Influence of MWCNT Coated Nickel on the Foaming Behavior of MWCNT Coated Nickel Reinforced AlMg4Si8 Foam by Powder Metallurgy Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
3. Result
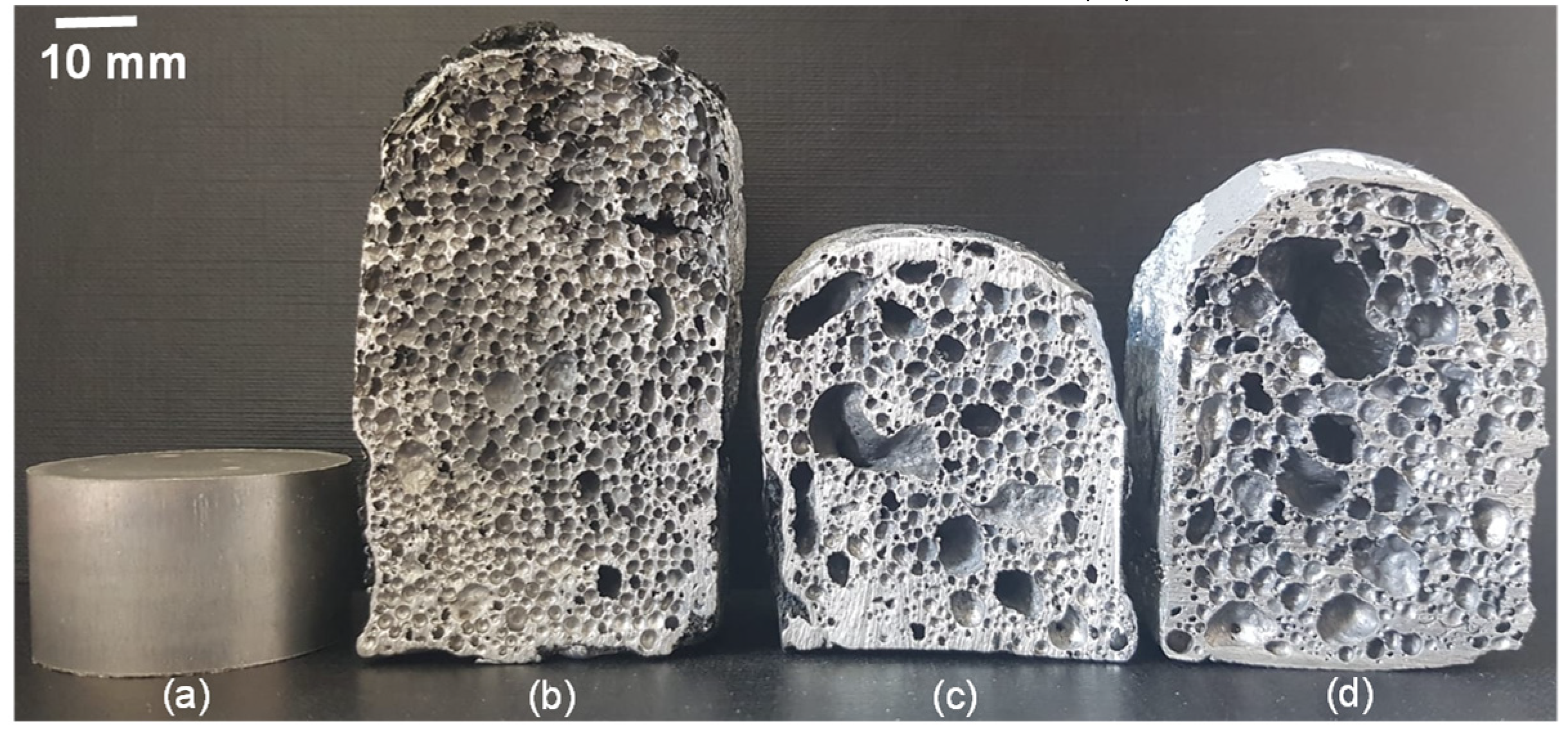
3.1. The Effect of Multi-Wall Carbon Nanotube Coated Nickel to Foaming Time on Morphology of AlMg4Si8 Foam
3.2. The Pore Size Distribution of Multi-Wall Carbon Nanotube Coated Nickel Reinforced AlMg4Si8
4. Discussion
| Pores Number: AlMg4Si8 (8 min) > MWCNT(Ni)–AlMg4Si8(8 min) > MWCNT(Ni)–AlMg4Si8 (9 min) |
| % Porosity: MWCNT(Ni)–AlMg4Si8 (8 min) < AlMg4Si88 (8 min) < MWCNT(Ni)–AlMg4Si8 (9 min) |
| Pore size: AlMg4Si8 (8 min) < MWCNT(Ni)–AlMg4Si8(8 min) < MWCNT(Ni)–AlMg4Si8(9 min) |
| Density: AlMg4Si8 (8 min) < MWCNT(Ni)–AlMg4Si8(9 min) < MWCNT(Ni)–AlMg4Si8(8 min) |
| The volumetric expansion coefficient: AlMg4Si8 (8 min) > MWCNT(Ni)–AlMg4Si8 (9 min) > MWCNT(Ni)–AlMg4Si8 (8 min) |
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cambroneroa, L.E.G.; Ruiz-Romana, J.M.; Corpasb, F.A.; Ruiz Prieto, J.M. Manufacturing of Al-Mg-Si alloy foam using calcium carbonate as foaming agent. J. Mater. Process. Technol. 2009, 209, 1803–1809. [Google Scholar] [CrossRef]
- Degischer, H.; Kriszt, B. Handbook of Cellular Metals: Production, Processing, Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Vienna, Austria, 2002. [Google Scholar]
- Duarte, I.; Ventura, E.; Olhero, S.; Ferreira, J.M. An effective approach to reinforced closed-cell Al-alloy foams with multiwalled carbon nanotubes. Carbon 2015, 95, 589–600. [Google Scholar] [CrossRef]
- Hipke, T.; Lange, G. Taschenbuch Für Aluminiumschäume, 1st ed.; Beuth Verlag GmbH: Berlin, Germany, 2014; ISBN 978-3-410-22071-8. [Google Scholar]
- Helwig, H.M.; Garcia-Moreno, F.; Banhart, J. A study of Mg and Cu additions on the foaming behavior of Al-Si alloys. J. Mater. Sci. 2011, 46, 5227–5236. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Asavavisitchai, S. Effects of TiB2 particle addition on the expansion, structure and mechanical properties of PM Al foams. Scr. Mater. 2004, 50, 115–119. [Google Scholar] [CrossRef]
- Afshar, M.; Mirbagheri, M.H.; Movahedi, N. Effect of SiC particle size on the mechanical properties of closed aluminum foams. Mater. Test. 2017, 59, 571–574. [Google Scholar] [CrossRef]
- Sharma, S.K.; Li, F.X.; Ko, G.D.; Kang, K.J. Strengthening effect of Cr2O3 thermally grown on alloy 617 foils at high temperature. J. Nucl. Mater. 2010, 405, 165–170. [Google Scholar] [CrossRef]
- Ip, S.W.; Sridhar, R.; Toguri, J.M.; Stephenson, T.F.; Warner, A.E.M. Wettability of nickel coated graphite by aluminum. Mater. Sci. Eng. 1998, 244, 31–38. [Google Scholar] [CrossRef]
- Koo, J.H. Polymer Nano Composite, Processing, Characterization, and Application; Mc Graw-Hill: New York, NY, USA, 2016. [Google Scholar]
- Zhang, Z.; Ding, J.; Xia, X.; Sun, X.; Song, K.; Zhao, W.; Liao, B. Fabrication and characterization of closed-cell aluminum foams with different contents of multi-walled carbon nanotubes. Mater. Des. 2015, 88, 359–365. [Google Scholar] [CrossRef]
- Cui, L.J.; Geng, H.Z.; Wang, W.Y.; Chen, L.T.; Gao, J. Functionalization of multi-wall carbon nanotubes to reduce the coefficient of the friction and improve the wear resistance of multi-wall carbon nanotube/epoxy composites. Carbon 2013, 54, 277–282. [Google Scholar] [CrossRef]
- Shin, A.; Han, J.H. Effects of acid treatment of carbon on electroless copper plating. Korean Inst. Surf. Eng. 2016, 49, 265–273. [Google Scholar] [CrossRef][Green Version]
- Damanik, F.S.; Lange, G. Uniformly dispersion of multi-walled carbon nanotubes in AlMg4Si8 foam by powder metallurgy. In Proceedings of the 3rd Asia Pacific Conference on Research in Industrial and Systems Engineering, Depok, Indonesia, 16–17 June 2020. [Google Scholar]
- Noharaa, L.B.; Filhob, G.P.; Noharac, E.L.; Kleinked, M.U.; Rezendee, M.C. Evaluation of MWCNT coated nickel surface treated by chemical and cold plasma processes. Mater. Res. 2005, 8, 281–286. [Google Scholar] [CrossRef]
- Kamath, R.; Chittappa, H.C.; Kumar, N. Electroless Nickel Coating of Short MWCNT coated nickel. In Proceedings of the International Conference on Emerging Trends in Engineering (ICETE -2014) @NITTE, Warangal, Telangana, India, 15–17 May 2014; pp. 169–173. [Google Scholar]
- Duarte, I.; Oliveira, M. Aluminum alloy foams: Production and properties. In Powder Metallurgy; IntechOpen: London, UK, 2012. [Google Scholar]
- Yunus, S.; Sefa-Ntiri, B.; Anderson, B.; Kumi, F.; Mensah-Amoah, P.; Sackey, S.S. Quantitative pore characterization of polyurethane foam with cost-effective imaging tools and image analysis: A proof-of-principle study. Polymers 2019, 11, 1879. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, J.S.; Hu, R.U.; Kou, H.C.; Lian, Z. Mechanical properties of porous titanium with different distributions of pore size. Trans. Nonferrous Met. Soc. Chin. 2013, 23, 2317–2322. [Google Scholar] [CrossRef]
- Bekoz, N.; Oktay, E. Mechanical properties of low alloy steel foams: Dependency on porosity and pore size. Mater. Sci. Eng. A 2013, 576, 82–90. [Google Scholar] [CrossRef]
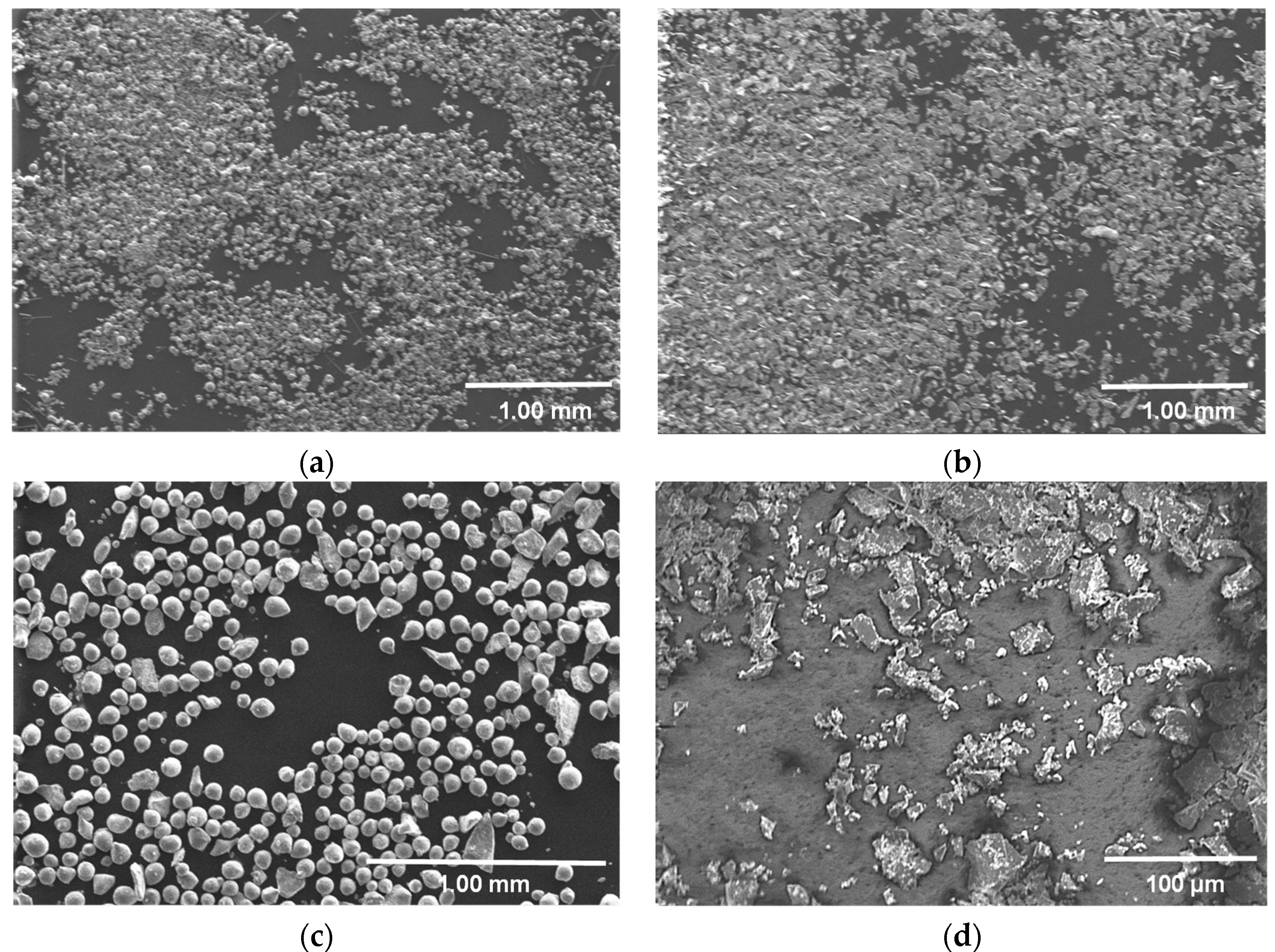
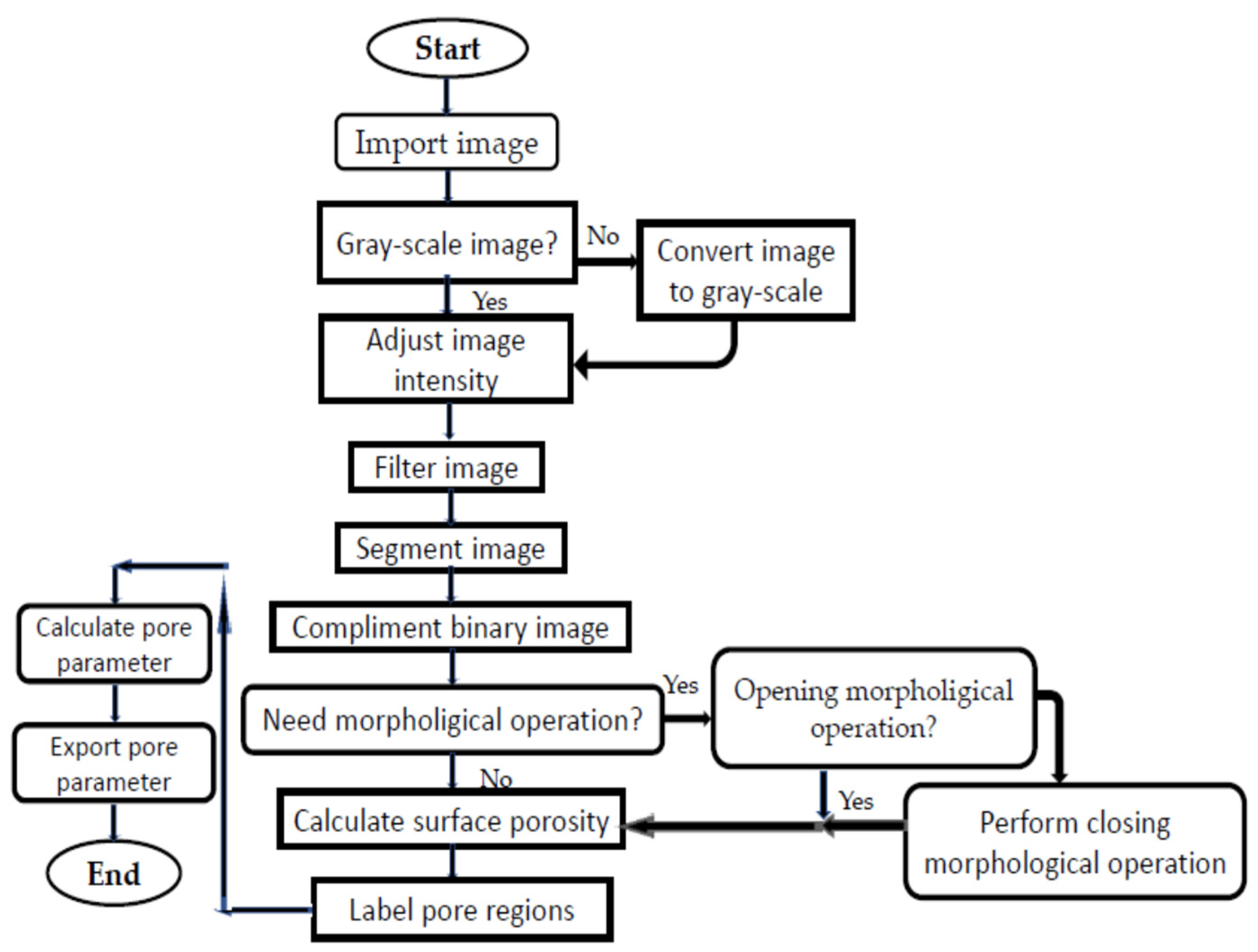
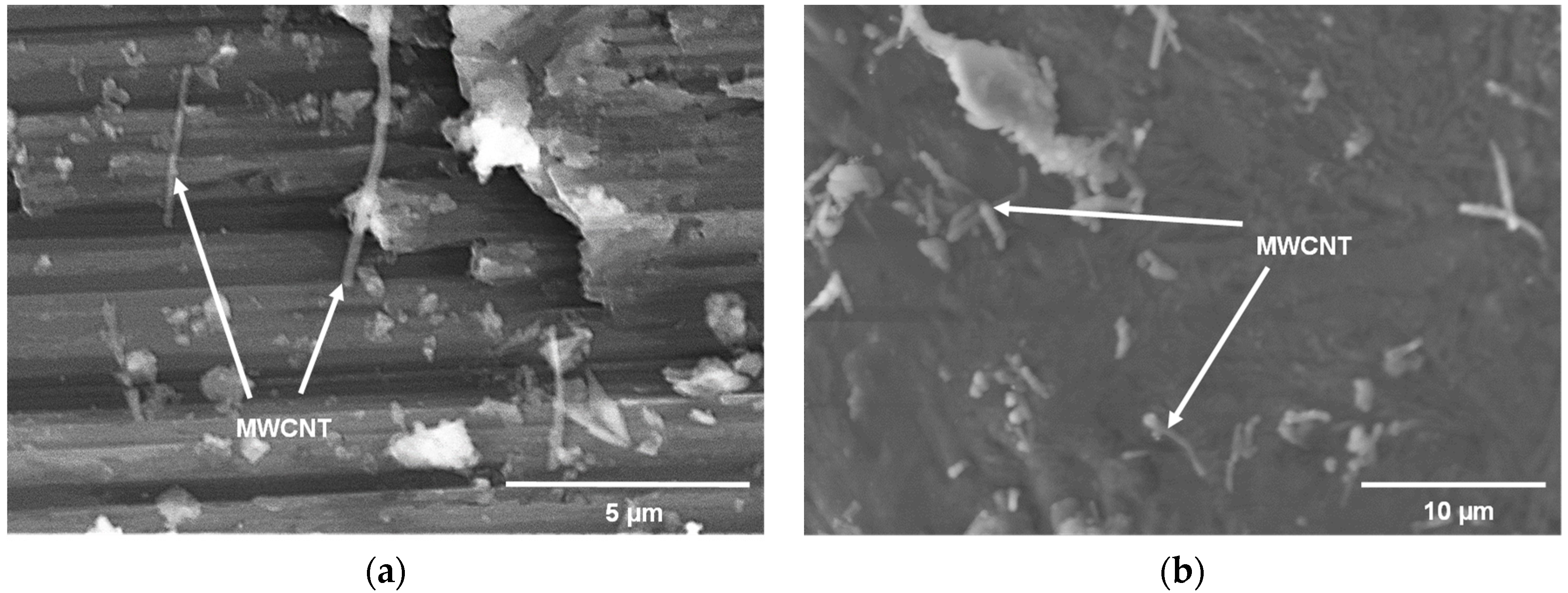

| Powder | Supplier | Purity | Size |
|---|---|---|---|
| Al | TLS Technik (Bitterfeld, Germany) | 99.7 | <150 μm |
| Mg | Laborladen.de (Hüfingen, Germany) | 99.8 | <40 μm |
| Si | - | >99.95 | <40 μm |
| TiH2 | Alfa Aesar | 99 | <45 μm |
| MWCNT | Sigma Aldrich | >90 | 5–9 μm |
| Foam Sample | Time (min) | Density (g/cm3) | Porosity (%) | The Volumetric Expansion Coefficient (1/°C) | Pore Number |
|---|---|---|---|---|---|
| AlMg4Si8 (a) | 8 | 0.5573 | 72.32 | 0.00693 | 2605 |
| MWCNT(Ni)-AlMg4Si8 (b) | 8 | 0.8368 | 67.81 | 0.00415 | 726 |
| MWCNT(Ni)-AlMg4Si8 (c) | 9 | 0.5581 | 78.55 | 0.00623 | 484 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damanik, F.S.; Lange, G. Influence of MWCNT Coated Nickel on the Foaming Behavior of MWCNT Coated Nickel Reinforced AlMg4Si8 Foam by Powder Metallurgy Process. Metals 2020, 10, 955. https://doi.org/10.3390/met10070955
Damanik FS, Lange G. Influence of MWCNT Coated Nickel on the Foaming Behavior of MWCNT Coated Nickel Reinforced AlMg4Si8 Foam by Powder Metallurgy Process. Metals. 2020; 10(7):955. https://doi.org/10.3390/met10070955
Chicago/Turabian StyleDamanik, Ferdinandus Sarjanadi, and Günther Lange. 2020. "Influence of MWCNT Coated Nickel on the Foaming Behavior of MWCNT Coated Nickel Reinforced AlMg4Si8 Foam by Powder Metallurgy Process" Metals 10, no. 7: 955. https://doi.org/10.3390/met10070955
APA StyleDamanik, F. S., & Lange, G. (2020). Influence of MWCNT Coated Nickel on the Foaming Behavior of MWCNT Coated Nickel Reinforced AlMg4Si8 Foam by Powder Metallurgy Process. Metals, 10(7), 955. https://doi.org/10.3390/met10070955





