Hydrogen Accumulation and Distribution in Titanium Coatings at Gas-Phase Hydrogenation
Abstract
:1. Introduction
2. Materials and Research Methods
3. Results and Discussion
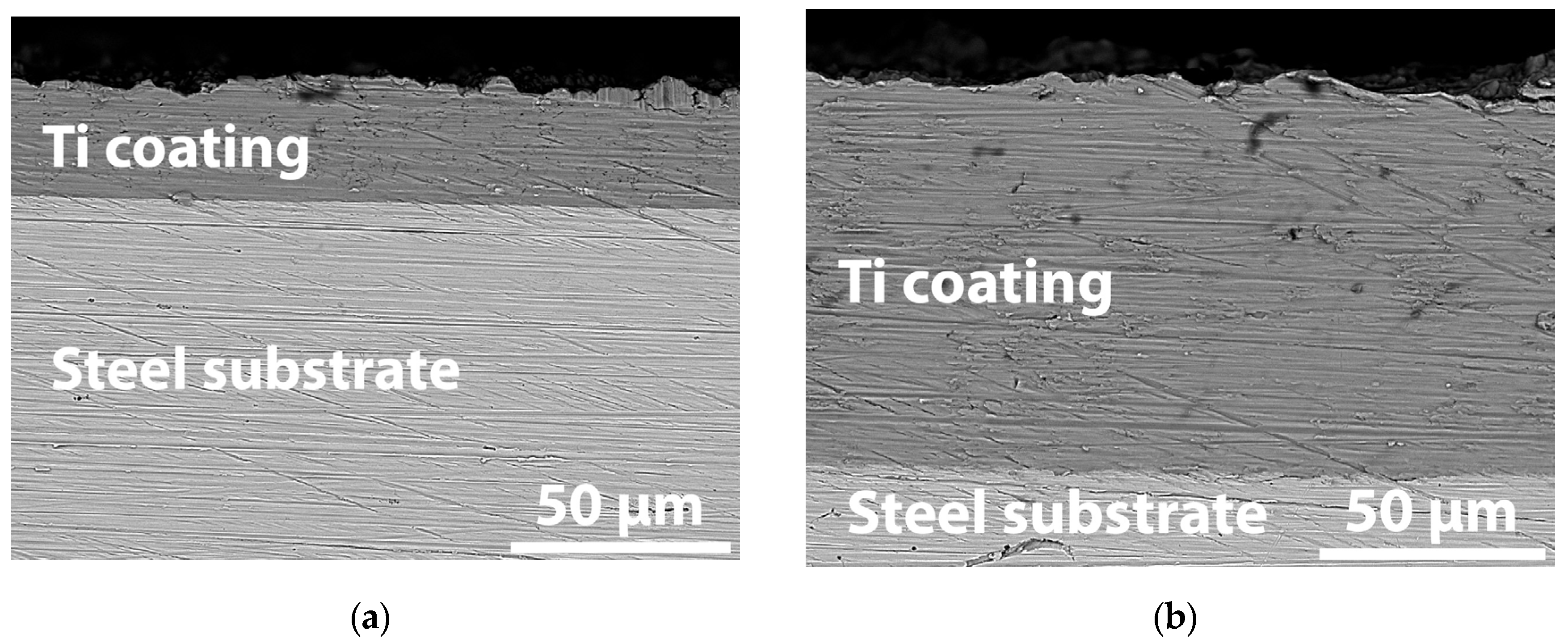
3.1. Coating Microstructure
3.2. Hydrogenation of Titanium Coatings at the Gas Reaction Controller Automated System
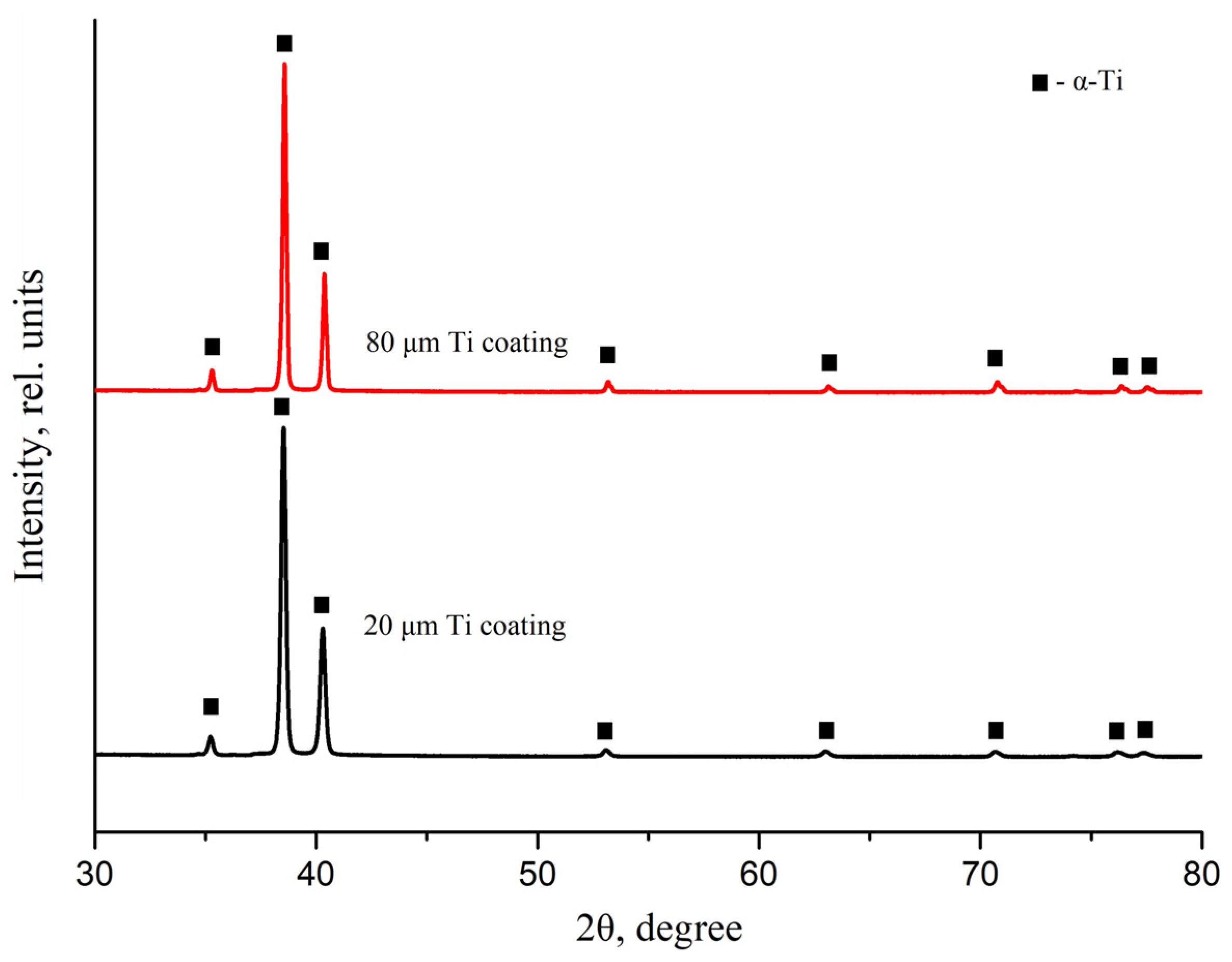
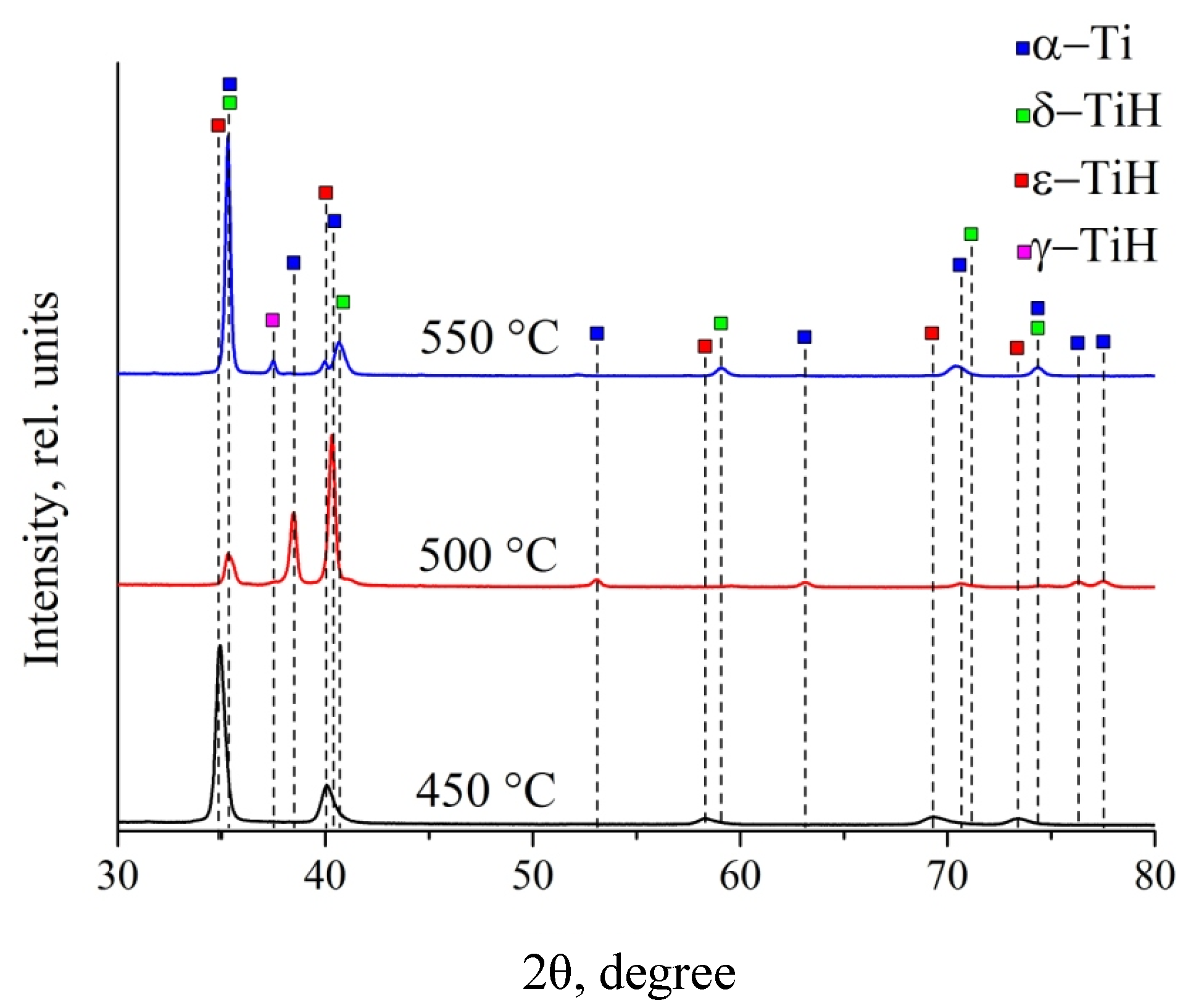
3.3. X-Ray Diffraction of Titanium Coatings
3.4. Determination of Hydrogen Content in Coatings by Melting in an Inert Gas Medium Using a RHEN 602 Hydrogen Analyzer
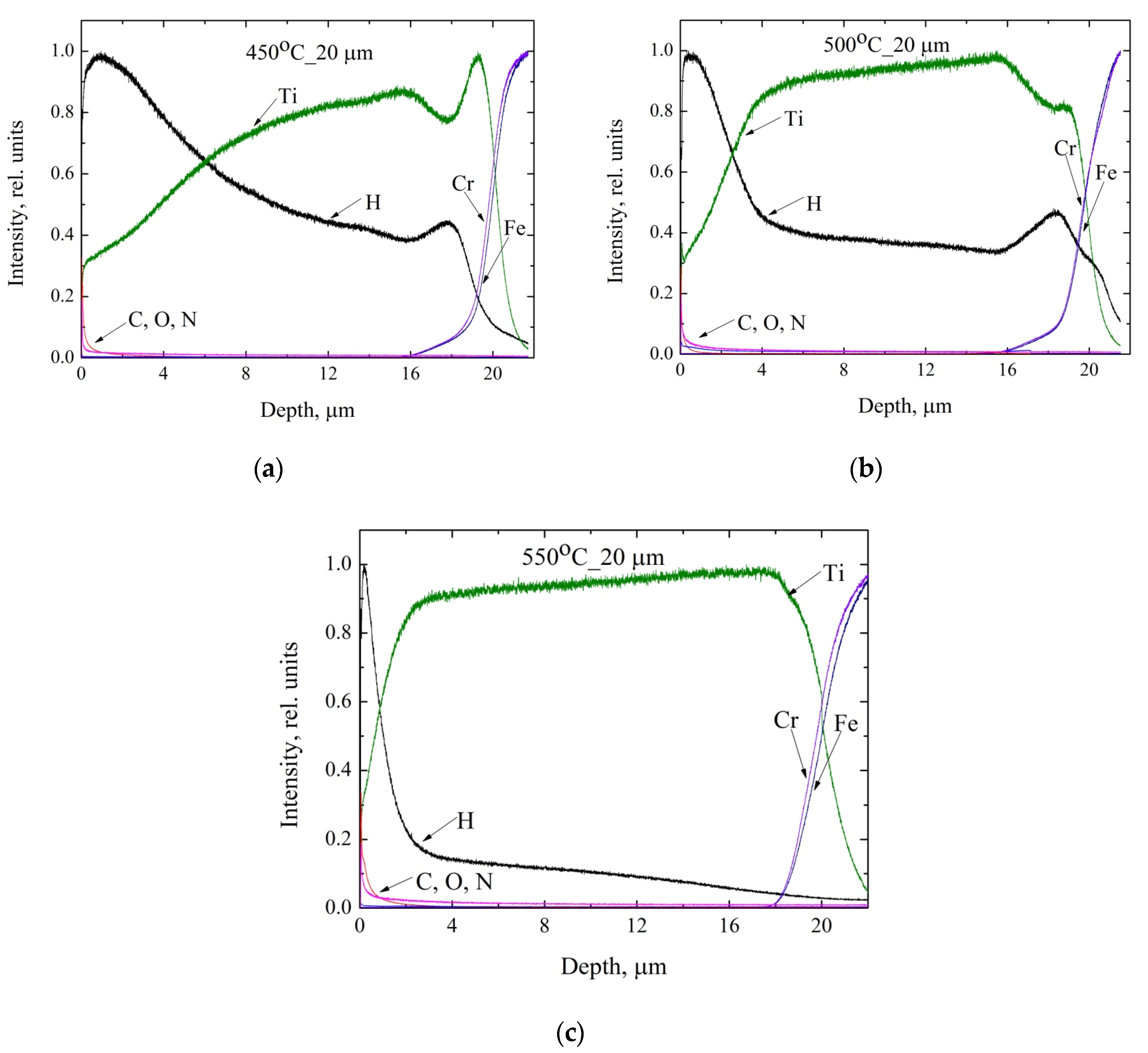
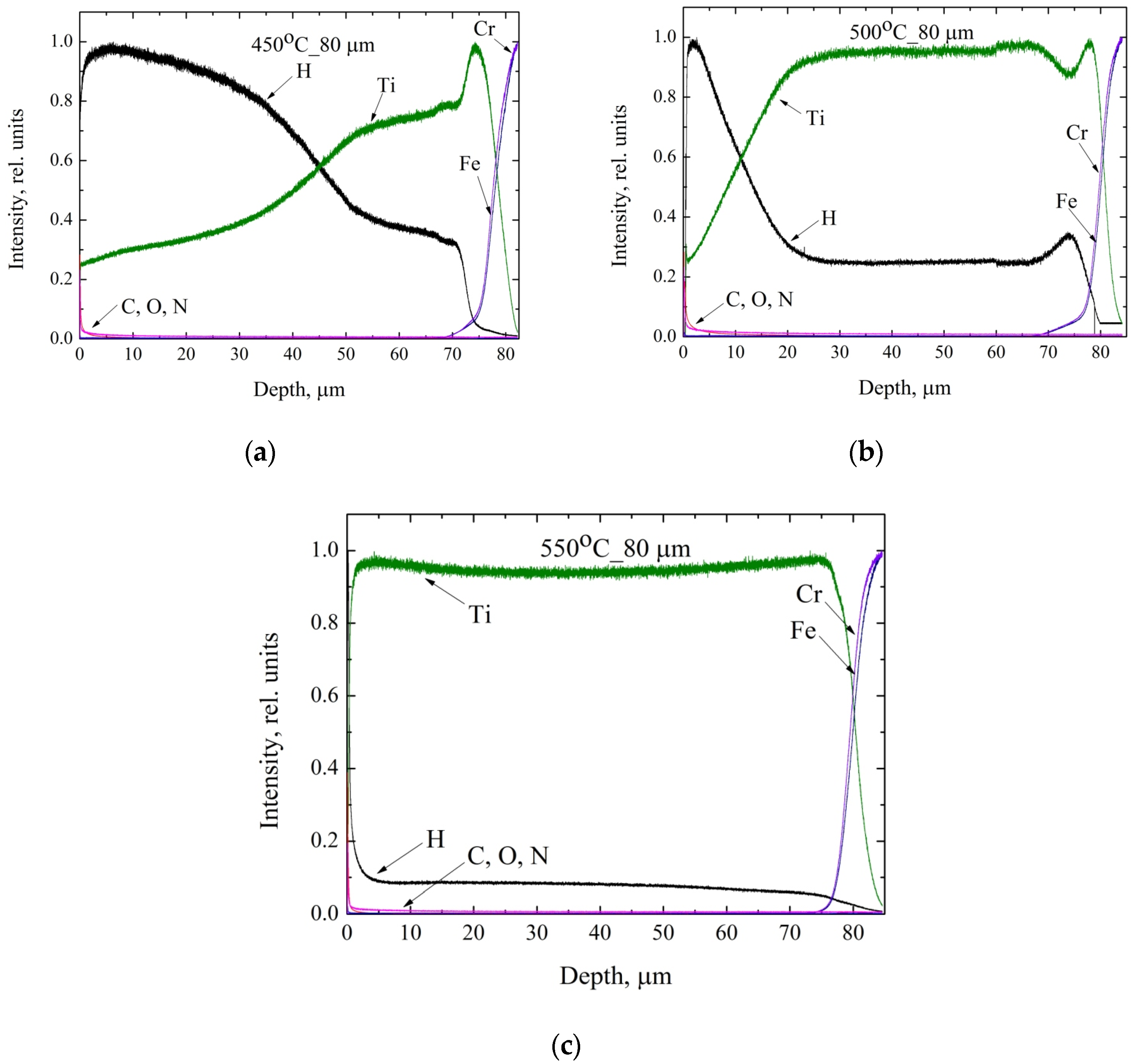
3.5. Glow-Discharge Optical Emission Spectroscopy Study of the Distribution of Hydrogen in Titanium Coating
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, J.; Jing, X.; Hu, B. The research status and development of hydrogen storage materials. Mater. Rev. 2007, 21, 118–120. [Google Scholar]
- Karpov, D. Film metal-hydride hydrogen accumulators: Potentials, production methods, prospects for application. In Proceedings of the International Conference on Innovative Applied Energy, Oxford, UK, 14–15 March 2019. Article 257 (ID:738), 38. [Google Scholar]
- Liu, W.; Aguey-Zinsou, K.F. Hydrogen storage properties of in-situ stabilised magnesium nanoparticles generated by electroless reduction with alkali metals. Int. J. Hydrog. Energy 2015, 40, 16948–16960. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Kumar, V.; Singh, S.K. High capacity hydrogen storage: Basic aspects, new developments and milestones. Nano Energy 2012, 1, 566–589. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. Mater. Int. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- Bliznakov, S.; Drenchev, N.; Drenchev, B.; Delchev, P.; Solsona, P.; Spassov, T. Electrochemical properties of nanocrystalline Mg2Ni-type alloys prepared by mechanical alloying. J. Alloys Compd. 2005, 404–406, 682–686. [Google Scholar] [CrossRef]
- Anik, M. Improvement of the electrochemical hydrogen storage performance of magnesium based alloys by various additive elements. Int. J. Hydrog. Energy 2012, 37, 1905–1911. [Google Scholar] [CrossRef]
- Ju, J.; Fu, H.; Lei, Y. Effect of Al addition on microstructure and properties of an Fe-B-Al alloy. Mater. Test. 2016, 58, 753–762. [Google Scholar] [CrossRef]
- Luo, Q.; An, X.H.; Pan, Y.B.; Zhang, X.; Zhang, J.Y.; Li, Q. The hydriding kinetics of Mg-Ni based hydrogen storage alloys: A comparative study on Chou model and Jander model. Int. J. Hydrog. Energy 2010, 35, 7842–7849. [Google Scholar] [CrossRef]
- Goo, N.H.; Lee, K.S. The electrochemical hydriding properties of Mg-Ni-Zr amorphous alloy. Int. J. Hydrog. Energy 2002, 27, 433–438. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, B.W.; Ma, Z.H.; Guo, S.H.; Qi, Y.; Wang, X.L. Improved hydrogen storage behaviours of nanocrystalline and amorphous Mg2Ni-type alloy by Mn substitution for Ni. Int. J. Hydrog. Energy 2010, 35, 11966–11974. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Ding, X.; Liu, D.; Zhang, Q.; Si, T. Microstructure characterization and hydrogen storage properties study of Mg2Ni0.92M0.08 (M = Ti, V, Fe or Si) alloys. Prog. Nat. Sci. Mater. Int. 2018, 28, 464–469. [Google Scholar] [CrossRef]
- Sun, H.; Feng, D.; Zhang, Y.; Ren, H. Gas hydrogen absorption and electrochemical properties of Mg 24 Ni 10 Cu 2 alloys improved by Y substitution, ball milling and Ni addition. Int. J. Hydrog. Energy 2019, 44, 5382–5388. [Google Scholar] [CrossRef]
- Grigorova, E.; Khristov, M.; Khrussanova, M.; Bobet, J.L.; Peshev, P. Effect of additives on the hydrogen sorption properties of mechanically alloyed composites based on Mg and Mg2Ni. Int. J. Hydrog. Energy 2005, 30, 1099–1105. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Besenbacher, F.; Jensen, T.R. Nanoconfined hydrides for energy storage. Nanoscale 2011, 3, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Korablov, D.; Besenbacher, F.; Jensen, T.R. Kinetics and thermodynamics of hydrogenation-dehydrogenation for Mg-25%TM (TM = Ti, Nb or V) composites synthesized by reactive ball milling in hydrogen. Int. J. Hydrog. Energy 2018, 43, 16804–16814. [Google Scholar] [CrossRef]
- Tarasov, B.P.; Arbuzov, A.A.; Mozhzhuhin, S.A.; Volodin, A.A.; Fursikov, P.V.; Lototskyy, M.V.; Yartys, V.A. Hydrogen storage behavior of magnesium catalyzed by nickel-graphene nanocomposites. Int. J. Hydrog. Energy 2019, 44, 29212–29223. [Google Scholar] [CrossRef]
- Lyu, J.; Lider, A.; Kudiiarov, V. Using ball milling for modification of the hydrogenation/dehydrogenation process in magnesium-based hydrogen storage materials: An overview. Metals 2019, 9, 768. [Google Scholar] [CrossRef] [Green Version]
- Cortez, J.J.; Castro, F.J.; Troiani, H.E.; Pighin, S.A.; Urretavizcaya, G. Kinetic improvement of H2 absorption and desorption properties in Mg/MgH2 by using niobium ethoxide as additive. Int. J. Hydrog. Energy 2019, 44, 11961–11969. [Google Scholar] [CrossRef]
- Crivello, J.C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Aguey-Zinsou, K.F.; Ares-Fernández, J.R. Hydrogen in magnesium: New perspectives toward functional stores. Energy Environ. Sci. 2010, 3, 526–543. [Google Scholar] [CrossRef]
- Suárez-Alcántara, K.; Palacios-Lazcano, A.F.; Funatsu, T.; Cabañas-Moreno, J.G. Hydriding and dehydriding in air-exposed MgFe powder mixtures. Int. J. Hydrog. Energy 2016, 41, 23380–23387. [Google Scholar] [CrossRef]
- Chen, X.; Zou, J.; Zeng, X.; Ding, W. Hydrogen storage properties of a Mg-La-Fe-H nano-composite prepared through reactive ball milling. J. Alloys Compd. 2017, 701, 208–214. [Google Scholar] [CrossRef]
- Shao, H.; Xin, G.; Zheng, J.; Li, X.; Akiba, E. Nanotechnology in Mg-based materials for hydrogen storage. Nano Energy 2012, 1, 590–601. [Google Scholar] [CrossRef]
- Lotoskyy, M.; Denys, R.; Yartys, V.A.; Eriksen, J.; Goh, J.; Nyamsi, S.N.; Sita, C.; Cummings, F. An outstanding effect of graphite in nano-MgH2-TiH2 on hydrogen storage performance. J. Mater. Chem. A 2018, 6, 10740–10754. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, J.Y. Structural relaxation and hydrogen solubility in an amorphous Pd80Si20 alloy. J. Appl. Phys. 1988, 63, 4758–4760. [Google Scholar] [CrossRef]
- Choo, W.Y.; Lee, J.Y. Hydrogen trapping phenomena in carbon steel. J. Mater. Sci. 1982, 17, 1930–1938. [Google Scholar] [CrossRef]
- Izumi, T.; Itoh, G. Thermal desorption spectroscopy study on the hydrogen trapping states in a pure aluminum. Mater. Trans. 2011, 52, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Pressouyre, G.M.; Bernstein, I.M. Example of the Effect of Hydrogen Trapping on Hydrogen Embrittlement. Metall. Trans. A Phys. Metall. Mater. Sci. 1981, 12, 835–844. [Google Scholar] [CrossRef]
- Tal-Gutelmacher, E.; Eliezer, D.; Abramov, E. Thermal desorption spectroscopy (TDS)-Application in quantitative study of hydrogen evolution and trapping in crystalline and non-crystalline materials. Mater. Sci. Eng. A 2007, 445–446, 625–631. [Google Scholar] [CrossRef]
- Karpov, D.A.; Litunovsky, V.N. A Battery for Storing Hydrogen in a Bound State and a Cartridge for the Battery. Patent RU 2606301, 1 October 2017. bul. #1. [Google Scholar]
- Karpov, D.A.; Litunovsky, V.N. Film hydrogen accumulator: Production method and application prospects. In Proceedings of the 13th International Conference “Films and Coatings—2017”, St. Petersburg, Russia, 18–20 April 2017; St. Petersburg, Polytechnic University Press: St. Petersburg, Russia, 2017; pp. 507–510. (In Russian). [Google Scholar]
- Léon, A.; Knystautas, E.J.; Huot, J.; Schulz, R. Hydrogenation characteristics of air-exposed magnesium films. J. Alloys Compd. 2002, 345, 158–166. [Google Scholar] [CrossRef]
- Ross, D.K. Hydrogen storage: The major technological barrier to the development of hydrogen fuel cell cars. Vacuum 2006, 10, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Kashkarov, E.B.; Nikitenkov, N.N.; Syrtanov, M.S.; Sutygina, A.N.; Shulepov, I.A.; Lider, A.M. Influence of plasma immersion titanium implantation on hydrogenation and mechanical properties of Zr-2.5Nb. Appl. Surf. Sci. 2016, 370, 142–148. [Google Scholar] [CrossRef]
- Kudiiarov, V.N.; Syrtanov, M.S.; Bordulev, Y.S.; Babikhina, M.N.; Lider, A.M.; Gubin, V.E.; Murashkina, T.L. The hydrogen sorption and desorption behavior in spherical powder of pure titanium used for additive manufacturing. Int. J. Hydrog. Energy 2017, 42, 15283–15289. [Google Scholar] [CrossRef]
- Messier, R.; Giri, A.P.; Roy, R.A. Revised structure zone model for thin film physical structure. J. Vac. Sci. Technol. A Vac. Surf. Films 1984, 2, 500–503. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Obrosov, A.; Sutygina, A.N.; Uludintceva, E.; Mitrofanov, A.; Weiß, S. Hydrogen permeation, and mechanical and tribological behavior, of CrNx coatings deposited at various bias voltages on IN718 by direct current reactive sputtering. Coatings 2018, 8, 66. [Google Scholar] [CrossRef] [Green Version]




| Sample | IArc, A | P, Pa | Ubias, V | Distance to the Substrate, cm | Thickness, μm | t, min | Deposition Rate, μm/min |
|---|---|---|---|---|---|---|---|
| Mode 1 | 75 | 0.16 | −50 | 30 | 19 ± 2 | 122 | 0.16 |
| Mode 2 | 100 | 0.16 | - | 15 | 78 ± 4 | 80 | 0.98 |
| Samples | 20 μm Ti Coating | 80 μm Ti Coating | ||||
|---|---|---|---|---|---|---|
| Temperature | 450 °C | 500 °C | 550 °C | 450 °C | 500 °C | 550 °C |
| Sorption rate, wt.%/min | 0.08 | 0.17 | 0.91 | 0.04 | 0.11 | 0.13 |
| Maximum hydrogen content, wt.% | 3.96 | 3.24 | 2.84 | 3.98 | 1.29 | 0.78 |
| Hydrogenation Temperature | Detected Phases | Phase Content, vol.% | Lattice Parameters, Å | Crystallite Size, nm |
|---|---|---|---|---|
| 450 °C | TiH_FCT | 100 | a = 3.190, c = 4.387 | 19 |
| 500 °C | Ti_HCP | 90.3 | a = 4.396 | 43 |
| TiH_FCC | 9.7 | a = 2.951, c = 4.700 | 31 | |
| 550 °C | TiH_FCC | 89.6 | - | - |
| TiH_FCT | 5.0 | - | - | |
| Ti_HCP | 5.4 | - | - |
| Hydrogenation Temperature | Detected Phases | Phase Content, vol.% | Lattice Parameters, Å | Crystallite Size, nm |
|---|---|---|---|---|
| 450 °C | TiH_FCT | 100 | a = 3.193, c = 4.401 | 17 |
| 500 °C | Ti_HCP | 52.5 | a = 2.948, c = 4.703 | 45 |
| TiH_FCC | 47.5 | a = 4.396 | 30 | |
| 550 °C | Ti_HCP | 80.9 | a = 2.949, c = 4.696 | 60 |
| TiH_FCC | 19.1 | a = 4.398 | 25 |
| Samples | 20 μm Ti Coating | 80 μm Ti Coating | ||||
|---|---|---|---|---|---|---|
| Temperature | 450 °C | 500 °C | 550 °C | 450 °C | 500 °C | 550 °C |
| The hydrogen content, wt.% | 3.82 | 3.12 | 2.76 | 3.84 | 1.13 | 0.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lider, A.; Kudiiarov, V.; Kashkarov, E.; Syrtanov, M.; Murashkina, T.; Lomygin, A.; Sakvin, I.; Karpov, D.; Ivanov, A. Hydrogen Accumulation and Distribution in Titanium Coatings at Gas-Phase Hydrogenation. Metals 2020, 10, 880. https://doi.org/10.3390/met10070880
Lider A, Kudiiarov V, Kashkarov E, Syrtanov M, Murashkina T, Lomygin A, Sakvin I, Karpov D, Ivanov A. Hydrogen Accumulation and Distribution in Titanium Coatings at Gas-Phase Hydrogenation. Metals. 2020; 10(7):880. https://doi.org/10.3390/met10070880
Chicago/Turabian StyleLider, Andrey, Viktor Kudiiarov, Egor Kashkarov, Maxim Syrtanov, Tatyana Murashkina, Anton Lomygin, Ivan Sakvin, Dmitri Karpov, and Alexander Ivanov. 2020. "Hydrogen Accumulation and Distribution in Titanium Coatings at Gas-Phase Hydrogenation" Metals 10, no. 7: 880. https://doi.org/10.3390/met10070880
APA StyleLider, A., Kudiiarov, V., Kashkarov, E., Syrtanov, M., Murashkina, T., Lomygin, A., Sakvin, I., Karpov, D., & Ivanov, A. (2020). Hydrogen Accumulation and Distribution in Titanium Coatings at Gas-Phase Hydrogenation. Metals, 10(7), 880. https://doi.org/10.3390/met10070880








