Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Emulsified Oil with Emulsifiers
2.2.2. Stability Tests of Emulsified Oil
2.2.3. KAX Conditioning and Agglomeration
2.2.4. Flotation Tests
3. Results and Discussion
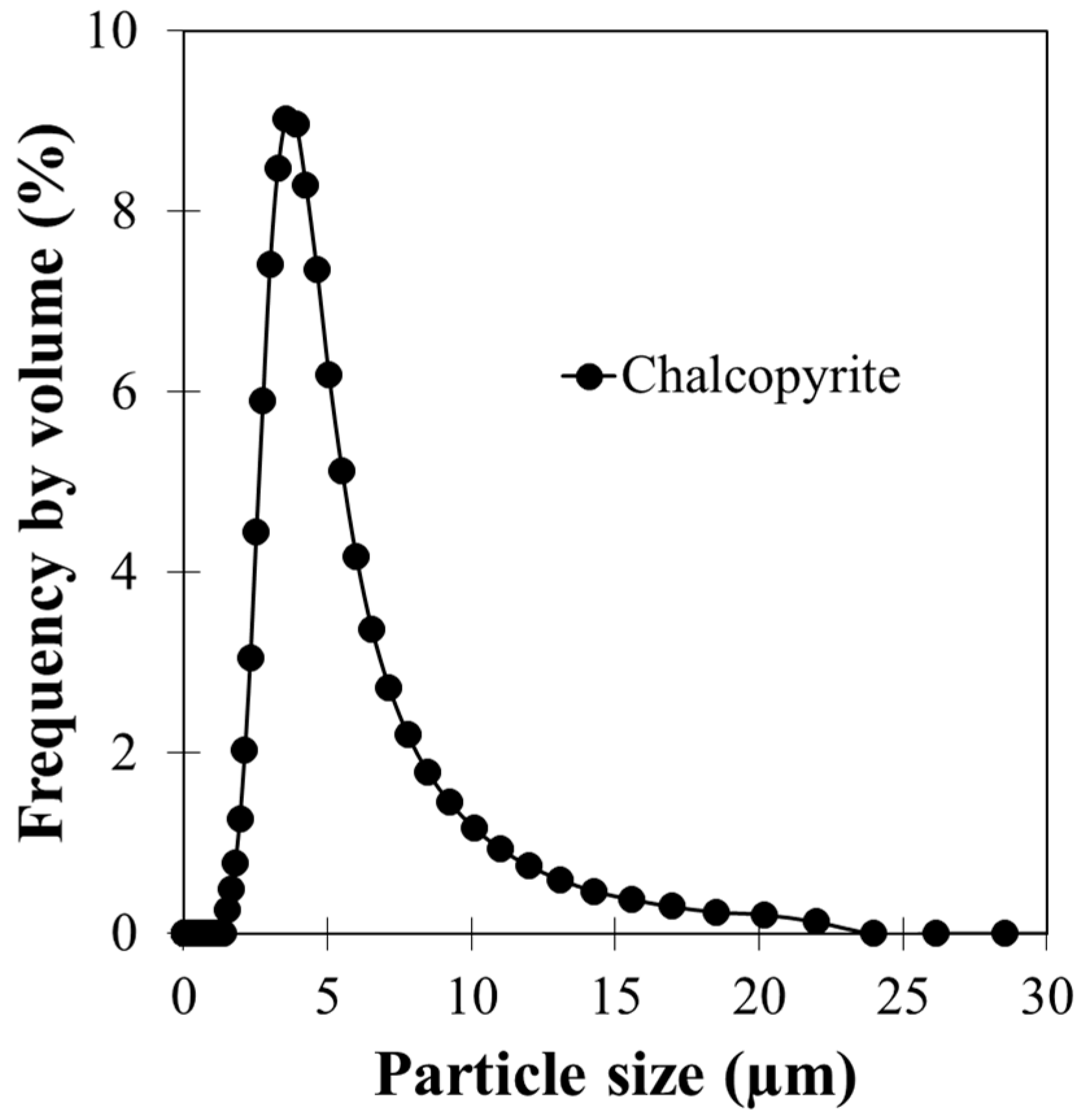
3.1. Characteristics of Chalcopyrite Samples
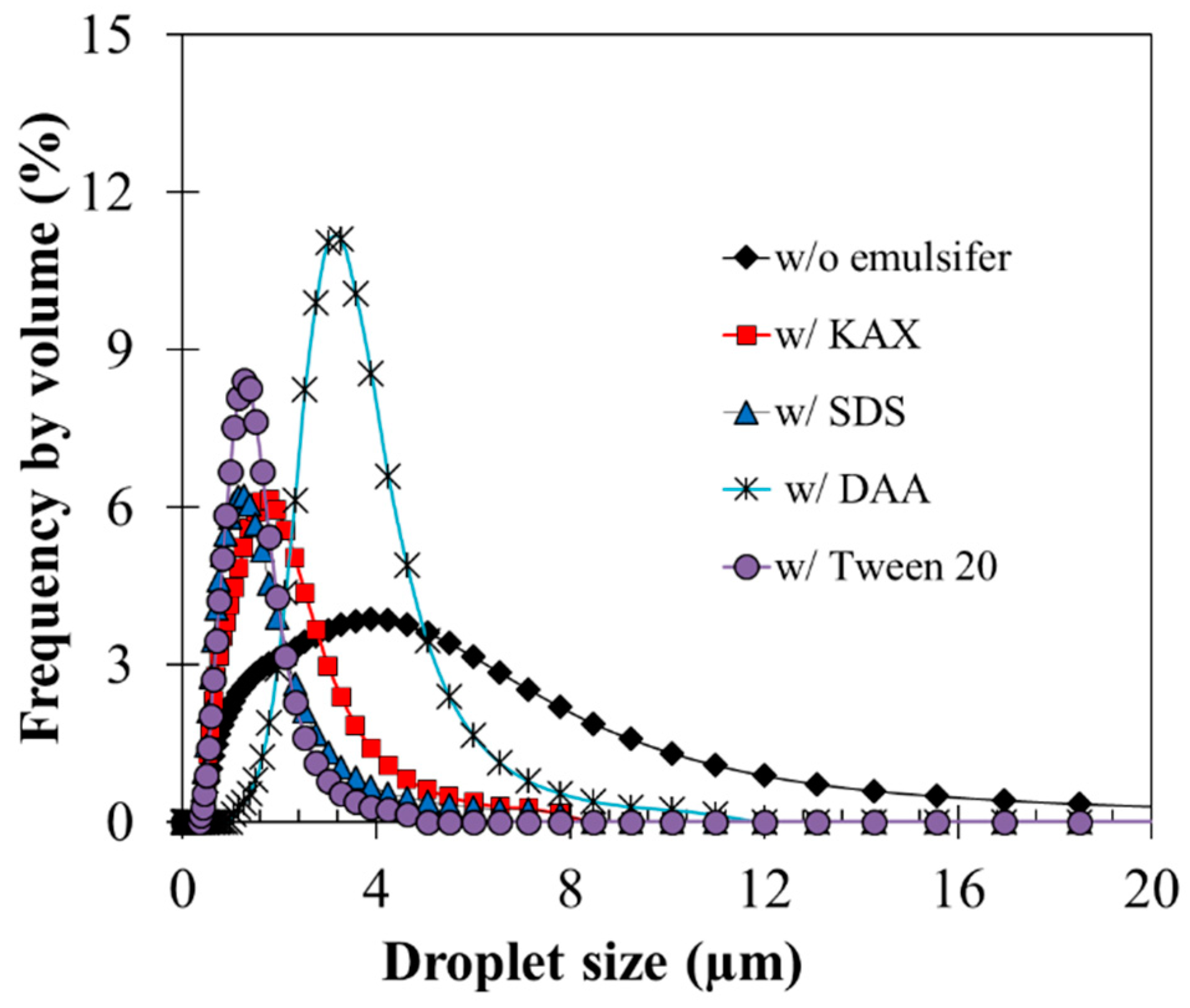
3.2. Effects of Emulsifiers on Emulsified Oil Droplet Size and Stability
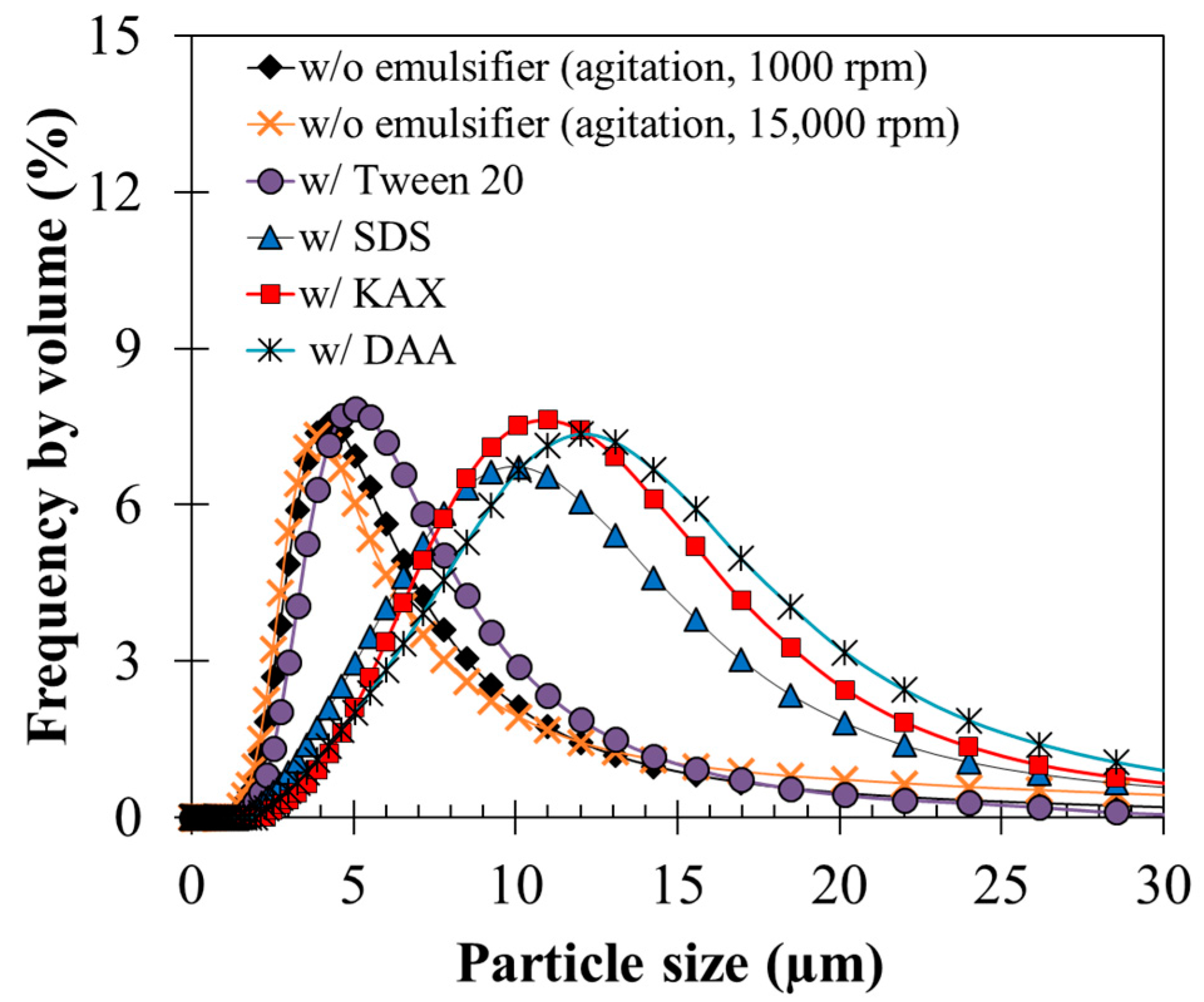
3.3. Effects of Emulsifiers on Agglomeration
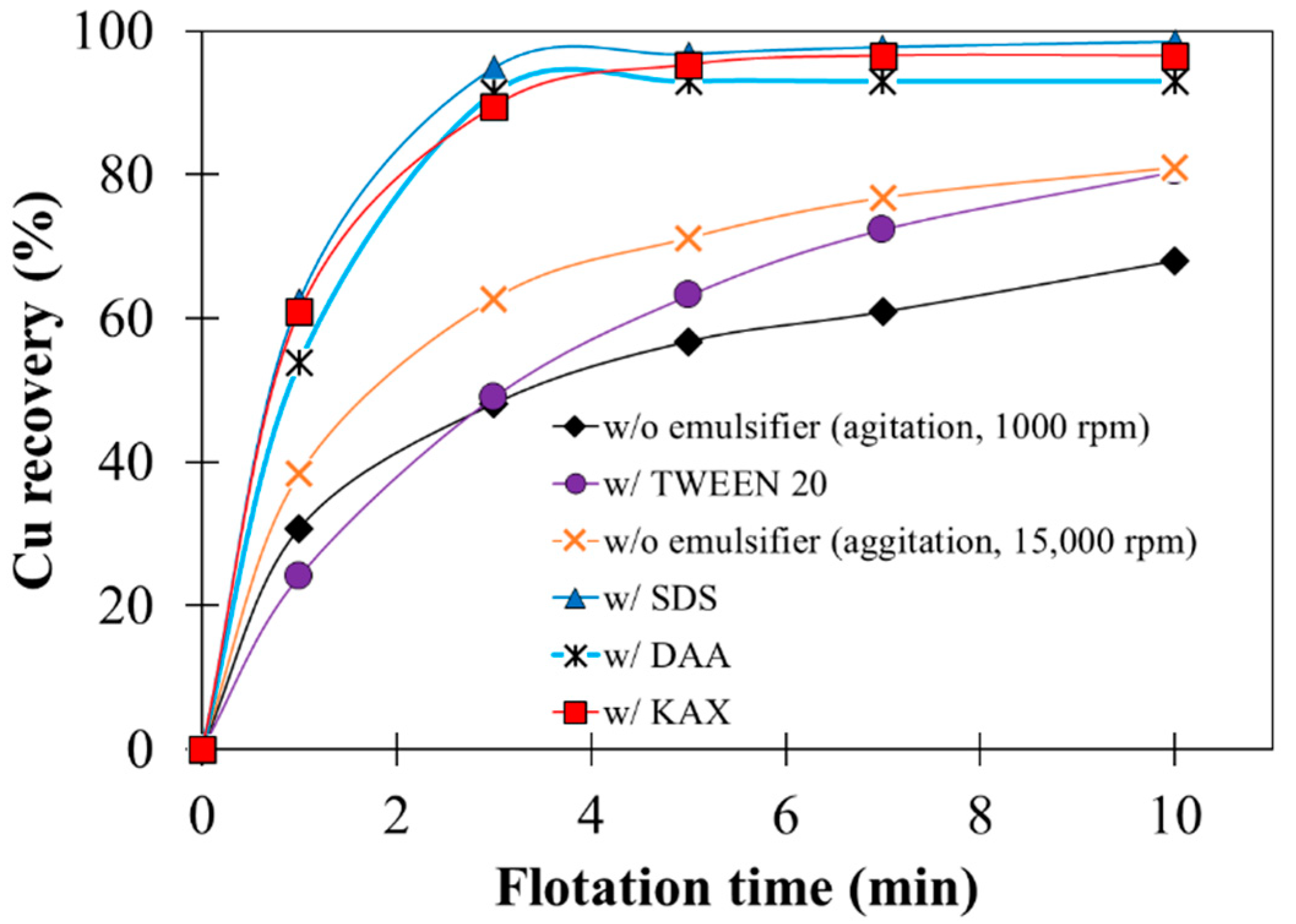
3.4. Effects of Emulsifiers on Flotation
4. Conclusions
- Addition of emulsifiers (e.g., SDS, KAX, DAA, and Tween 20) reduced the size of oil droplets and maintained the stability of emulsified oil longer.
- Addition of emulsifiers (SDS, KAX, and DAA) to emulsified oil contributed to the formation of bigger agglomerates, thus improving Cu recovery by agglomeration–flotation even at low agitation strength and shorter agglomeration time.
- When emulsified oil with emulsifiers is used, specialized equipment with higher agitation strength during agglomeration is not required, and thus the process could be easily integrated into existing flotation circuits.
Author Contributions
Funding
Conflicts of Interest
References
- Brown, T.J.; Bide, T.; Walters, A.S.; Idoine, N.E.; Shaw, R.A.; Hannis, S.D.; Lusty, P.A.J.; Kendall, R.; MacKenzie, A.C. World Mineral Production 2005-09; British Geological Survey: Nottingham, UK, 2011; pp. 29–31. [Google Scholar]
- Richards, J.P.; Boyce, A.J.; Pringle, M.S. Geologic Evolution of the Escondida Area, Northern Chile: A Model for Spatial and Temporal Localization of Porphyry Cu Mineralization. Econ. Geol. Bull. Soc. Econ. Geol. 2001, 96, 271–305. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Segawa, T.; Jeon, S.; Park, I.; Tabelin, C.B.; Hiroyoshi, N. Depression of Lead-Activated Sphalerite by Pyrite via Galvanic Interactions: Implications to the Selective Flotation of Complex Sulfide Ores. Miner. Eng. 2020, 152, 106367. [Google Scholar] [CrossRef]
- Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration-Flotation of Finely Ground Chalcopyrite and Quartz: Effects of Agitation Strength during Agglomeration Using Emulsified Oil on Chalcopyrite. Minerals 2020, 10, 380. [Google Scholar] [CrossRef]
- Ito, M.; Saito, A.; Murase, N.; Phengsaart, T.; Kimura, S.; Tabelin, C.B.; Hiroyoshi, N. Development of Suitable Product Recovery Systems of Continuous Hybrid Jig for Plastic-Plastic Separation. Miner. Eng. 2019, 141, 105839. [Google Scholar] [CrossRef]
- Ito, M.; Takeuchi, M.; Saito, A.; Murase, N.; Phengsaart, T.; Tabelin, C.B.; Hiroyoshi, N.; Tsunekawa, M. Improvement of Hybrid Jig Separation Efficiency Using Wetting Agents for the Recycling of Mixed-Plastic Wastes. J. Mater. Cycles Waste Manag. 2019, 21, 1376–1383. [Google Scholar] [CrossRef]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsumrankul, R.; Kitajima, N.; Park, I.; Hiroyoshi, N. Gold Recovery from Shredder Light Fraction of E-Waste Recycling Plant by Flotation-Ammonium Thiosulfate Leaching. Waste Manag. 2018, 77, 195–202. [Google Scholar] [CrossRef]
- Jeon, S.; Ito, M.; Tabelin, C.B.; Pongsumrankul, R.; Tanaka, S.; Kitajima, N.; Saito, A.; Park, I.; Hiroyoshi, N. A Physical Separation Scheme to Improve Ammonium Thiosulfate Leaching of Gold by Separation of Base Metals in Crushed Mobile Phones. Miner. Eng. 2019, 138, 168–177. [Google Scholar] [CrossRef]
- Phengsaart, T.; Ito, M.; Azuma, A.; Tabelin, C.B.; Hiroyoshi, N. Jig Separation of Crushed Plastics: The Effects of Particle Geometry on Separation Efficiency. J. Mater. Cycles Waste Manag. 2020, 22, 787–800. [Google Scholar] [CrossRef]
- Phengsaart, T.; Ito, M.; Hamaya, N.; Tabelin, C.B.; Hiroyoshi, N. Improvement of Jig Efficiency by Shape Separation, and a Novel Method to Estimate the Separation Efficiency of Metal Wires in Crushed Electronic Wastes Using Bending Behavior and “Entanglement Factor”. Miner. Eng. 2018, 129, 54–62. [Google Scholar] [CrossRef]
- Seng, S.; Tabelin, C.B.; Makino, Y.; Chea, M.; Phengsaart, T.; Park, I.; Hiroyoshi, N.; Ito, M. Improvement of Flotation and Suppression of Pyrite Oxidation Using Phosphate-Enhanced Galvanic Microencapsulation (GME) in a Ball Mill with Steel Ball Media. Miner. Eng. 2019, 143, 105931. [Google Scholar] [CrossRef]
- Jiangang, F.; Kaida, C.; Hui, W.; Chao, G.; Wei, L. Recovering Molybdenite from Ultrafine Waste Tailings by Oil Agglomerate Flotation. Miner. Eng. 2012, 39, 133–139. [Google Scholar] [CrossRef]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle–Bubble Collision Models—a Review. Adv. Colloid Interface Sci. 2000, 85, 231–256. [Google Scholar] [CrossRef]
- Miettinen, T.; Ralston, J.; Fornasiero, D. The Limits of Fine Particle Flotation. Miner. Eng. 2010, 23, 420–437. [Google Scholar] [CrossRef]
- Trahar, W.J.; Warren, L.J. The Flotability of Very Fine Particles—A Review. Int. J. Miner. Process. 1976, 3, 103–131. [Google Scholar] [CrossRef]
- Finch, J.A. Column Flotation: A Selected Review— Part IV: Novel Flotation Devices. Miner. Eng. 1995, 8, 587–602. [Google Scholar] [CrossRef]
- Yoon, R.H. Microbubble Flotation. Miner. Eng. 1993, 6, 619–630. [Google Scholar] [CrossRef]
- Bhaskar Raju, G.; Khangaonkar, P.R. Electro-Flotation of Chalcopyrite Fines. Int. J. Miner. Process. 1982, 9, 133–143. [Google Scholar] [CrossRef]
- Rodrigues, R.T.; Rubio, J. DAF–Dissolved Air Flotation: Potential Applications in the Mining and Mineral Processing Industry. Int. J. Miner. Process. 2007, 82, 1–13. [Google Scholar] [CrossRef]
- Warren, L.J. Shear-Flocculation of Ultrafine Scheelite in Sodium Oleate Solutions. J. Colloid Interface Sci. 1975, 50, 307–318. [Google Scholar] [CrossRef]
- Rubio, J.; Hoberg, H. The Process of Separation of Fine Mineral Particles by Flotation with Hydrophobic Polymeric Carrier. Int. J. Miner. Process. 1993, 37, 109–122. [Google Scholar] [CrossRef]
- Sresty, G.C.; Somasundaran, P. Selective Flocculation of Synthetic Mineral Mixtures Using Modified Polymers. Int. J. Miner. Process. 1980, 6, 303–320. [Google Scholar] [CrossRef]
- Alonso, M.I.; Valdés, A.F.; Martínez-Tarazona, R.M.; Garcia, A.B. Coal Recovery from Coal Fines Cleaning Wastes by Agglomeration with Vegetable Oils: Effects of Oil Type and Concentration. Fuel 1999, 78, 753–759. [Google Scholar] [CrossRef]
- Slaghuis, J.H.; Ferreira, L.C. Selective Spherical Agglomeration of Coal. Fuel 1987, 66, 1427–1430. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Liu, Q.; Bolin, N.J. Flotation of Sulphide Minerals 1990 Polysaccharides in Flotation of Sulphides. Part I. Adsorption of Polysaccharides onto Mineral Surfaces. Int. J. Miner. Process. 1991, 33, 223–234. [Google Scholar] [CrossRef]
- Bensley, C.N.; Swanson, A.R.; Nicol, S.K. The Effect of Emulsification on the Selective Agglomeration of Fine Coal. Int. J. Miner. Process. 1977, 4, 173–184. [Google Scholar] [CrossRef]
- Van Netten, K.; Moreno-Atanasio, R.; Galvin, K.P. Fine Particle Beneficiation through Selective Agglomeration with an Emulsion Binder. Ind. Eng. Chem. Res. 2014, 53, 15747–15754. [Google Scholar] [CrossRef]
- Sahinoglu, E.; Uslu, T. Use of Ultrasonic Emulsification in Oil Agglomeration for Coal Cleaning. Fuel 2013, 113, 719–725. [Google Scholar] [CrossRef]
- Johnson, N.W. Liberated 0–10μm Particles from Sulphide Ores, Their Production and Separation—Recent Developments and Future Needs. Miner. Eng. 2006, 19, 666–674. [Google Scholar] [CrossRef]
- Stang, M.; Karbstein, H.; Schubert, H. Adsorption Kinetics of Emulsifiers at Oil—Water Interfaces and Their Effect on Mechanical Emulsification. Chem. Eng. Process. Process Intensif. 1994, 33, 307–311. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An Experimental Study of Stability of Oil–Water Emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Oh, S.G.; Shah, D.O. Effect of counterions on the interfacial tension and emulsion droplet size in the oil/water/dodecyl sulfate system. J. Phys. Chem. 1993, 97, 284–286. [Google Scholar] [CrossRef]
- Darelius, A.; Rasmuson, A.; Björn, I.N.; Folestad, S. High shear wet granulation modelling—a mechanistic approach using population balances. Powder Technol. 2005, 160, 209–218. [Google Scholar] [CrossRef]
- Kunieda, H.; Hanno, K.; Yamaguchi, S.; Shinoda, K. The Three-Phase Behavior of a Brine/Ionic Surfactant/Nonionic Surfactant/Oil System: Evaluation of the Hydrophile-Lipophile Balance (HLB) of Ionic Surfactant. J. Colloid Interface Sci. 1985, 107, 129–137. [Google Scholar] [CrossRef]
- Kunieda, H.; Ishikawa, N. Evaluation of the Hydrophile-Lipophile Balance (HLB) of Nonionic Surfactants. II. Commercial-Surfactant Systems. J. Colloid Interface Sci. 1985, 107, 122–128. [Google Scholar] [CrossRef]




| Elements | Cu | Fe | S | Zn | Si | Ca |
|---|---|---|---|---|---|---|
| wt% | 26 | 27 | 26 | 0.8 | 6 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hornn, V.; Ito, M.; Shimada, H.; Tabelin, C.B.; Jeon, S.; Park, I.; Hiroyoshi, N. Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals 2020, 10, 912. https://doi.org/10.3390/met10070912
Hornn V, Ito M, Shimada H, Tabelin CB, Jeon S, Park I, Hiroyoshi N. Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals. 2020; 10(7):912. https://doi.org/10.3390/met10070912
Chicago/Turabian StyleHornn, Vothy, Mayumi Ito, Hiromasa Shimada, Carlito Baltazar Tabelin, Sanghee Jeon, Ilhwan Park, and Naoki Hiroyoshi. 2020. "Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation" Metals 10, no. 7: 912. https://doi.org/10.3390/met10070912
APA StyleHornn, V., Ito, M., Shimada, H., Tabelin, C. B., Jeon, S., Park, I., & Hiroyoshi, N. (2020). Agglomeration–Flotation of Finely Ground Chalcopyrite Using Emulsified Oil Stabilized by Emulsifiers: Implications for Porphyry Copper Ore Flotation. Metals, 10(7), 912. https://doi.org/10.3390/met10070912







