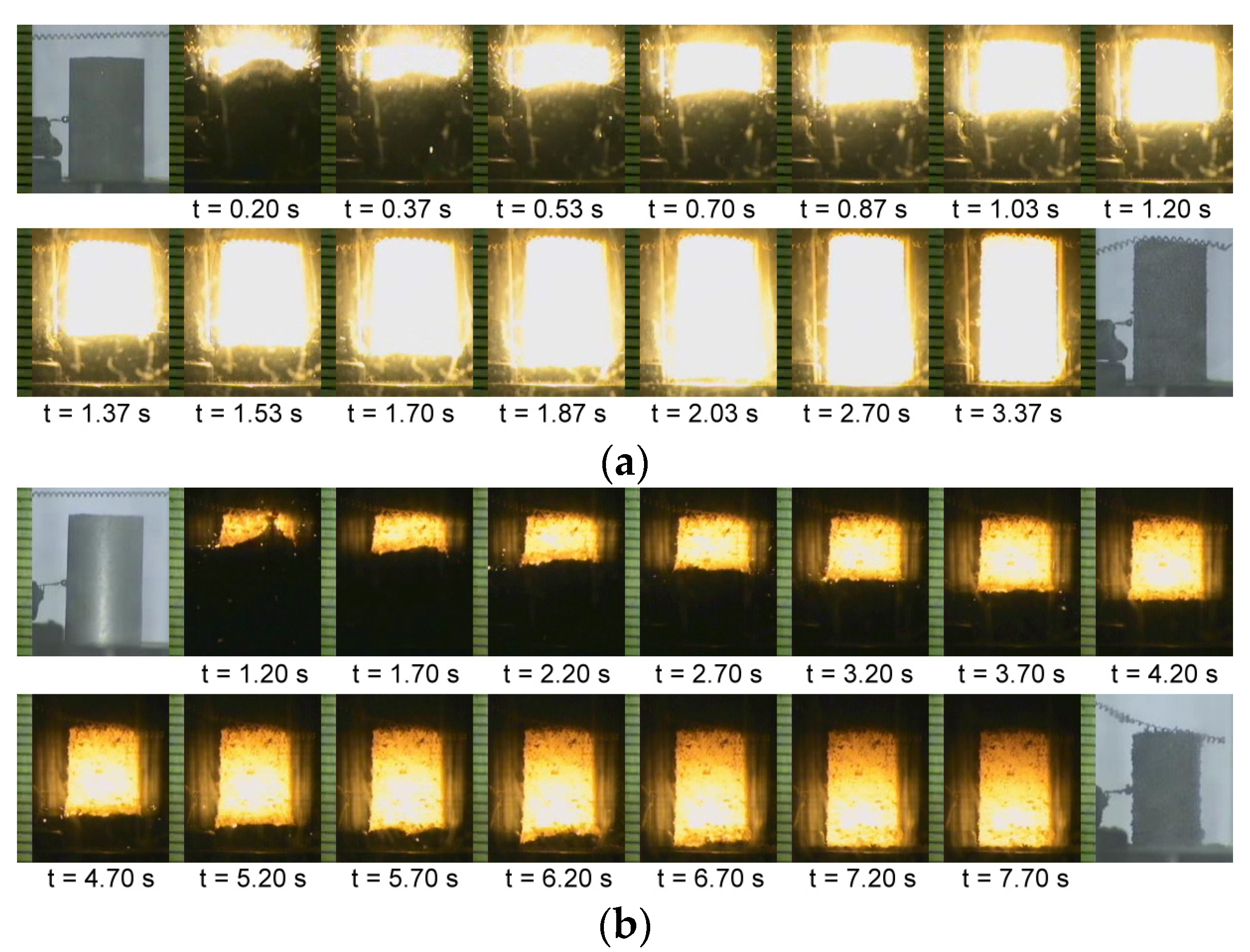
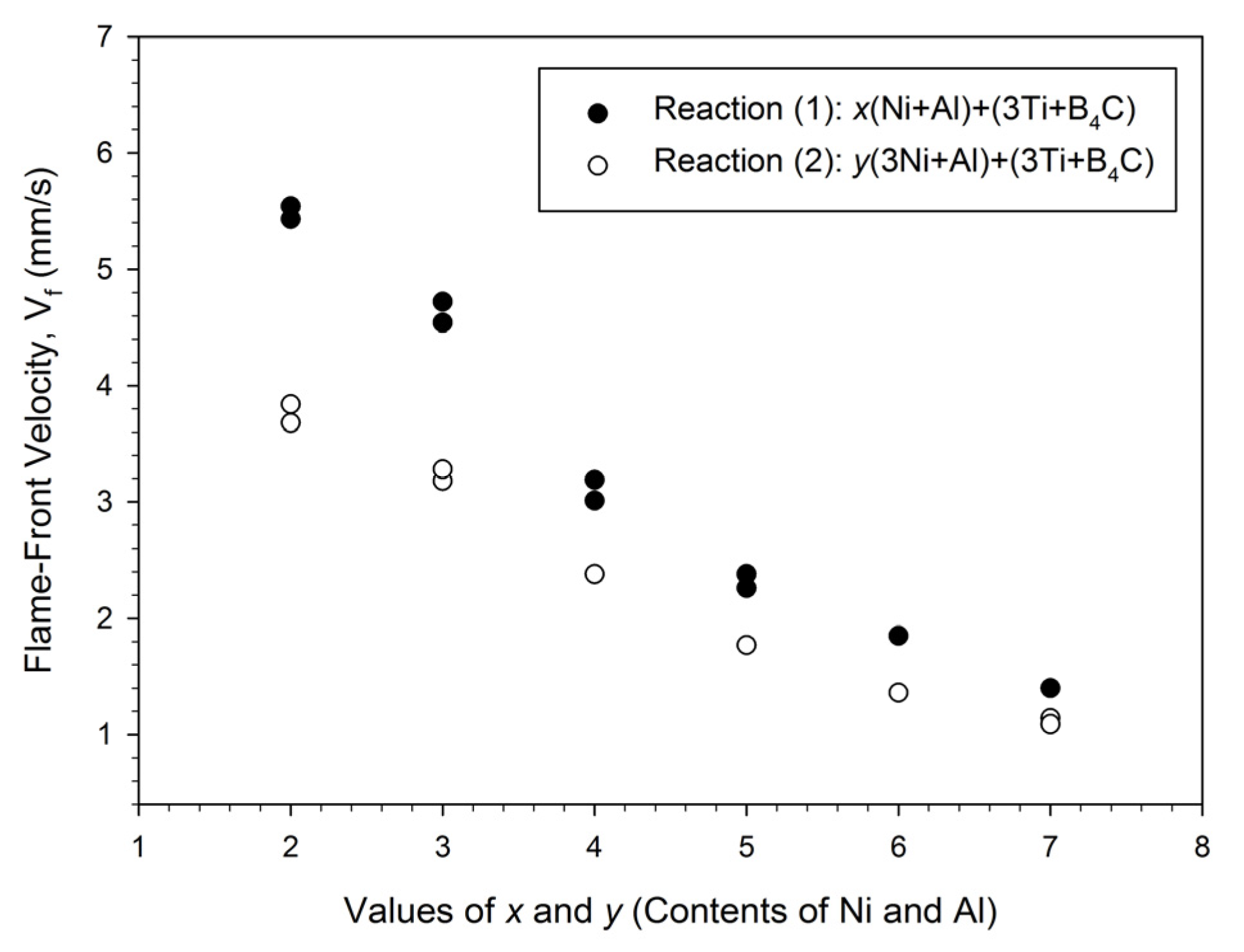
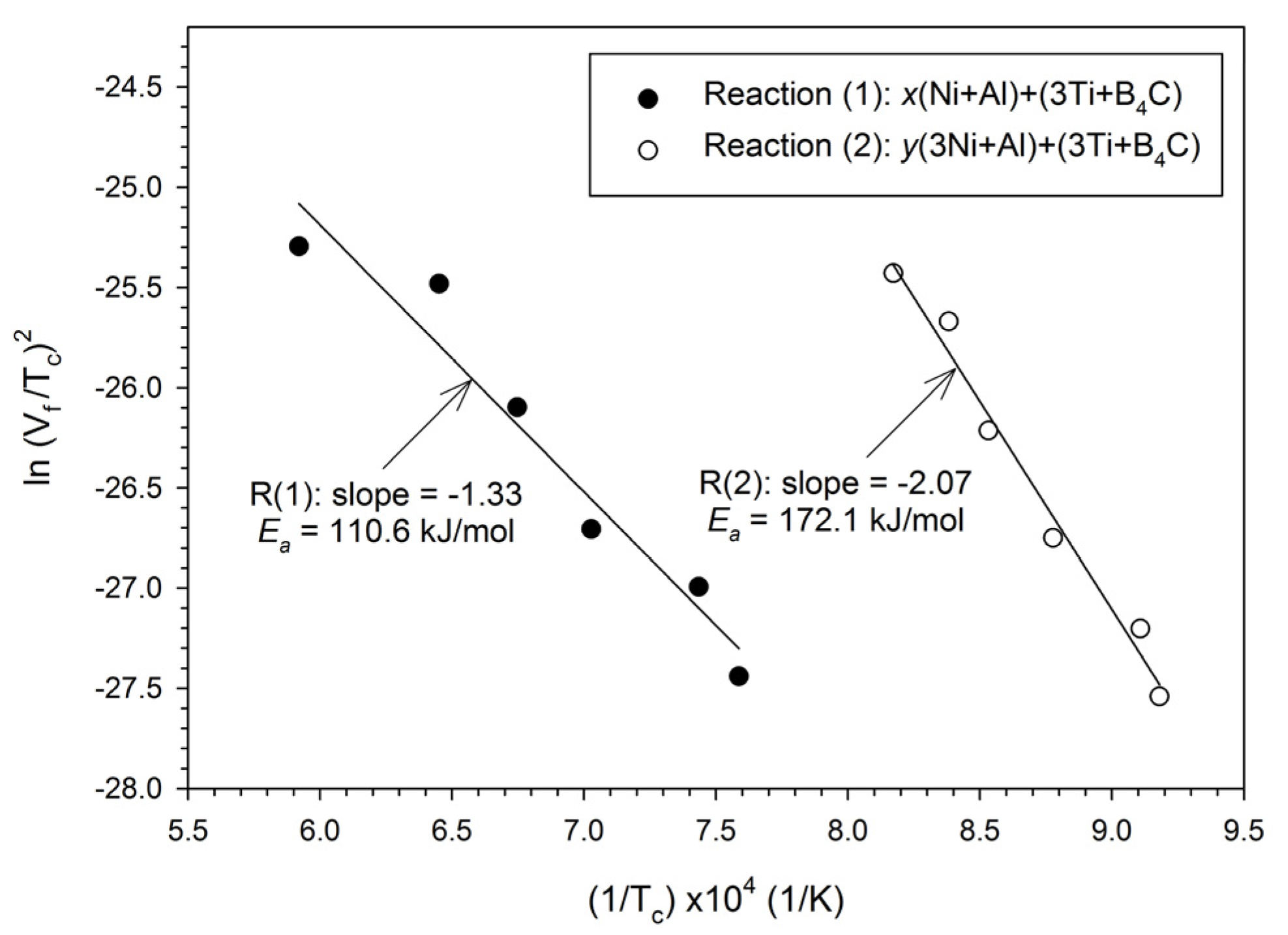
3.1. Self-Propagating Combustion Characteristics and Kinetics
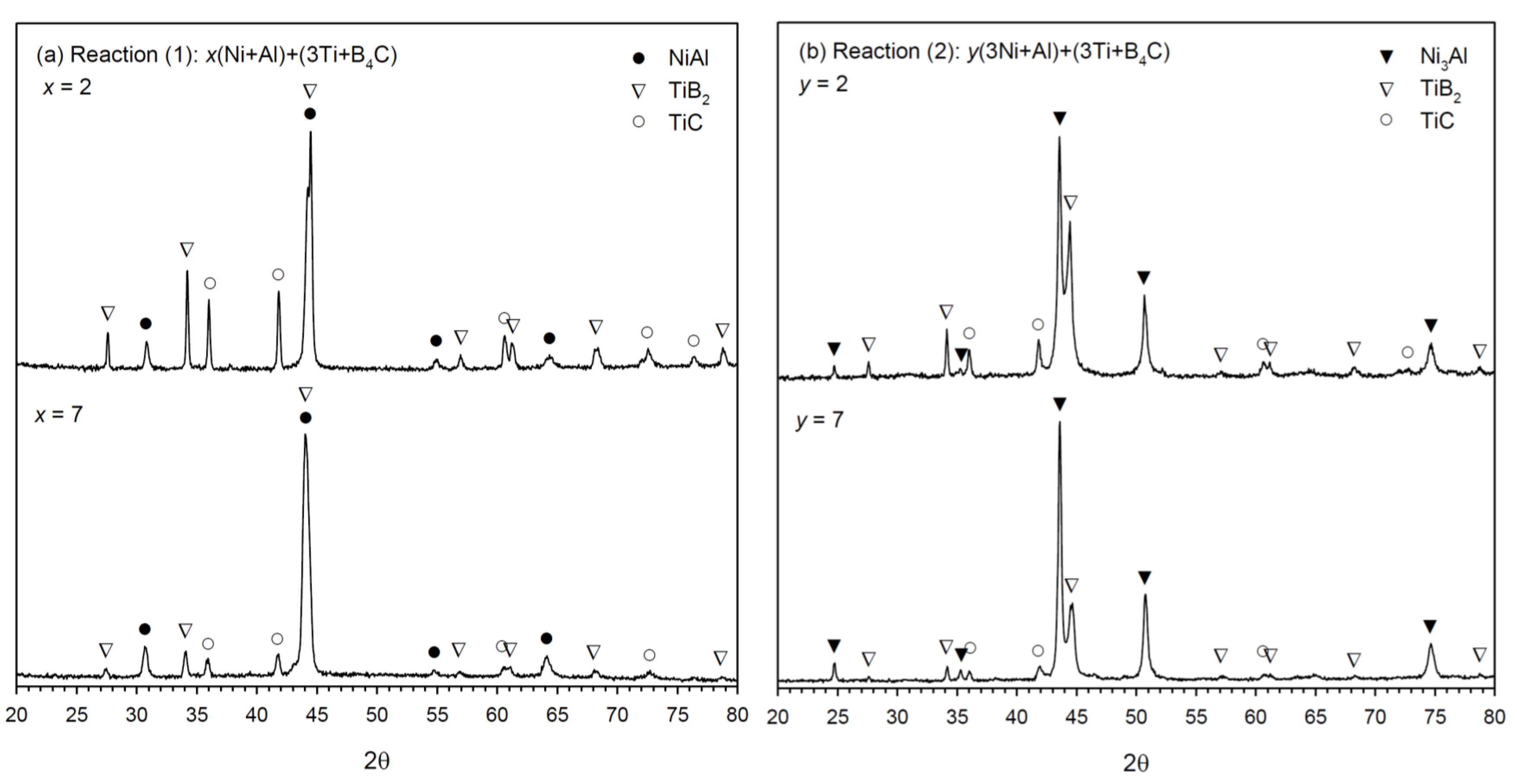
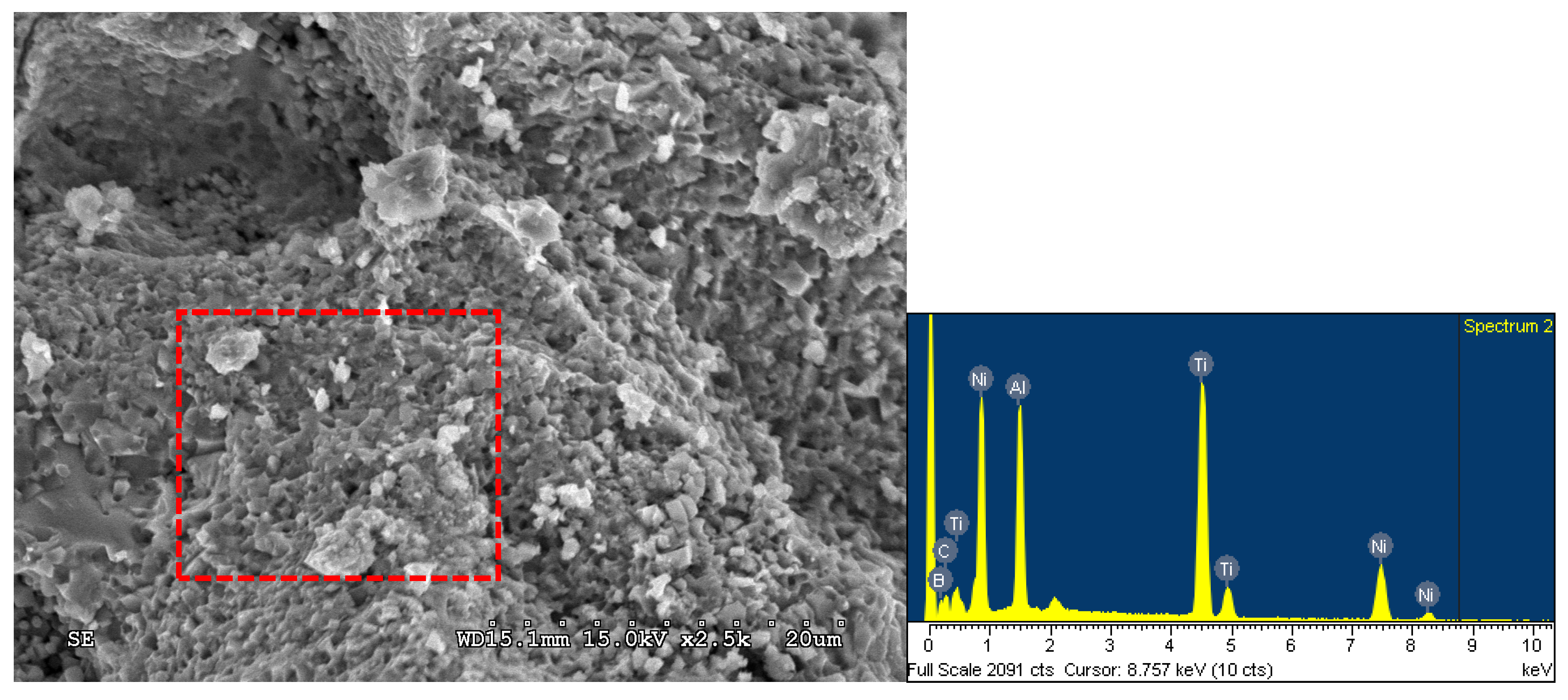
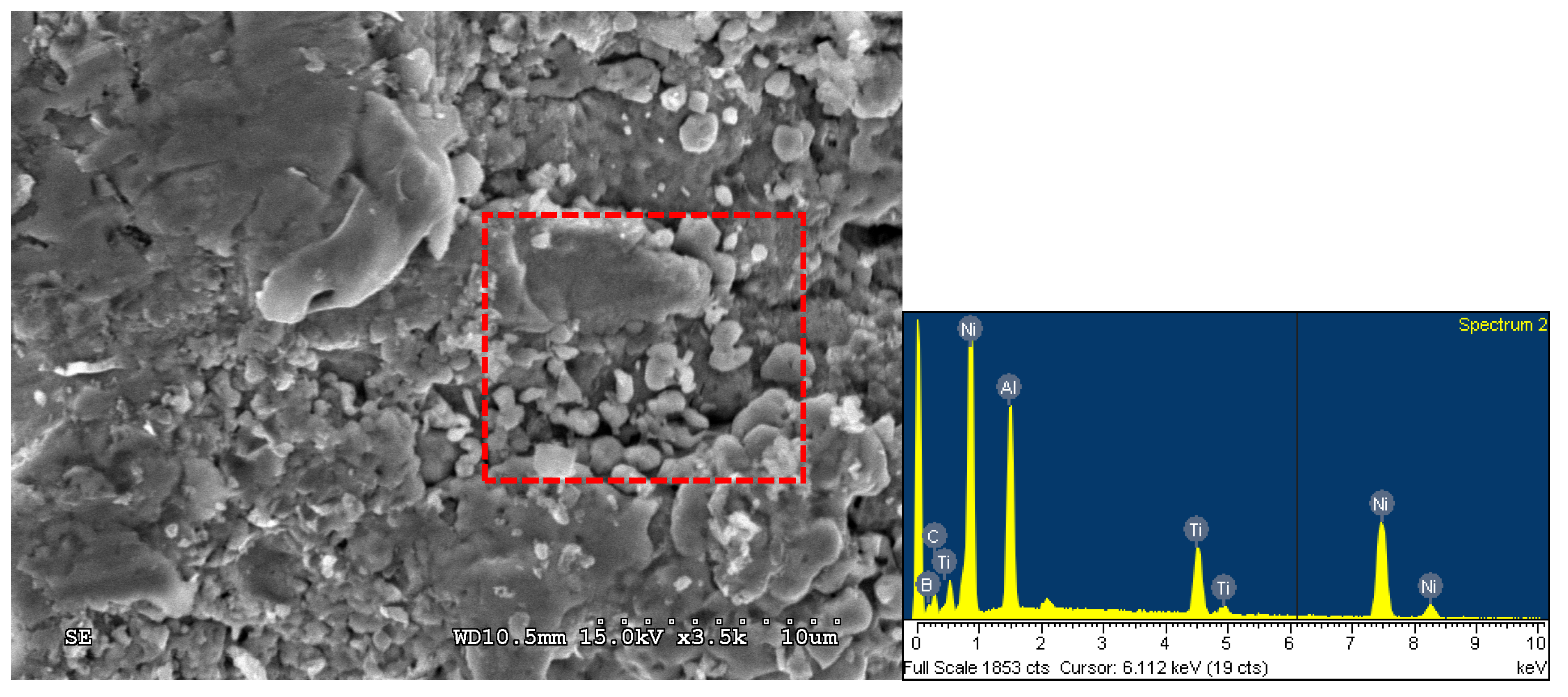
3.2. Phase Constituents and Properties of Synthesized Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bochenek, K.; Basista, M. Advances in processing of NiAl intermetallic alloys and composites for high temperature aerospace applications. Prog. Aerosp. Sci. 2015, 79, 136–146. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Inui, H.; Ito, K. High-temperature structural intermetallics. Acta Mater. 2000, 48, 307–322. [Google Scholar] [CrossRef]
- Cinca, N.; Lima, C.R.C.; Guilemany, J.M. An overview of intermetallics research and application: Status of thermal spray coatings. J. Mater. Res. Technol. 2013, 2, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.; Santos, O.S.; Alves, W.; Lima, M.S.F.; Silva, M.M.D. Fiber laser surface melting of a NiTi superelastic alloy: Influence on structural and mechanical properties. Metals 2019, 9, 1268. [Google Scholar] [CrossRef] [Green Version]
- Morsi, K. Review: Reaction synthesis processing of Ni–Al intermetallic materials. Mater. Sci. Eng. A 2001, 299, 1–15. [Google Scholar] [CrossRef]
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Wang, S.; Xi, Y.; Li, H.; Bowen, P. Fractographic study on naturally initiated short fatigue cracks in a near-lamellar TiAl alloy at room temperature. Metals 2019, 9, 1101. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.; Wang, F.; Ai, T.T.; Zhu, J.F. Microstructure and mechanical properties of in situ TiAl/Ti2AlC composites prepared by reactive hot pressing. Ceram. Int. 2014, 40, 8165–8171. [Google Scholar] [CrossRef]
- Bhaumik, S.K.; Divakar, C.; Rangaraj, L.; Singh, A.K. Reaction sintering of NiAl and TiB2–NiAl composites under pressure. Mater. Sci. Eng. A 1998, 257, 341–348. [Google Scholar] [CrossRef]
- Anvari, S.Z.; Karimzadeh, F.; Enayati, M.H. Synthesis and characterization of NiAl–Al2O3 nanocomposite powder by mechanical alloying. J. Alloys Compd. 2009, 477, 178–181. [Google Scholar] [CrossRef]
- Yeh, C.L.; Ke, C.Y. Combustion synthesis of FeAl-based composites from thermitic and intermetallic reactions. Crystals 2019, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Merzhanov, A.G. Combustion and explosion processes in physical chemistry and technology of inorganic materials. Russ. Chem. Rev. 2003, 72, 289–310. [Google Scholar] [CrossRef]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2017, 62, 203–239. [Google Scholar] [CrossRef]
- Çamurlu, H.E.; Maglia, F. Self-propagating high-temperature synthesis of ZrB2 or TiB2 reinforced Ni–Al composite powder. J. Alloys Compd. 2009, 478, 721–725. [Google Scholar] [CrossRef]
- Riyadi, T.W.B.; Zhang, T.; Marchant, D.; Zhu, X. NiAl–TiC–Al2O3 composites formed by self-propagation high-temperature synthesis process: Combustion behavior, microstructure, and properties. J. Alloys Compd. 2019, 805, 104–112. [Google Scholar] [CrossRef]
- Yeh, C.L.; Su, S.H. In situ formation of TiAl-TiB2 composite by SHS. J. Alloys Compd. 2006, 407, 150–156. [Google Scholar] [CrossRef]
- Yeh, C.L.; Shen, Y.G. Formation of TiAl-Ti2AlC in situ composites by combustion synthesis. Intermetallics 2009, 17, 169–173. [Google Scholar] [CrossRef]
- Yeh, C.L.; Li, R.F. Formation of TiAl-Ti5Si3 and TiAl-Al2O3 in situ composites by combustion synthesis. Intermetallics 2008, 16, 64–70. [Google Scholar] [CrossRef]
- Kopit, Y. The ability of systems based on Ni, Al and Ti to be synthesized by self-propagating high-temperature synthesis (SHS). Intermetallics 2001, 9, 387–393. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, C.; Qu, W.; He, X.; Kvanin, V.L. Self-propagating high temperature combustion synthesis of TiC/TiB2 ceramic-matrix composites. Compos. Sci. Technol. 2002, 62, 2037–2041. [Google Scholar]
- Yeh, C.L.; Chen, Y.L. Combustion synthesis of TiC-TiB2 composites. J. Alloys Compd. 2008, 463, 373–377. [Google Scholar] [CrossRef]
- Merzhanov, A.G. Solid flames: Discoveries, concepts, and horizons of cognition. Combust. Sci. Technol. 1994, 98, 307–336. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF Themochemical Tables, 4th ed.; American Institute of Physics: Woodbury, NY, USA, 1998; pp. 276–278, 655–657. [Google Scholar]
- Yeh, C.L.; Chen, Y.C. Formation of Mo5Si3/Mo3Si–MgAl2O4 composites via self-propagating high-temperature synthesis. Molecules 2020, 25, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, W.B.; Kirihara, S.; Miyamoto, Y.; Matsuura, K.; Kudoh, M. Development of freeform fabrication method for Ti–Al–Ni intermetallics. Intermetallics 2002, 10, 879–885. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, Y.L. An experimental study on self-propagating high-temperature synthesis in the Ta-B4C system. J. Alloys Compd. 2009, 478, 163–167. [Google Scholar] [CrossRef]
- Evans, A.G.; Charles, E.A. Fracture toughness determinations by indentation. J. Am. Ceram. Soc. 1976, 59, 371–372. [Google Scholar] [CrossRef]
- Rios, C.T.; Coelho, A.A.; Batista, W.W.; Gonçalves, M.C.; Caram, R. ISE and fracture toughness evaluation by Vickers hardness testing of an Al3Nb-Nb2Al-AlNbNi in situ composite. J. Alloys Compd. 2009, 472, 65–70. [Google Scholar] [CrossRef]
- Varma, A.; Rogachev, A.S.; Mukasyan, A.S.; Hwang, S. Combustion synthesis of advanced materials: Principals and applications. Adv. Chem. Eng. 1998, 24, 79–225. [Google Scholar]
- Yeh, C.L.; Wang, H.J. A comparative study on combustion synthesis of Ta-B compounds. Ceram. Int. 2011, 37, 1569–1573. [Google Scholar] [CrossRef]
- Belin-Ferré, E. Basics of Thermodynamics and Phase Transitions in Complex Intermetallics; World Scientific Publishing Co. Inc.: Singapore, 2008. [Google Scholar]
- Hibino, A.; Matsuoka, S.; Kiuchi, M. Synthesis and sintering of Ni3Al intermetallic compound by combustion synthesis process. J. Mater. Process. Technol. 2001, 112, 127–135. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Wuchina, E.J.; Lee, W.E.; Zhou, Y. Ultra-High Temperature Ceramics: Materials for Extreme Environment Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany; New York, NY, USA, 2014. [Google Scholar]
- Mitra, R. Intermetallic Matrix Composites; Woodhead Publishing: Cambridge, UK, 2017; pp. 1–18. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Ke, C.-Y. Intermetallic/Ceramic Composites Synthesized from Al–Ni–Ti Combustion with B4C Addition. Metals 2020, 10, 873. https://doi.org/10.3390/met10070873
Yeh C-L, Ke C-Y. Intermetallic/Ceramic Composites Synthesized from Al–Ni–Ti Combustion with B4C Addition. Metals. 2020; 10(7):873. https://doi.org/10.3390/met10070873
Chicago/Turabian StyleYeh, Chun-Liang, and Chih-Yao Ke. 2020. "Intermetallic/Ceramic Composites Synthesized from Al–Ni–Ti Combustion with B4C Addition" Metals 10, no. 7: 873. https://doi.org/10.3390/met10070873




