Biosorption of Rare Earth Elements by Different Microorganisms in Acidic Solutions
Abstract
1. Introduction
2. Material and Methods
2.1. Microorganisms and Cultivation Conditions
2.2. Biosorption Experiments with Suspended Biomass
2.3. Biosorption Conditions Experiments with Immobilized Biomass
2.4. Phylogenetic Analysis
3. Results and Discussion
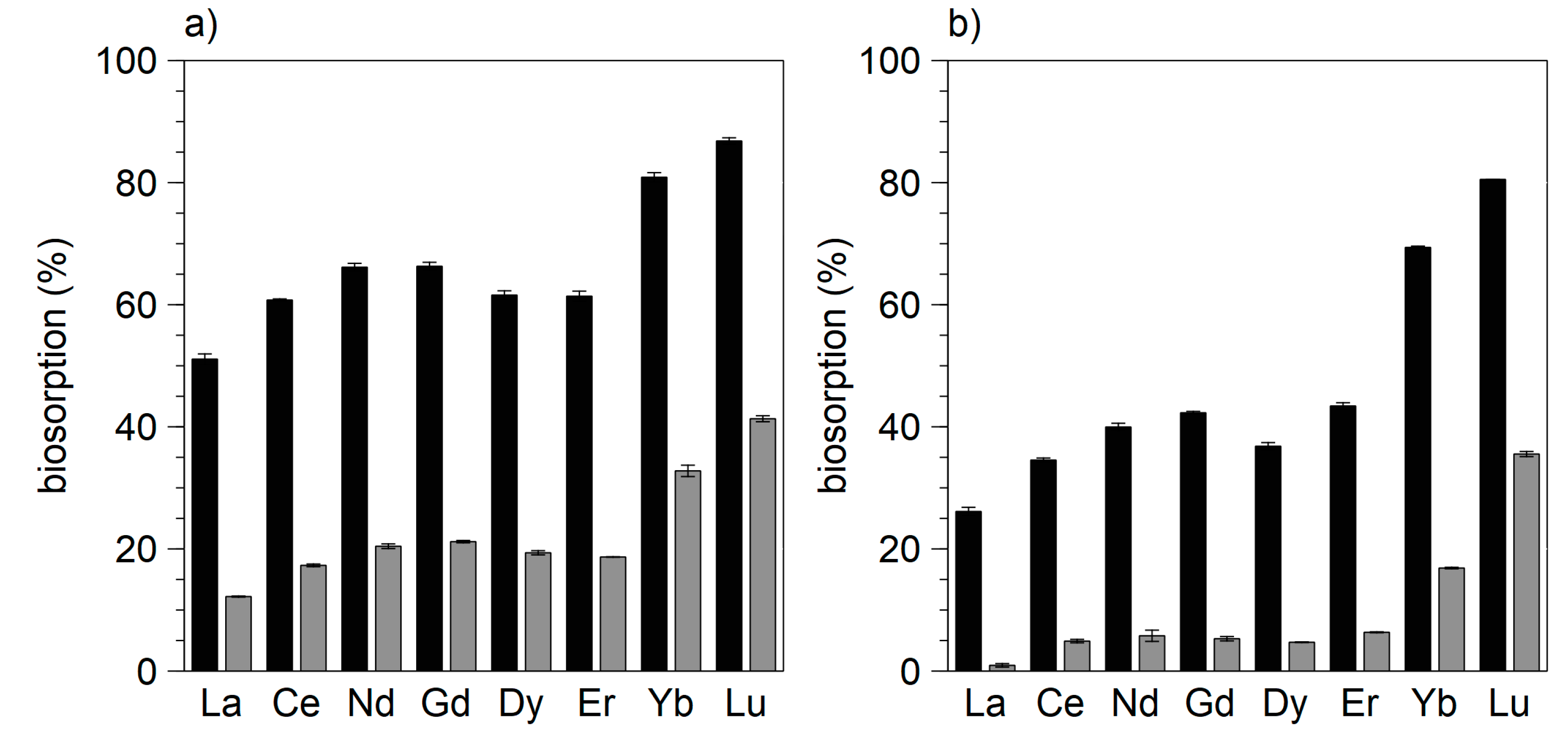
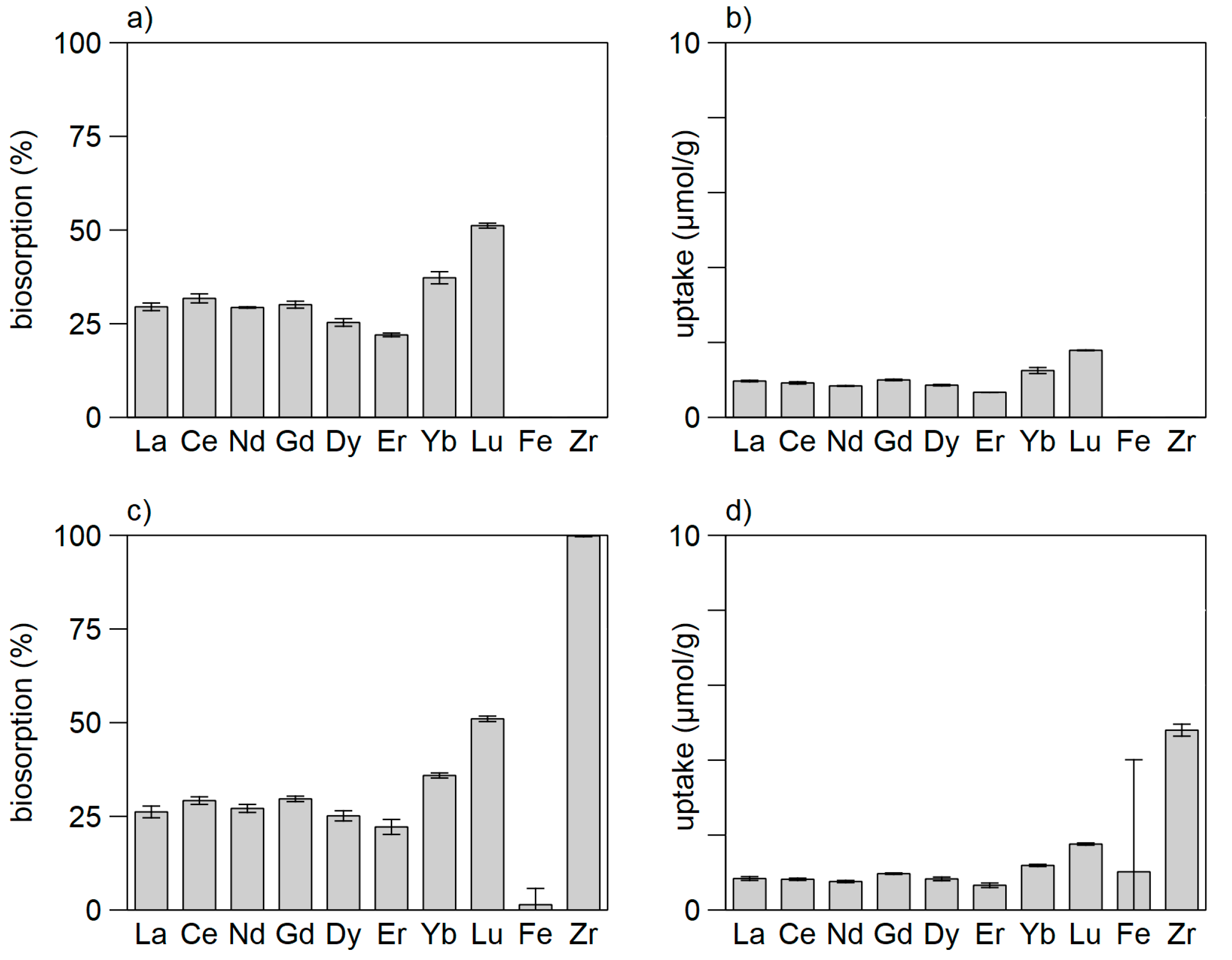
3.1. Biosorption of REE by Bacteria
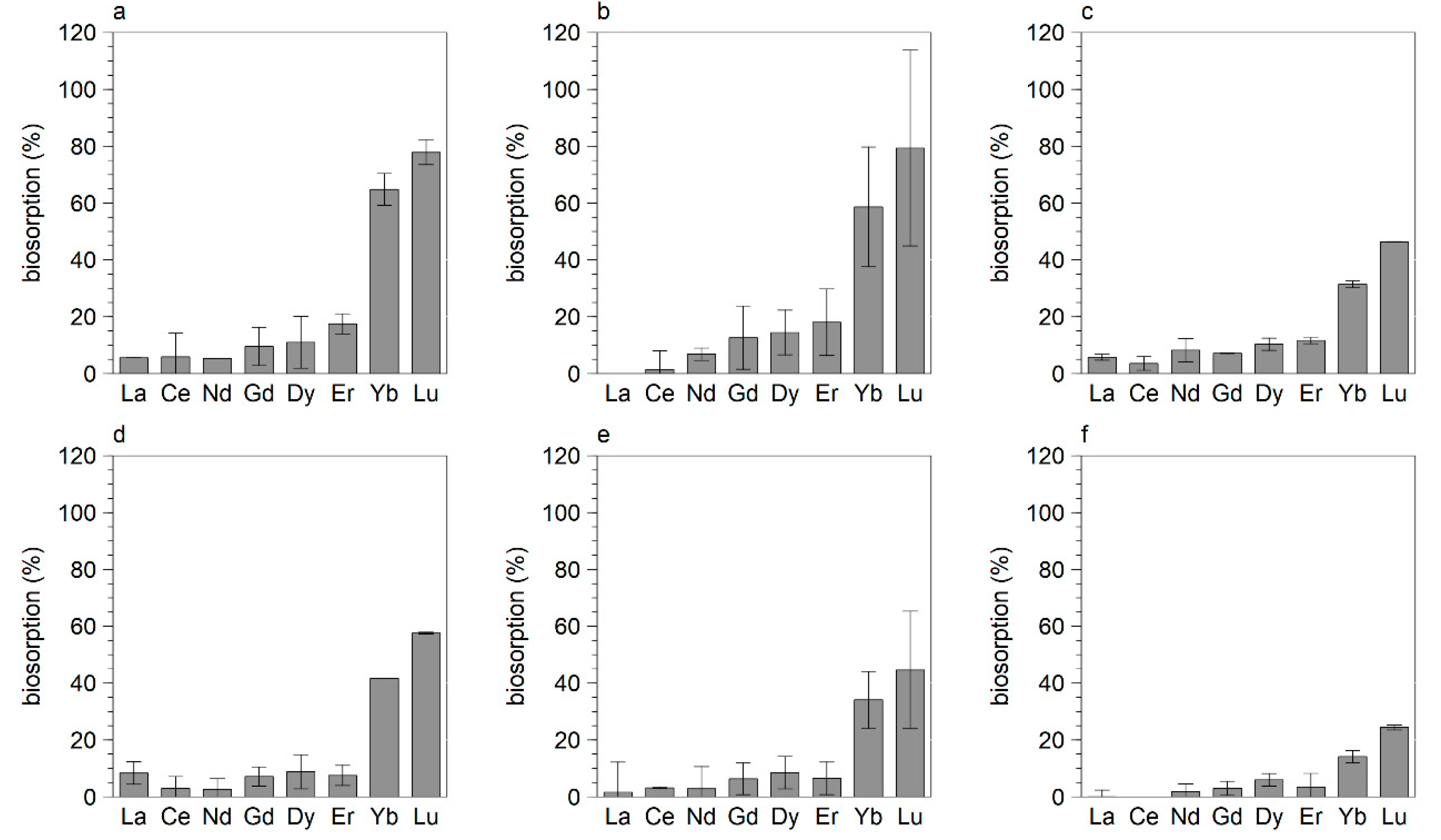
3.2. Biosorption of REE by Eukaryotes
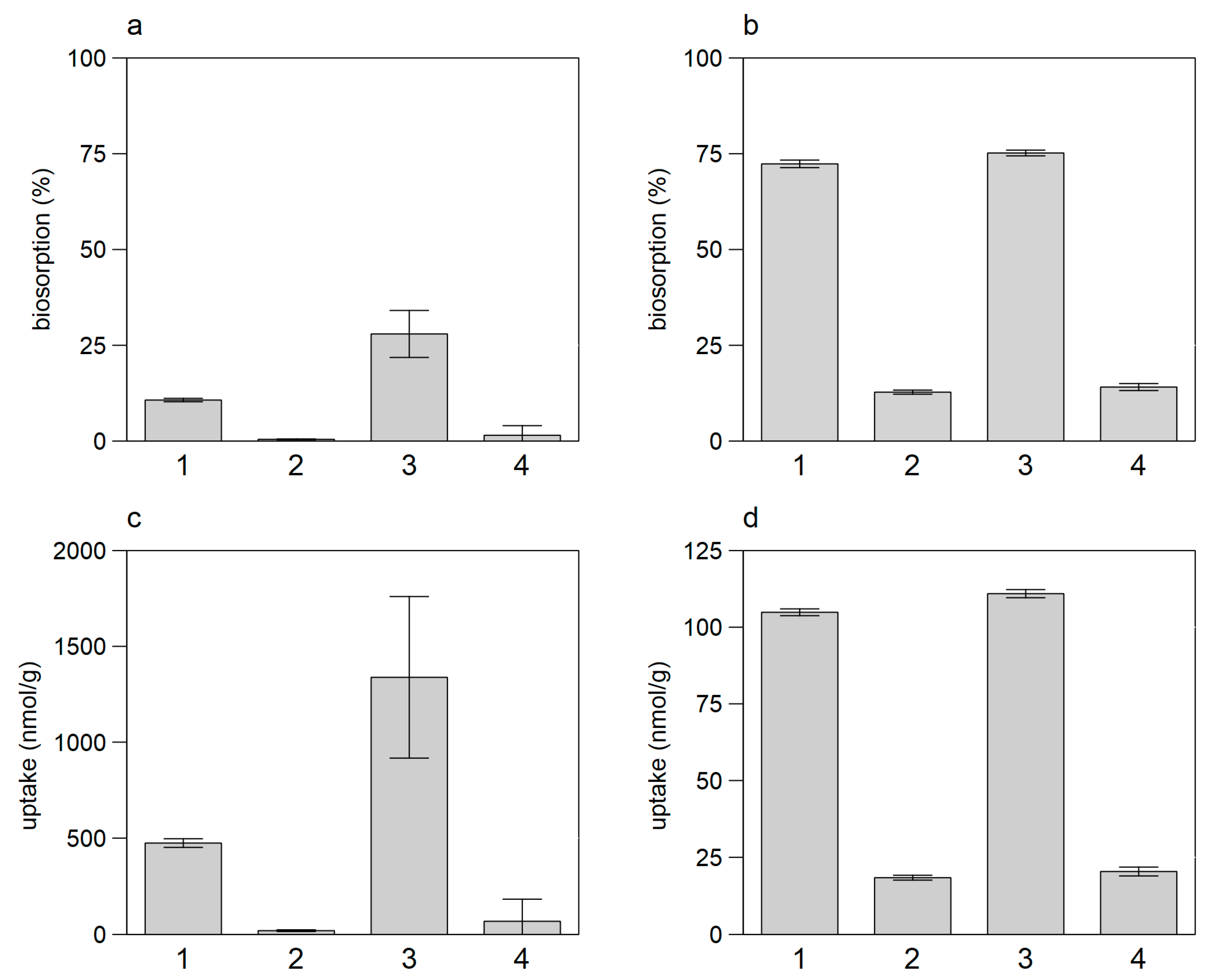
3.3. Tests with Dead vs. Living Biomass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsezos, M. Biosorption: A mechanistic approach. Adv. Biochem. Eng. Biotechnol. 2014, 141, 173–209. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2008, 84, 13–28. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process--a review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Lee, J.-C. Biometallurgical recovery of metals from waste electrical and electronic equipment: A review. ChemBioEng Rev. 2014, 1, 148–169. [Google Scholar] [CrossRef]
- Andrès, Y.; Gérente, C. Removal of rare earth elements and precious metal species by biosorption. In Microbial Biosorption of Metals; Kotrba, P., Mackova, M., Macek, T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 179–196. ISBN 978-94-007-0443-5. [Google Scholar]
- Akcil, A. Critical and Rare Earth Elements. Recovery from Secondary Resources; CRC Press, Taylor et Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2020; ISBN 9780367086473. [Google Scholar]
- Andrès, Y.; MacCordick, H.J.; Hubert, J.-C. Adsorption of several actinide (Th, U) and lanthanide (La, Eu, Yb) ions by Mycobacterium smegmatis. Appl. Microbiol. Biotechnol. 1993, 39. [Google Scholar] [CrossRef]
- Karavaiko, G.I.; Kareva, A.S.; Avakian, Z.A.; Zakharova, V.I.; Korenevsky, A.A. Biosorption of scandium and yttrium from solutions. Biotechnol. Lett. 1996, 18, 1291–1296. [Google Scholar] [CrossRef]
- Texier, A.C.; Andrès, Y.; Cloirec, P.L. Selective Biosorption of Lanthanide (La, Eu) Ions by Mycobacterium smegmatis. Environ. Technol. 1997, 18, 835–841. [Google Scholar] [CrossRef]
- Texier, A.-C.; Andrès, Y.; Le Cloirec, P. Selective biosorption of lanthanide (La, Eu, Yb) ions by Pseudomonas aeruginosa. Environ. Sci. Technol. 1999, 33, 489–495. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Garcia, O.; Melnikov, P. Neodymium biosorption from acidic solutions in batch system. Process Biochem. 2000, 36, 441–444. [Google Scholar] [CrossRef]
- Haferburg, G.; Merten, D.; Büchel, G.; Kothe, E. Biosorption of metal and salt tolerant microbial isolates from a former uranium mining area. Their impact on changes in rare earth element patterns in acid mine drainage. J. Basic Microbiol. 2007, 47, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, B.T.; Mosselmans, J.F.W.; Magennis, M.; Atkinson, K.D.; Tourney, J.; Olive, V.; Ellam, R.M. Macroscopic and spectroscopic analysis of lanthanide adsorption to bacterial cells. Geochim. Cosmochim. Acta 2009, 73, 3134–3147. [Google Scholar] [CrossRef]
- Minoda, A.; Sawada, H.; Suzuki, S.; Miyashita, S.-I.; Inagaki, K.; Yamamoto, T.; Tsuzuki, M. Recovery of rare earth elements from the sulfothermophilic red alga Galdieria sulphuraria using aqueous acid. Appl. Microbiol. Biotechnol. 2015, 99, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Horiike, T.; Yamashita, M. A new fungal isolate, Penidiella sp. strain T9, accumulates the rare earth element dysprosium. Appl. Environ. Microbiol. 2015, 81, 3062–3068. [Google Scholar] [CrossRef]
- Bonificio, W.D.; Clarke, D.R. Rare-earth separation using bacteria. Environ. Sci. Technol. Lett. 2016, 3, 180–184. [Google Scholar] [CrossRef]
- Takahashi, Y.; Châtellier, X.; Hattori, K.H.; Kato, K.; Fortin, D. Adsorption of rare earth elements onto bacterial cell walls and its implication for REE sorption onto natural microbial mats. Chem. Geol. 2005, 219, 53–67. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, M.; Yamamoto, Y.; Tanaka, K. EXAFS study on the cause of enrichment of heavy REEs on bacterial cell surfaces. Geochim. Cosmochim. Acta 2010, 74, 5443–5462. [Google Scholar] [CrossRef]
- Markai, S.; Andrès, Y.; Montavon, G.; Grambow, B. Study of the interaction between europium (III) and Bacillus subtilis: Fixation sites, biosorption modeling and reversibility. J. Colloid Interface Sci. 2003, 262, 351–361. [Google Scholar] [CrossRef]
- Saitoh, N.; Fujimori, R.; Nakatani, M.; Yoshihara, D.; Nomura, T.; Konishi, Y. Microbial recovery of gold from neutral and acidic solutions by the baker’s yeast Saccharomyces cerevisiae. Hydrometallurgy 2018, 181, 29–34. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-green algae with chlorophyllide C1,2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Webster, G.; Newberry, C.J.; Fry, J.C.; Weightman, A.J. Assessment of bacterial community structure in the deep sub-seafloor biosphere by 16S rDNA-based techniques: A cautionary tale. J. Microbiol. Methods 2003, 55, 155–164. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Mullen, M.D.; Wolf, D.C.; Ferris, F.G.; Beveridge, T.J.; Flemming, C.A.; Bailey, G.W. Bacterial sorption of heavy metals. Appl. Environ. Microbiol. 1989, 55, 3143–3149. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, H.; Koide, R.; Yoshikawa, R.; Warabino, Y.; Yamamoto, H. Adsorption of rare earth ions onto the cell walls of wild-type and lipoteichoic acid-defective strains of Bacillus subtilis. Appl. Microbiol. Biotechnol. 2013, 97, 3721–3728. [Google Scholar] [CrossRef] [PubMed]
- Park, D.M.; Reed, D.W.; Yung, M.C.; Eslamimanesh, A.; Lencka, M.M.; Anderko, A.; Fujita, Y.; Riman, R.E.; Navrotsky, A.; Jiao, Y. Bioadsorption of rare earth elements through cell surface display of lanthanide binding tags. Environ. Sci. Technol. 2016, 50, 2735–2742. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Kümmerer, K.; Helmers, E. Hospital effluents as a source of gadolinium in the aquatic environment. Environ. Sci. Technol. 2000, 34, 573–577. [Google Scholar] [CrossRef]
- Tepe, N.; Romero, M.; Bau, M. High-technology metals as emerging contaminants: Strong increase of anthropogenic gadolinium levels in tap water of Berlin, Germany, from 2009 to 2012. Appl. Geochem. 2014, 45, 191–197. [Google Scholar] [CrossRef]
- Kulaksız, S.; Bau, M. Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare earth element distribution in rivers. Earth Planet. Sci. Lett. 2013, 362, 43–50. [Google Scholar] [CrossRef]
- Vriens, B.; Voegelin, A.; Hug, S.J.; Kaegi, R.; Winkel, L.H.E.; Buser, A.M.; Berg, M. Quantification of element fluxes in wastewaters: A nationwide survey in Switzerland. Environ. Sci. Technol. 2017, 51, 10943–10953. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Bau, M.; Merschel, G.; Tepe, N. Anthropogenic gadolinium in tap water and in tap water-based beverages from fast-food franchises in six major cities in Germany. Sci. Total Environ. 2019, 687, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-R.; Byrne, R.H. Carbonate complexation of yttrium and the rare earth elements in natural waters. Geochim. Cosmochim. Acta 2004, 68, 691–699. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of chitin: Solvents, solution behaviors and their related mechanisms. In Solubility of Polysaccharides; Xu, Z., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3649-1. [Google Scholar]




| Organism | Taxonomy | Medium | Tempe-Rature | Type of Flasks | rpm on the Shaker | Incuba-Tion Time (Days) | Reference |
|---|---|---|---|---|---|---|---|
| Leisingeria methylo-halidivorans DSM 14336 | Bacteria (gram-negative, Roseobacter clade | Marine Broth medium (Difco, MB2216) | 20 °C | Bullying flasks | 80 | 2 | [17] |
| Phaeobacter inhibens DSM 17395 | Bacteria (gram-negative, Roseobacter clade | Marine Broth medium (Difco, MB2216) | 20 °C | Bullying flasks | 80 | 2 | [17] |
| Bacillius subtilis ATCC 7003 | Bacteria (gram-positive) | FP medium | 30 °C | Erlen-meyer flasks | 120 | 2–4 | [18,19,20] |
| Pezicomy-cotina sp. | Eukarya (fungus) | FP medium | 30 °C | Erlen-meyer flasks | 120 | ~10 | BGR culture M8 |
| Fusarium sp. | Eukarya (fungus) | malt medium | 30 °C | Erlen-meyer flasks | 120 | 2–4 | BGR culture |
| Catenulostro-ma chromo-blastomyces CBS 597.97 | Eukarya (fungus) | malt medium | 30 °C, | Erlen-meyer flasks | 120 | 2–4 | [16] |
| Pichia sp. | Eukarya (fungus, yeast) | malt medium | 30 °C, | Erlen-meyer flasks | 120 | 2–4 | BGR culture |
| Pichia naganishii [MB#320511] | Eukarya (fungus, yeast) | NB medium | 30 °C, | Erlen-meyer flasks | 120 | 2–4 | BGR culture |
| Saccharo-myces cerevisiae | Eukarya (fungus, yeast) | Sabouraud medium | 30 °C, | Erlen-meyer flasks | 120 | 2–4 | [21] |
| Galderia sulphuraria | Eukarya, Rhodo-phyta (algae) | Cyanidium medium and 2× Allens medium | 20 °C, 30 °C sunlight and dark | Baffled shake flasks | 200 | ~14 | [15] |
| Messastrum gracilis | Eukarya, Chloro-phyta (algae) | WC medium | 20 °C, 30 °C, sunlight and dark | Baffled shake flasks | 200 | ~14 | [12] |
| Organism | Result |
|---|---|
| Saccharomyces cerevisiae | Tested with BM of 5, 10 and 20 g/L, at a BM of 5 g/L biosorption was <50%, biosorption was 100% at a BM of 10 g/L, preference for Gd, Dy, Yb, Lu |
| Pichia naganishii [MB#320511] | Tested for La, Nd, Dy, Yb at a BM of 2.5, 5 and 10 g/L. At a BM >5 g/L, biosorption >80%. |
| Pichia sp. | Preference for Ce, Nd, Gd, Dy |
| Catenulostroma chromoblastomyces CBS 597.97 | Preference for Ce, Nd, Gd, Dy, at a BM of 7.5 g/L ~30% biosorption |
| Pezicomycotina sp. | Tested with BM of 2, 5 and 10 g/L, biosorption <20%, preference for Gd, Yb, Lu |
| Fusarium sp. | Preference for Nd, Gd, Gy, Lu, tested for 4 elements (La, Nd, Dy, Yb), BM 4 g/L~40%, BM 5 g/L biosorption ~100% |
| Messastrum gracilis | Literature data not confirmed [12] |
| Galdieria sulphuraria | Literature data not confirmed [15] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breuker, A.; Ritter, S.F.; Schippers, A. Biosorption of Rare Earth Elements by Different Microorganisms in Acidic Solutions. Metals 2020, 10, 954. https://doi.org/10.3390/met10070954
Breuker A, Ritter SF, Schippers A. Biosorption of Rare Earth Elements by Different Microorganisms in Acidic Solutions. Metals. 2020; 10(7):954. https://doi.org/10.3390/met10070954
Chicago/Turabian StyleBreuker, Anja, Simon F. Ritter, and Axel Schippers. 2020. "Biosorption of Rare Earth Elements by Different Microorganisms in Acidic Solutions" Metals 10, no. 7: 954. https://doi.org/10.3390/met10070954
APA StyleBreuker, A., Ritter, S. F., & Schippers, A. (2020). Biosorption of Rare Earth Elements by Different Microorganisms in Acidic Solutions. Metals, 10(7), 954. https://doi.org/10.3390/met10070954






