Preparing Sc-Bearing Master Alloy Using Aluminum–Magnesium Thermoreduction Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, Chemical Reagent and Equipment
2.2. Procedure
3. Results and Discussion
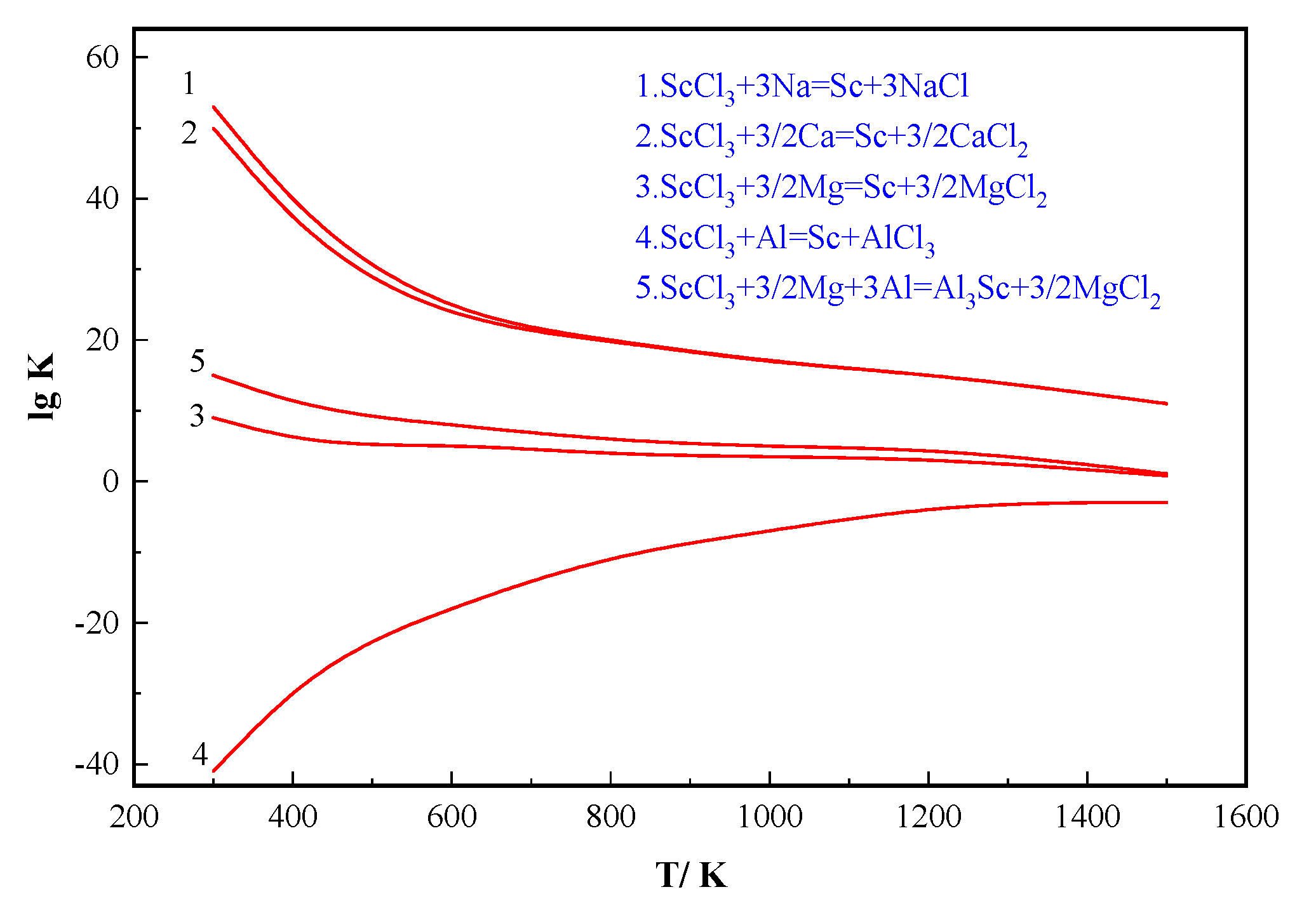
3.1. Thermodynamic Calculation of Reduction Reactions
3.2. Small Test of Preparing Al–Mg–Sc Master Alloy
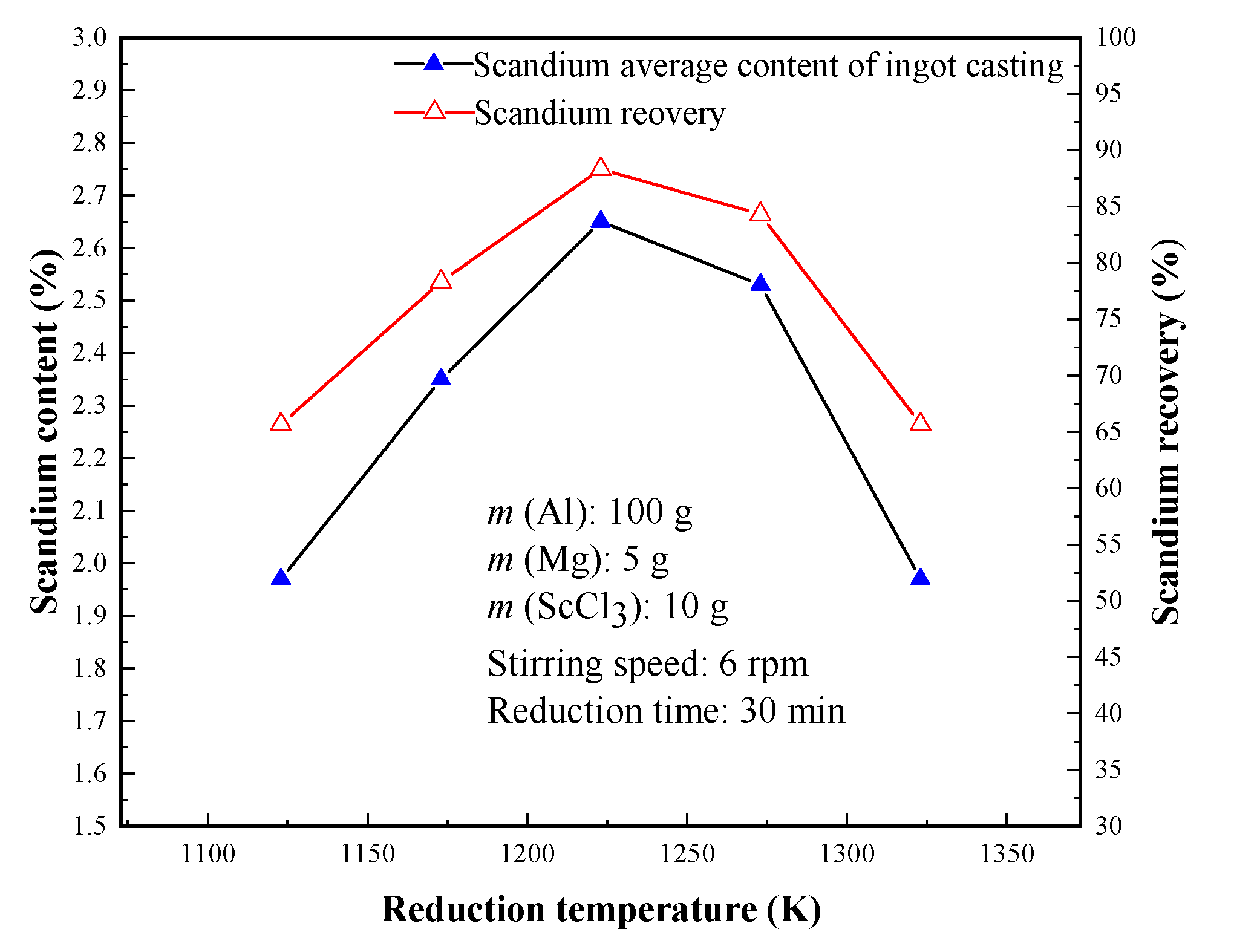
3.2.1. Effect of Reduction Temperature
3.2.2. Effect of ScCl3 Molten Salt Dosage
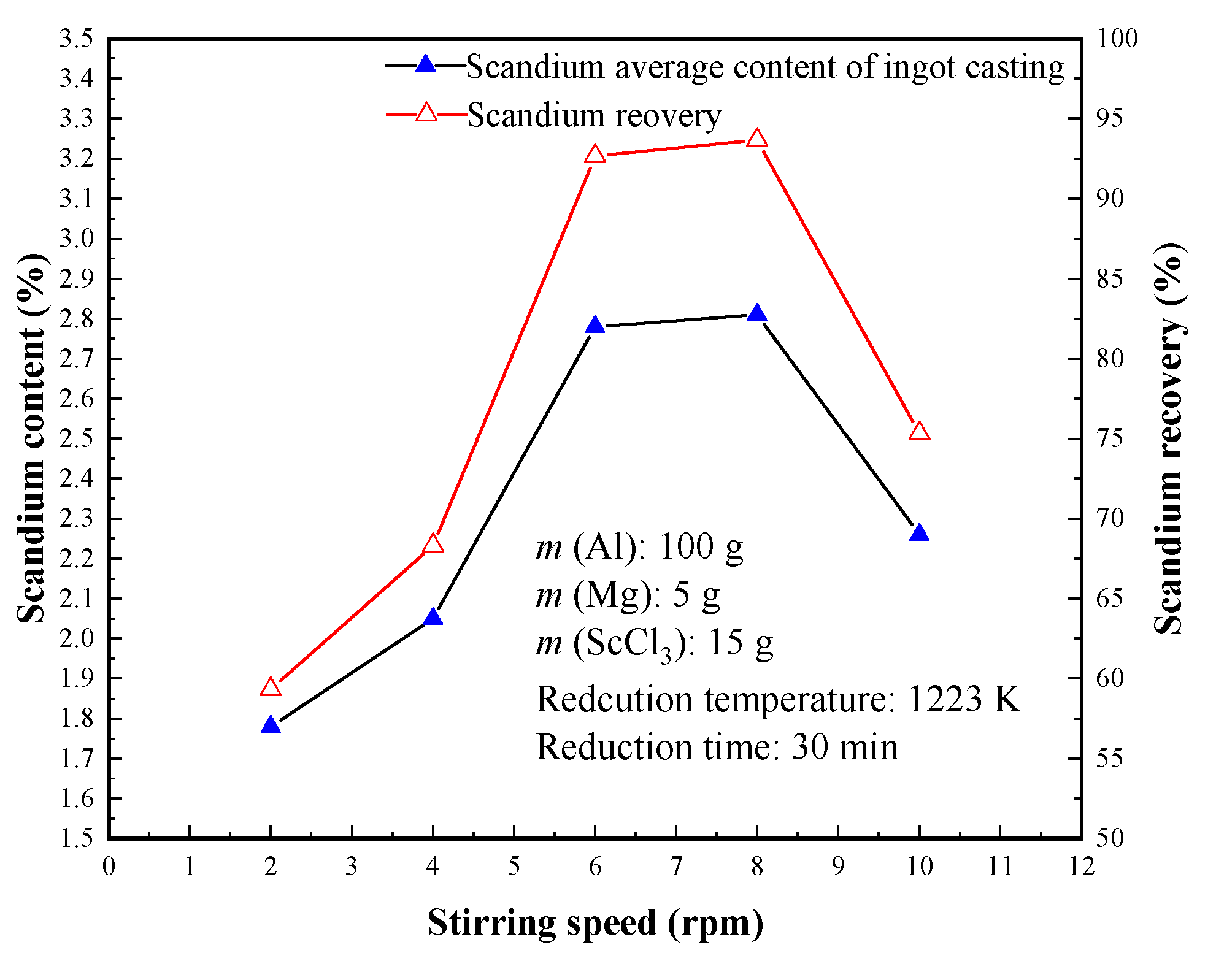
3.2.3. Effect of Stirring Speed (Rotation per Minute)
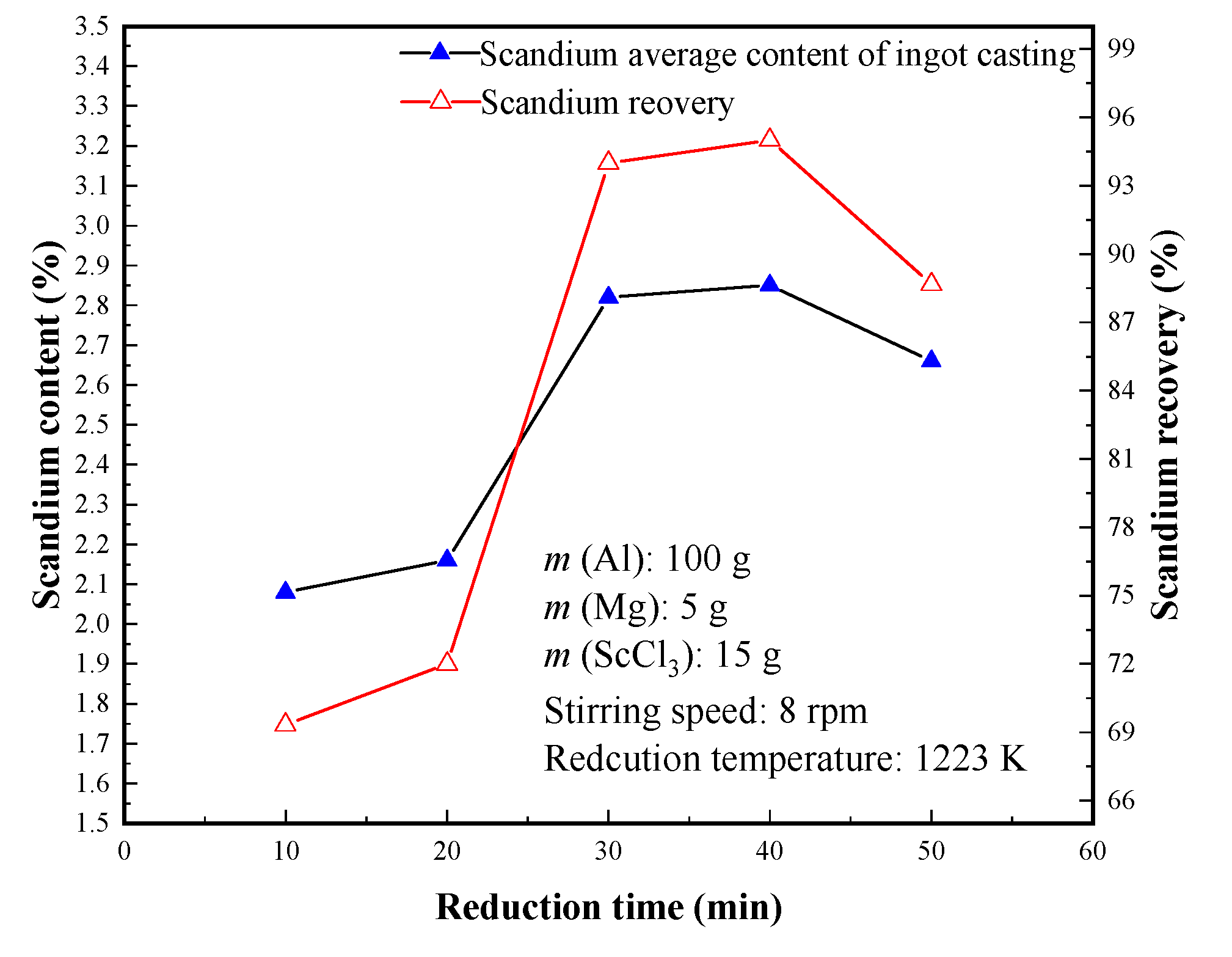
3.2.4. Effect of Reduction Time
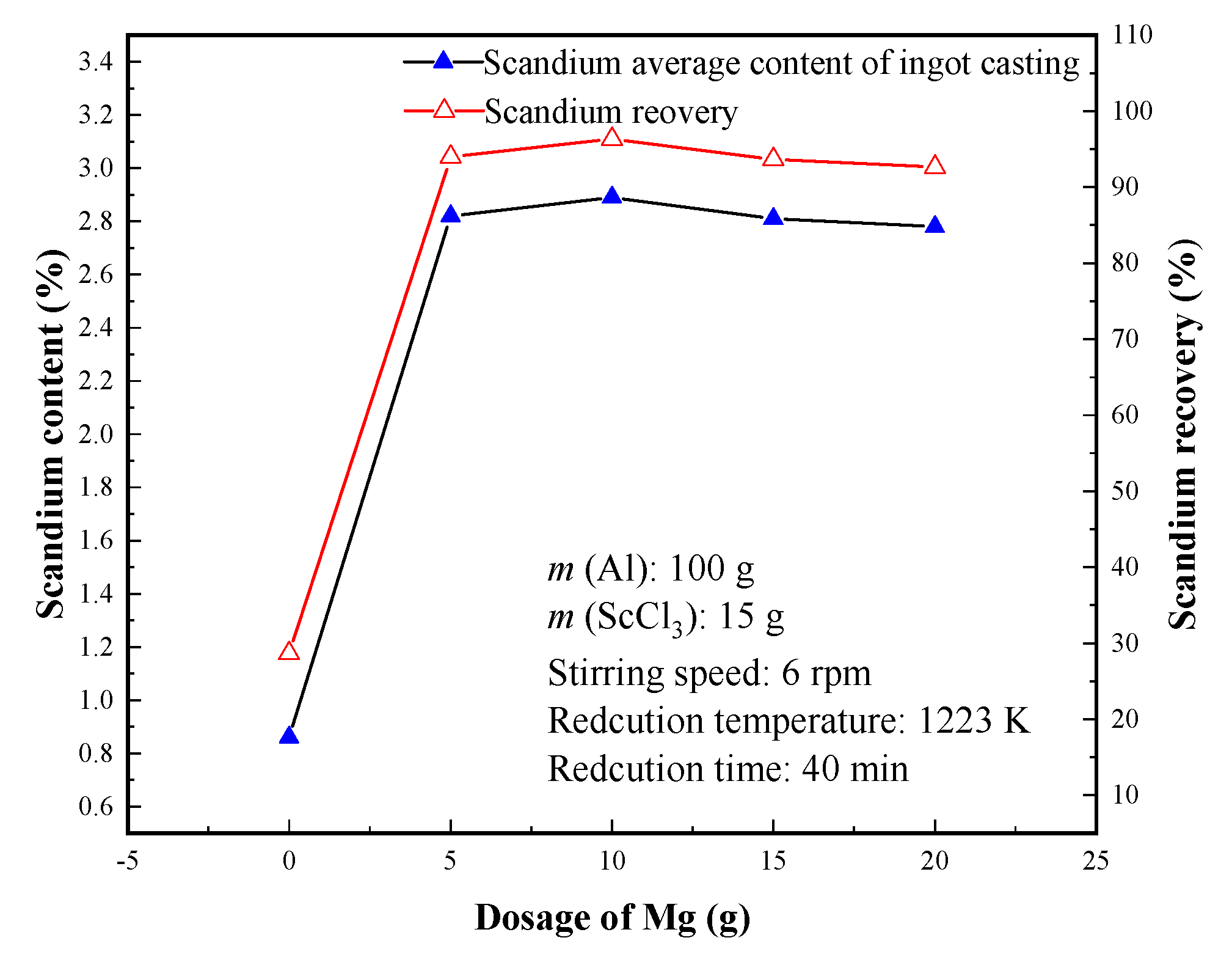
3.2.5. Effect of Mg Dosage
3.2.6. Repeated Test
3.3. Scandium Segregation Analysis of Small Test Ingot Castings
3.4. Scale-Up Test and Scandium Segregation Analysis in Ingot Castings
- (1)
- Stirring by Blowing Argon Method
- (2)
- ScCl3 Molten Salt Bell Jar by Argon Stirring Method
3.5. Middle Test of Preparing Al–Mg–Sc Master Alloy
4. Conclusions
- (1)
- Thermodynamic calculations show that ScCl3 can be reduced by magnesium to scandium metal at 1000–1200 K—and when aluminum exists, the equilibrium concentration of ScCl3 can be greatly reduced to the formation of Al3Sc compound;
- (2)
- The small test results of preparing Sc-bearing master alloy show that Sc-bearing master alloy with scandium content of 2.89%,and scandium recovery of 96.22% was obtained under the technological conditions used: m(Al): m(Mg): m(ScCl3) = 10:1:1.5, stirring speed of eight rpm, reduction temperature of 1223 K, reduction time of 40 min;
- (3)
- Because the process conditions determined by the small-scale test could not meet the requirements of the expanded test, the expanded test optimized the process conditions determined by the small-scale test, changed the stirring mode to argon blowing stirring, changed the molten salt adding mode to bell jar pressing, and finally determined the process conditions for the preparation of aluminum, magnesium and scandium intermediate alloy. The scandium recovery of Al–Mg–Sc master alloy reached to 96.78%, which was basically superior to the index of small scale test;
- (4)
- Under the intermediate alloy, the process conditions were determined, which was carried out among 10 kg level preparation of Al–Mg–Sc master alloy test. The Al–Mg–Sc master alloy ingot contained 2.90% Sc, 5.73% Mg, 0.0058% Cu, 0.29%, 0.029% Ti, 0.13% Fe, 0.075% Zn, 0.025% Na, and 96.72% recovered scandium. The main content of impurity elements are lower and Al–Mg–Sc master alloy indicator was ideal. The scandium recovery approaches to 100%, and the preparation of Sc-bearing master alloy by this method is simple and can reduce the production cost greatly.
Author Contributions
Funding
Conflicts of Interest
References
- Bian, M.Z.; Huang, X.S.; Mabuchi, M.; Chino, Y. Compositional optimization of Mg-Zn-Sc sheet alloys for enhanced room temperature stretch formability. J. Alloys Compd. 2020, 818, 152891. [Google Scholar] [CrossRef]
- Fujii, S.S.; Suzuki, E.C.; Inazu, N.M.; Tsubaki, S.T.; Fukushima, J.; Takizawa, H.S.; Wada, Y.J. Microwave irradiation process for Al-Sc alloy production. Sci. Rep. 2020, 10, 2689. [Google Scholar] [CrossRef] [PubMed]
- Ryabtsev, S.I.; Polonskyy, V.A.; Sukhova, O.V. Effect of scandium on the structure and corrosion properties of vapor-deposited nanostructured quasicrystalline Al-Cu-Fe films. Powder Metall. Met. Ceram. 2020, 58, 567–575. [Google Scholar] [CrossRef]
- Ganiev, I.N.; Norova, M.T.; Eshov, B.B.; Ibrokhimov, N.F.; Ibrokhimov, S.Z. Effect of scandium additions on the temperature dependences of the heat capacity and thermodynamic functions of aluminum-manganese alloys. Phys. Met. Metallogr. 2020, 121, 21–27. [Google Scholar] [CrossRef]
- Coy, M.; Russell, K.; Heidelberger, E.; Sanders, P.; Langan, T. Effect of scandium on cast iron microstructure. Int. J. Metalcast. 2020, 14, 275–277. [Google Scholar] [CrossRef]
- Khrustalyov, A.P.; Kozulin, A.A.; Zhukov, I.A.; Khmeleva, M.G.; Vorozhtsov, A.B.; Eskin, D.; Chankitmunkong, S.; Platov, V.V.; Vasilyev, S.V. Influence of titanium diboride particle size on structure and mechanical properties of an Al-Mg Alloy. Metals 2019, 10, 1030. [Google Scholar] [CrossRef]
- Liu, L.; Jia, Y.Y.; Jiang, J.T.; Zhang, B.; Li, G.A.; Shao, W.Z.; Zhen, L. The effect of Cu and Sc on the localized corrosion resistance of Al-Zn-Mg-X alloys. J. Alloys Compd. 2019, 799, 1–14. [Google Scholar] [CrossRef]
- Guo, Z.C.; Liu, X.; Xue, J.L. Fabrication of Al-Si-Sc alloy bearing AlSi2Sc2 phase using ultrasonically assisted molten salt electrolysis. J. Alloys Compd. 2019, 797, 883–889. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Babaniaris, S.; Langan, T.J. Micro-segregation and precipitates in as-solidified Al-Sc-Zr-(Mg)-(Si)-(Cu) alloys. Mater. Charact. 2019, 154, 353–362. [Google Scholar] [CrossRef]
- Ali, S.R.A.S.; Hussein, A.H.A.; Nofal, A.; Elnaby, S.I.H.; Elgazzar, H. A contribution to laser cladding of Ti-6Al-4V titanium alloy. Metall. Res. Technol. 2019, 116, 634. [Google Scholar]
- Rogachev, S.O.; Naumova, E.A.; Sundeev, R.V.; Tabachkova, N.Y. Structural and phase transformations in a new eutectic Al-Ca-Mn-Fe-Zr-Sc alloy induced by high pressure torsion. Mater. Lett. 2019, 243, 161–164. [Google Scholar] [CrossRef]
- Pasebani, S.; Samimi, P.; Saber, M. Effects of scandium and hafnium solute additions on microstructure thermal stability in nanostructured ferritic alloys. Mater. Charact. 2019, 151, 216–220. [Google Scholar] [CrossRef]
- Garcia-Martino, A.; Prieto, M.M. Practical thermal model for a radiant tubes annealing furnace. Metall. Res. Technol. 2020, 117, 109. [Google Scholar] [CrossRef]
- Liu, X.; Guo, Z.C.; Xue, J.L.; Zhang, C. Effects of synergetic ultrasound on the Sc yield and primary Al3Sc in the Al-Sc alloy prepared by the molten salts electrolysis. Ultrason. Sonochem. 2019, 52, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tocci, M.; Pola, A.; Girelli, L.; Lollio, F.; Montesano, L.; Gelfi, M. Wear and Cavitation Erosion Resistance of an AlMgSc Alloy Produced by DMLS. Metals 2019, 9, 308. [Google Scholar] [CrossRef]
- Yang, Y.; Licavoli, J.J.; Sanders, P.G. Improved strengthening in supersaturated Al-Sc-Zr alloy via melt-spinning and extrusion. J. Alloys Compd. 2020, 826, 154185. [Google Scholar] [CrossRef]
- Xiao, J.H.; Peng, Y.; Ding, W.; Chen, T.; Zou, K.; Wang, Z. Recovering scandium from scandium rough concentrate using roasting-hydrolysis-leaching process. Processes 2020, 8, 365. [Google Scholar] [CrossRef]
- Jiang, F.; Li, H.G.; Yin, Z.M. Thermodynamic calculation on metallic thermoreduction during preparation of aluminum-rare master alloys. Trans. Nonferrous Met. Soc. China. 2001, 11, 18–21. [Google Scholar]
- Shen, M.F. Physical Chemistry of Metallurgy; Higher Education Press: Beijing, China, 2017; pp. 247–254. [Google Scholar]
- Lin, C.Z.; Bai, Z.H.; Zhang, Z.R. Handbook of Thermodynamic Data on Minerals and Related Compounds; Science Press: Beijing, China, 1985; pp. 149–166. [Google Scholar]
- Aleksandrovskii, S.V. Study on thermoanalysis process of scandium halids. J. Appl. Chem. 1997, 70, 1761–1766. [Google Scholar]
- Xiao, J.H.; Ding, W.; Peng, Y.; Chen, T.; Zou, K.; Wang, Z. Extraction of nickel from garnierite laterite ore using roasting and magnetic separation with calcium chloride and iron concentrate. Minerals 2020, 10, 352. [Google Scholar] [CrossRef]
- Xiao, J.H.; Zou, K.; Ding, W.; Peng, Y.; Chen, T. Extraction of lead and zinc from a rotary kiln oxidizing roasting cinder. Metals 2020, 10, 465. [Google Scholar] [CrossRef]
- Fatimah, S.; Yoon, D.K.; Ko, Y.G. Role of V2O5 particles on the microstructures and corrosion behavior of Al-Mg-Si alloy via plasma electrolysis. J. Mater. Process. Technol. 2020, 284, 116757. [Google Scholar] [CrossRef]


| Raw Materials | Composition (%) | ||||
|---|---|---|---|---|---|
| Fe | Si | Ca | P | Ti | |
| Aluminum ingot | 0.126 | 0.684 | 0.045 | 0.001 | 0.059 |
| Magnesium ingot | 0.062 | 0.027 | 0.019 | 0.001 | 0.068 |
| Scandium chloride | 0.015 | 0.062 | 0.034 | 0.003 | 0.012 |
| Slag removal agent | 0.033 | 0.044 | 0.018 | 0.004 | 0.031 |
| Refining agent | 0.061 | 0.145 | 0.017 | 0.003 | 0.049 |
| Covering agent | 0.038 | 0.275 | 0.021 | 0.002 | 0.026 |
| Repeat | Average scandium Content in Ingot Casting (g) | Scandium Recovery (%) |
|---|---|---|
| 1 | 2.86 | 95.33 |
| 2 | 2.84 | 94.66 |
| 3 | 2.93 | 97.66 |
| 4 | 2.92 | 97.33 |
| 5 | 2.89 | 96.33 |
| 6 | 2.88 | 96.00 |
| Average | 2.89 | 96.22 |
| Range () | 0.09 | 3.00 |
| Arithmetic mean error () | 0.027 | 0.89 |
| Sum square variation () | 0.006 | 6.59 |
| Average deviation () | 0.001 | 1.32 |
| Standard deviation () | 0.032 | 1.15 |
| Number | Scandium Content (%) | Average Value between A and B | Scandium Recovery (%) | ||
|---|---|---|---|---|---|
| Top (A) | Lower (B) | Middle (C) | |||
| 1 | 2.56 | 4.26 | 2.68 | 2.27 | 98.08 |
| 2 | 2.27 | 3.75 | 2.76 | 2.46 | 93.67 |
| 3 | 2.31 | 3.89 | 2.61 | 2.51 | 94.33 |
| 4 | 1.96 | 4.05 | 2.85 | 2.71 | 96.42 |
| 5 | 2.11 | 4.23 | 2.56 | 2.19 | 92.42 |
| Average | 2.24 | 4.04 | 2.69 | 2.43 | 94.98 |
| Number | Scandium Content (%) | Scandium Recovery (%) | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | Average between A and B | ||
| 1 | 1.76 | 2.06 | 2.53 | 2.69 | 2.69 | 1.91 | 78.20 |
| 2 | 1.66 | 2.19 | 2.66 | 2.77 | 2.69 | 1.93 | 79.80 |
| 3 | 1.57 | 2.25 | 2.47 | 2.88 | 2.68 | 1.91 | 79.00 |
| 4 | 1.97 | 2.06 | 2.36 | 2.54 | 2.33 | 2.02 | 75.07 |
| Average | 1.27 | 2.24 | 2.51 | 2.77 | 2.57 | 1.76 | 75.73 |
| Number | Scandium Content (%) | Scandium Recovery (%) | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | Average between A and B | ||
| 1 | 1.94 | 3.03 | 2.66 | 2.52 | 2.74 | 2.49 | 85.93 |
| 2 | 1.89 | 3.17 | 2.62 | 2.79 | 2.81 | 2.53 | 88.53 |
| 3 | 1.92 | 3.26 | 2.57 | 2.78 | 2.82 | 2.59 | 89.00 |
| 4 | 1.87 | 2.98 | 2.66 | 2.59 | 2.79 | 2.43 | 85.93 |
| Average | 1.91 | 3.11 | 2.63 | 2.67 | 2.79 | 2.51 | 85.93 |
| Number | Scandium Content (%) | Scandium Recovery (%) | |||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | Average between A and B | ||
| 1 | 2.76 | 3.26 | 2.93 | 2.79 | 2.89 | 3.01 | 97.53 |
| 2 | 2.66 | 3.12 | 2.86 | 2.87 | 2.96 | 2.89 | 96.47 |
| 3 | 2.57 | 3.15 | 2.89 | 2.92 | 2.92 | 2.86 | 96.33 |
| 4 | 2.68 | 3.26 | 2.66 | 2.98 | 2.94 | 2.97 | 96.80 |
| Average | 2.67 | 3.20 | 2.84 | 2.89 | 2.93 | 2.93 | 96.78 |
| Alloy Ingots | Position | Content (%) | Scandium Recovery (%) | |
|---|---|---|---|---|
| Sc | Mg | |||
| First | Top | 2.68 | 6.12 | 97.17 |
| Lower | 3.15 | 5.44 | ||
| Second | Top | 2.37 | 6.25 | 96.33 |
| Lower | 3.41 | 5.26 | ||
| Third | Top | 2.51 | 4.96 | 96.67 |
| Lower | 3.29 | 6.33 | ||
| Average | 2.90 | 5.73 | 96.72 | |
| Alloy Ingots | Position | Content (%) | |||||
|---|---|---|---|---|---|---|---|
| Cu | Si | Ti | Fe | Zn | Na | ||
| First | Top | 0.0052 | 0.25 | 0.033 | 0.14 | 0.0067 | 0.0027 |
| Lower | 0.0061 | 0.29 | 0.025 | 0.13 | 0.0079 | 0.0019 | |
| Second | Top | 0.0054 | 0.31 | 0.028 | 0.15 | 0.0088 | 0.0022 |
| Lower | 0.0059 | 0.28 | 0.027 | 0.12 | 0.0076 | 0.0029 | |
| Third | Top | 0.0058 | 0.32 | 0.031 | 0.11 | 0.0069 | 0.0025 |
| Lower | 0.0063 | 0.28 | 0.029 | 0.15 | 0.0072 | 0.0026 | |
| Average | 0.0058 | 0.29 | 0.029 | 0.13 | 0.0075 | 0.0025 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Ding, W.; Peng, Y.; Chen, T.; Zou, K. Preparing Sc-Bearing Master Alloy Using Aluminum–Magnesium Thermoreduction Method. Metals 2020, 10, 960. https://doi.org/10.3390/met10070960
Xiao J, Ding W, Peng Y, Chen T, Zou K. Preparing Sc-Bearing Master Alloy Using Aluminum–Magnesium Thermoreduction Method. Metals. 2020; 10(7):960. https://doi.org/10.3390/met10070960
Chicago/Turabian StyleXiao, Junhui, Wei Ding, Yang Peng, Tao Chen, and Kai Zou. 2020. "Preparing Sc-Bearing Master Alloy Using Aluminum–Magnesium Thermoreduction Method" Metals 10, no. 7: 960. https://doi.org/10.3390/met10070960
APA StyleXiao, J., Ding, W., Peng, Y., Chen, T., & Zou, K. (2020). Preparing Sc-Bearing Master Alloy Using Aluminum–Magnesium Thermoreduction Method. Metals, 10(7), 960. https://doi.org/10.3390/met10070960






