Fatigue Corrosion Behavior of Friction Welded Dissimilar Joints in Different Testing Conditions
Abstract
:1. Introduction
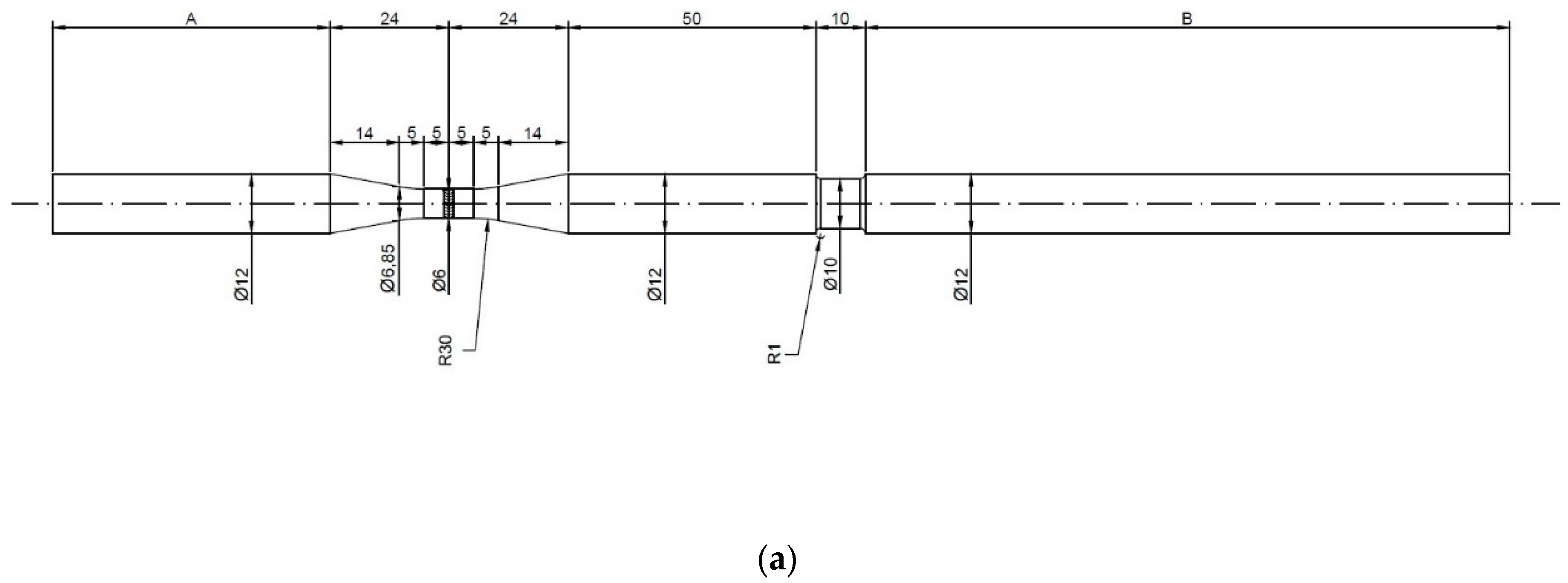
2. Materials and Methods
3. Results and Discussion
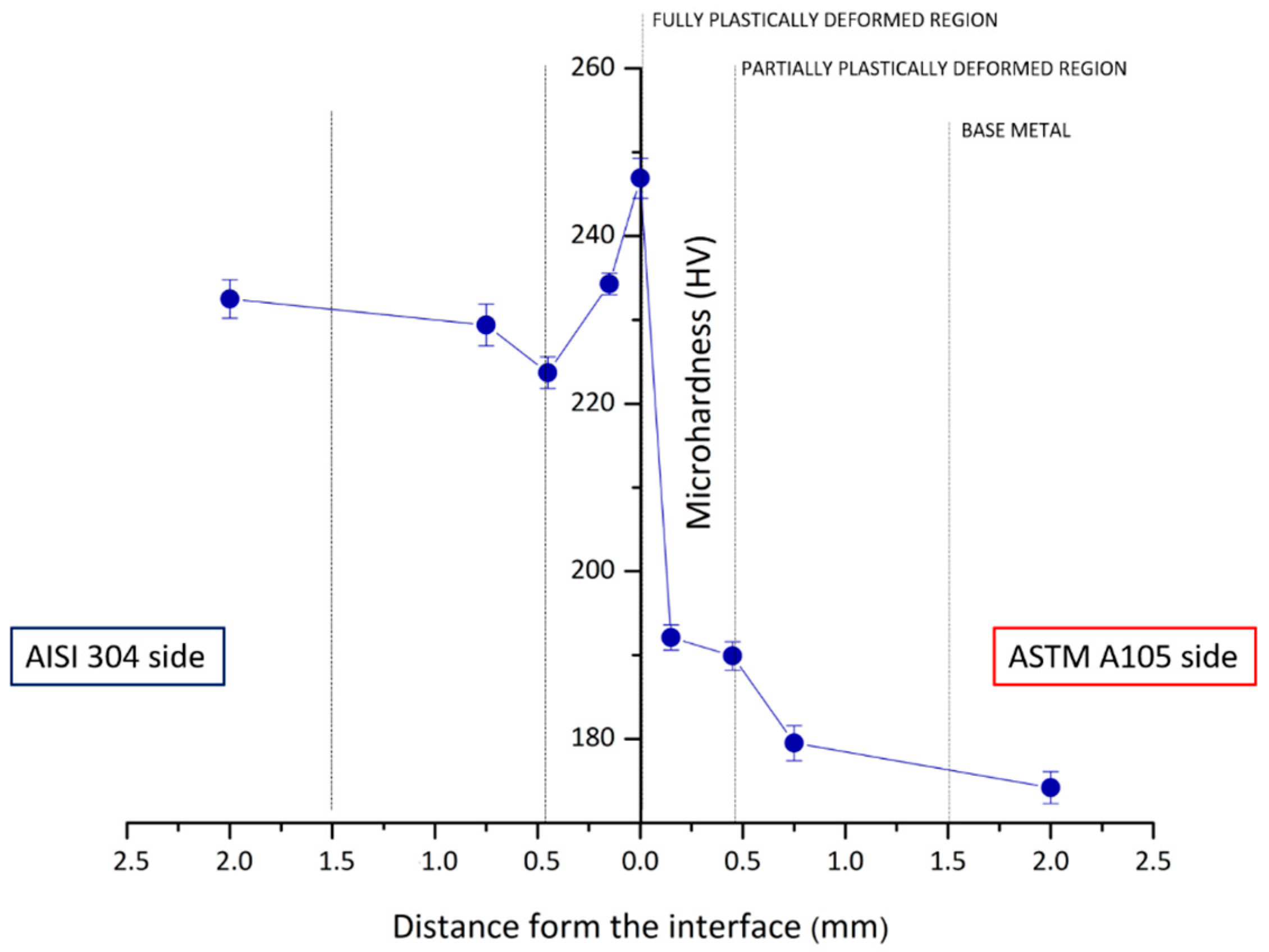
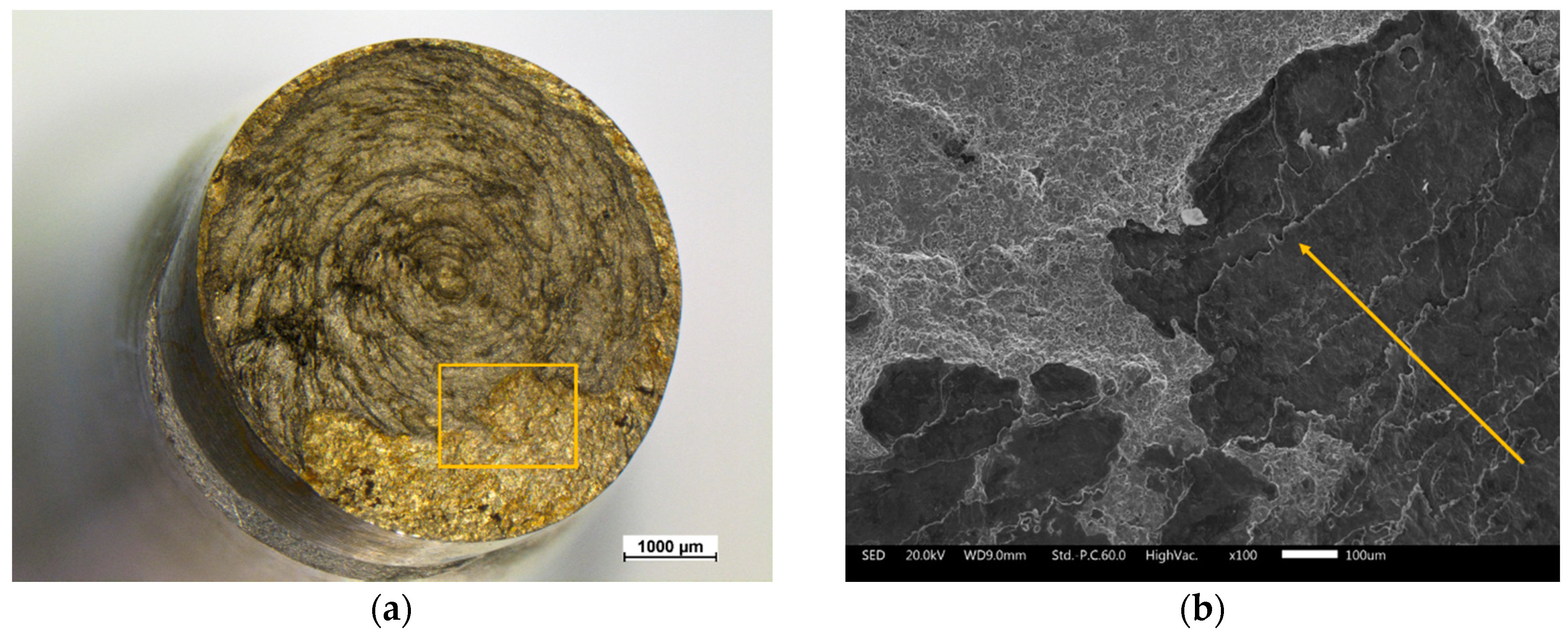
3.1. Morphological and Metallographic Analysis
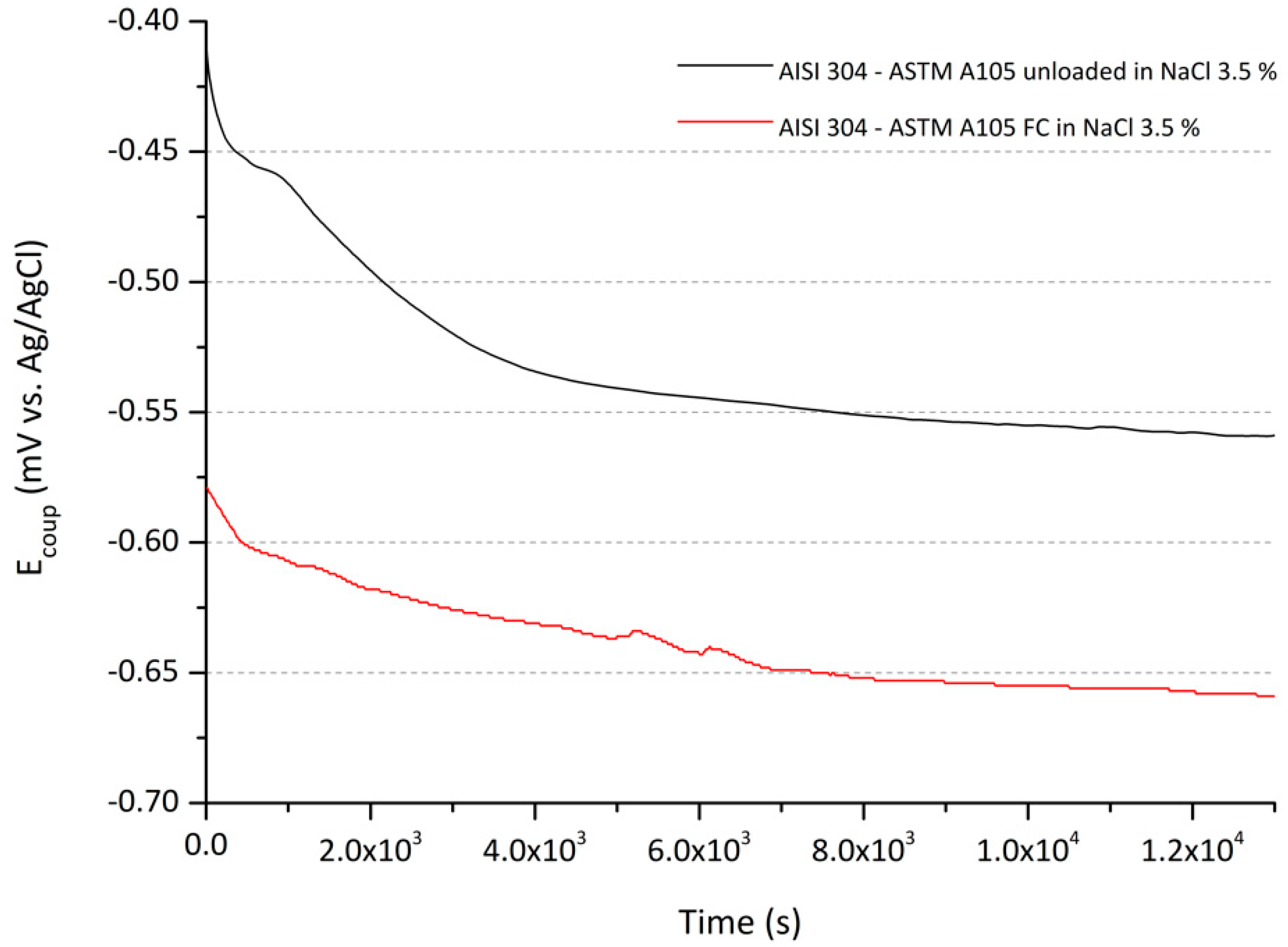
3.2. Electrochemical Analysis
3.3. Fatigue Testing
Electrochemical Characterization during Fatigue Experiments
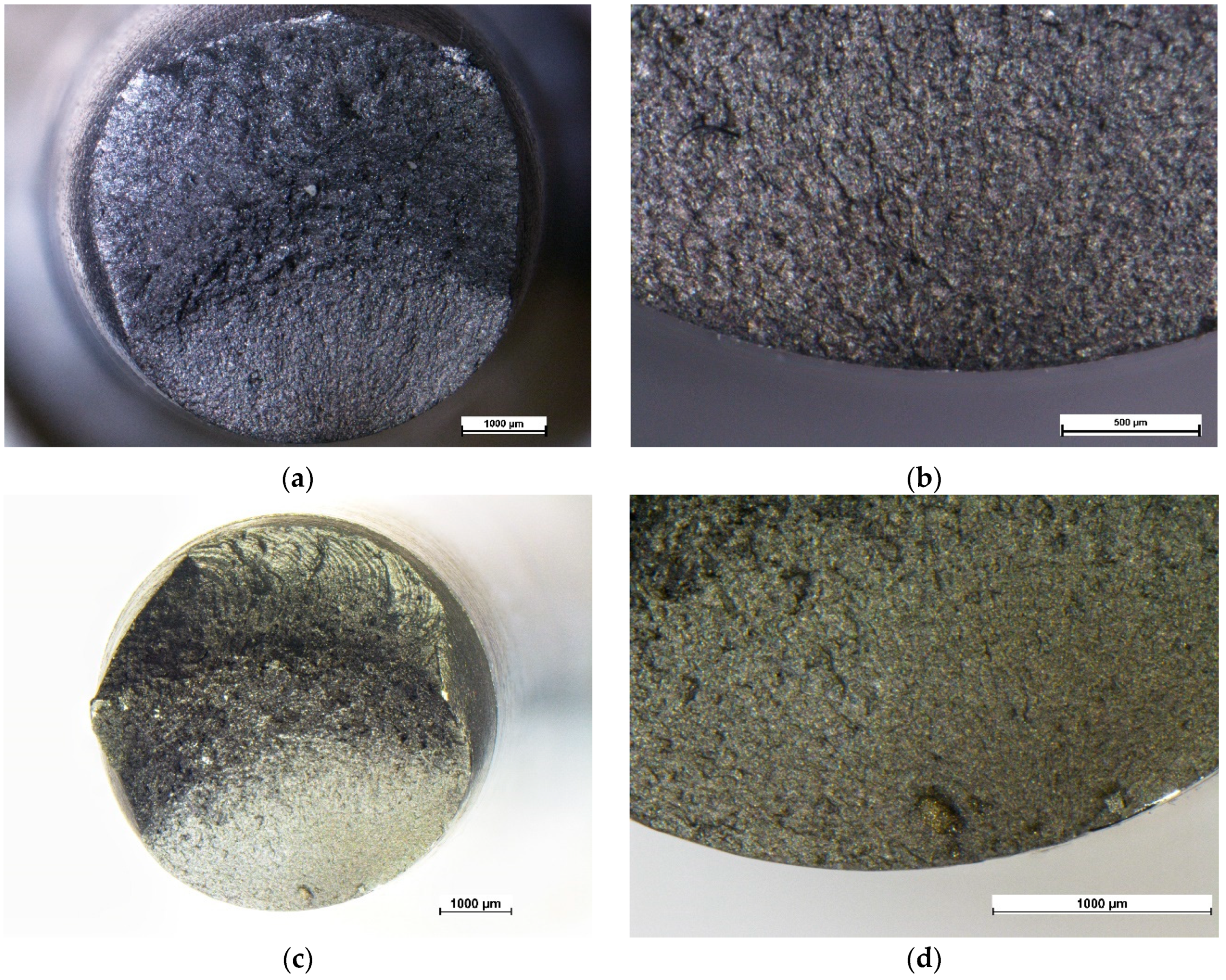
3.4. Failure Analysis
3.4.1. Fatigue in Air
3.4.2. Fatigue Corrosion
3.4.3. Fatigue Corrosion under Cathodic Protection
3.4.4. Accelerated Fatigue Corrosion
4. Conclusions
- The home-built setup proved to be effective to be adapted to the study of fatigue corrosion behavior of the samples under investigation, with the possibility of monitoring the progression of the tests by electrochemical measurements.
- The tests highlighted an evident coupling between environmental and mechanical testing conditions by the local electrochemical environment. The cathodic protection of the samples is effective in improving their fatigue behavior, in particular at low stress values. On the contrary, fatigue corrosion or even anodic activation worsen the behavior of the samples.
- The failure analysis of the samples tested in different conditions showed that in all cases their failure occurs near the welded interface, in the plastically deformed regions and not in the parent metal. A significant difference was observed among the fracture surface produced by different testing conditions thus confirming that the different severity of the test conditions may change the local damage mechanisms.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sakundarini, N.; Taha, Z.; Abdul-Rashid, S.H.; Ghazila, R.A.R. Optimal Multi-material Selection for Lightweight Design of Automotive Body Assembly Incorporating Recyclability. Mater. Des. 2013, 50, 846–857. [Google Scholar] [CrossRef]
- Varelis, G.E.; Papatheocharis, T.; Karamanos, S.A.; Perdikaris, P.C. Structural behavior and design of high-strength steel welded tubular connections under extreme loading. Mar. Struct. 2020, 71, 102701. [Google Scholar] [CrossRef]
- Wirsching, P.H. Fatigue reliability in welded joints of offshore structures. Int. J. Fatigue 1980, 2, 77–83. [Google Scholar] [CrossRef]
- Fan, Y.; Jiang, D.; Mo, D.; Zhu, D.; Luo, Z. A review on dissimilar metals’ welding methods and mechanisms with interlayer. Int. J. Adv. Manuf. Technol. 2019, 102, 2845–2863. [Google Scholar]
- Uday, M.B.; Ahmad Fauzi, M.N.; Zuhailawati, H.; Ismail, A.B. Advances in friction welding process: A review. Sci. Technol. Weld. Join. 2010, 15, 534–558. [Google Scholar] [CrossRef]
- Martinsen, K.; Hu, S.J.; Carlson, B.E. Joining of dissimilar materials. CIRP Ann. Manuf. Technol. 2015, 64, 679–699. [Google Scholar] [CrossRef] [Green Version]
- Cai, W.; Daehn, G.; Li, G.; Mishra, R.; Vivek, A.; Khan, H.; Komarasamy, M. A state-of-the-art review on solid state metal joining. J. Manuf. Sci. Eng. 2019, 141, 031012. [Google Scholar] [CrossRef]
- Wenya, L.; Vairis, A.; Preuss, M.; Tiejun, M. Linear and rotary friction welding review. Int. Mater. Rev. 2015, 61, 71–100. [Google Scholar]
- Vairis, A.; Papazafeiropoulos, G.; Tsainis, A. A comparison between friction stir welding, linear friction welding and rotary friction welding. Adv. Manuf. 2016, 4, 296–304. [Google Scholar] [CrossRef]
- Klomp, J.T.; De With, G. Strong metal-ceramic joints. Mater. Manuf. Process. 1993, 8, 129–157. [Google Scholar] [CrossRef]
- Meshram, S.D.; Mohandas, T.; Reddy, G.M. Friction welding of dissimilar pure metals. J. Mater. 2007, 184, 330–337. [Google Scholar] [CrossRef]
- Rupinder, S.; Ranvijay, K.; Feo, L.; Fraternali, F. Friction welding of dissimilar plastic/polymer materials with metal powder reinforcement for engineering applications. Compos. Part B Eng. 2016, 101, 77–86. [Google Scholar]
- Arivazhagan, N.; Surendra, S.; Prakash, S.; Reddy, G.M. Investigation on AISI 304 austenitic stainless steel to AISI 4140 low alloy steel dissimilar joints by gas tungsten arc, electron beam and friction welding. Mater. Des. 2011, 32, 3036–3050. [Google Scholar] [CrossRef]
- Carlone, P.; Astarita, A. Dissimilar metal welding. Metals 2019, 9, 1206. [Google Scholar] [CrossRef] [Green Version]
- Sathiya, P.; Aravindan, S.; Noorul Haq, A.; Paneerselvam, K. Optimization of friction welding parameters using evolutionary computational techniques. J. Mater. 2009, 209, 2576–2584. [Google Scholar] [CrossRef]
- Nascimento, M.P.; Souza, R.C.; Pigatin, W.L.; Voorwald, J.H.C. Effects of surface treatments on the fatigue strength of AISI 4340 aeronautical steel. Int. J. Fatigue 2001, 23, 607–618. [Google Scholar] [CrossRef]
- Shushan, S.M.; Charles, E.A.; Congleton, J. The environment assisted cracking of diffusion bonded stinless steel to carbon steel joints in an aqueous chloride solution. Corros. Sci. 1996, 38, 673–686. [Google Scholar] [CrossRef]
- Sarkari Khorrami, M.; Mostafaei, M.A.; Pouraliakbar, H.; Kokabi, A.H. Study on microstructure and mechanical characteristics of low-carbon steel and ferritic stainless steel joints. Mater. Sci. Eng. A 2014, 608, 35–45. [Google Scholar] [CrossRef]
- Prasad, K.S.; Rao, C.S.; Rao, D.N. A review on welding of AISI 304L austenitic stainless steel. J. Manuf. Sci. Prod. 2012, 14, 1–11. [Google Scholar] [CrossRef]
- Maddox, S.J. Fatigue behaviour of welded joints. In Advances in Fatigue Science and Technology; Branco, C.M., Rosa, L.G., Eds.; Springer: Dordrecht, The Netherlands, 1989; Volume 159. [Google Scholar]
- Akiniwa, Y.; Miyamoto, N.; Tsuru, H.; Tanaka, K. Notch effect on fatigue strength reduction of bearing steel in the very high cycle regime. Int. J. Fatigue 2006, 28, 1555–1565. [Google Scholar] [CrossRef]
- Casavola, C.; Giordano, V.; Pappalettere, P.; Pruncu, C.I. Influence of geometrical shape of specimen in fatigue life characterization on welded joint in titanium alloy. In Proceedings of the Twelfth Meeting New Trends in Fatigue and Fracture, Brasov, Romania, 27–30 May 2012. [Google Scholar]
- Alam, M.M.; Barsoum, Z.; Jonsén, P.; Kaplan, A.F.H.; Häggblad, H.Å. The influence of surface geometry and topography on the fatigue cracking behaviour of laser hybrid welded eccentric fillet joints. Appl. Surf. Sci. 2010, 256, 1936–1945. [Google Scholar] [CrossRef]
- Bertini, L.; Fontanari, V.; Straffelini, G. Influence of post weld treatments on the fatigue behaviour of Al-alloy welded joints. Int. J. Fatigue 1998, 20, 749–755. [Google Scholar] [CrossRef]
- Sabzi, M.; Dezfuli, S.M. Post weld heat treatment of hypereutectoid hadfield steel: Characterization and control of microstructure, phase equilibrium, mechanical properties and fracture mode of welding joint. J. Manuf. Process. 2018, 34, 313–328. [Google Scholar] [CrossRef]
- Revie, R.W. Environmental cracking of metals. In Modern Aspects of Electrochemistry; Conway, B.E., Bockris, J.O., Eds.; Springer: Boston, MA, USA, 1994; Volume 26. [Google Scholar]
- Andersen, P.L. ASM Handbook—Vol. 19. Corrosion Fatigue Testing; ASM International: Geauga County, OH, USA, 1998. [Google Scholar]
- Schijve, J. Fatigue of Structures and Materials; Springer: Dordrecht, The Netherlands, 2009; pp. 457–479. [Google Scholar]
- Andersen, P.L.; Jones, R.H. Stress Corrosion Cracking—Material Performance and Evaluation; ASM International: Geauga County, OH, USA, 1992. [Google Scholar]
- Sieradzki, K.; Newman, R.C. Stress-corrosion cracking. J. Phys. Chem. Solids 1987, 48, 1101–1113. [Google Scholar] [CrossRef]
- ASTM International. ASTM A276 Standard, Standard Specification for Stainless Steel Bars and Shapes; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM International. ASTM A105 Standard, Standard Specification for Carbon Steel Forgings for Piping Applications; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- UNI Ente Nazionale Italiano di Unificazione. UNI EN ISO 11782-1 Standard, Corrosion of Metals and Alloys—Corrosion Fatigue Testing Cycles to Failure Testing; UNI Ente Nazionale Italiano di Unificazione: Milano, Italy, 2008. [Google Scholar]
- Fontana, M.G. Corrosion Engineering; Tata McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- ASTM International. ASTM G8-96 Standard, Standard Test Methods for Cathodic Disbonding of Pipeline Coatings; ASTM International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- Ananthapadmanaban, D.; Seshagiri Rao, V.; Abraham, N.; Prasad Ra, K. A study of mechanical properties of friction welded mild steel to stainless steel joints. Mater. Des. 2009, 30, 2642–2646. [Google Scholar] [CrossRef]
- Özdemir, N. Investigation of the mechanical properties of friction-welded joints between AISI 304L and AISI 4340 steel as a function rotational speed. Mater. Lett. 2005, 59, 2504–2509. [Google Scholar] [CrossRef]
- Sahin, M. Joining with friction welding of high-speed steel and medium-carbon steel. J. Mater. Process. Technol. 2005, 168, 202–210. [Google Scholar] [CrossRef]
- Yoon, H.-K.; Kong, Y.-S.; Kim, S.-J.; Kohyama, A. Mechanical properties of friction welds of RAFs (JLF-1) to SUS304 steels as measured by the acoustic emission technique. Fusion Eng. Des. 2006, 81, 945–950. [Google Scholar] [CrossRef]
- Rowlands, D.P. The Mechanical Properties of Stainless Steel. In Sassda Stainless Steel Information Series No. 3; Southern Africa Stainless Steel Development Association: Sandton, South Africa, 2006. [Google Scholar]
- MT Acciai Speciali. Acciai al Carbonio. Available online: http://www.mtacciai.com/it/acciai/al-carbonio/astm-a105 (accessed on 21 July 2020).
- Sachs, N.W. Understanding the Surface Features of Fatigue Fractures: How They Describe the Failure Cause and the Failure History. J. Fail. Anal. Prev. 2005, 2, 11–15. [Google Scholar] [CrossRef]








| Alloying Element | AISI 304 | ASTM A105 2 |
|---|---|---|
| C | 0.08 1 | 0.35 1 |
| Mn | 2.00 1 | 0.60–1.05 |
| P | 0.045 1 | 0.035 1 |
| S | 0.03 1 | 0.040 1 |
| Si | 1.00 1 | 0.10–0.35 |
| Cr | 18.00–20.00 | 0.30 1 |
| Ni | 8.00–10.50 | 0.40 1 |
| Mo | - | 0.12 1 |
| Cu | - | 0.40 1 |
| V | - | 0.08 1 |
| Fe | Bal. | Bal. |
| Test Name | Description | Solution | Potential |
|---|---|---|---|
| F | Fatigue in air | N/A | N/A |
| FC | Fatigue corrosion | 3.5 wt% NaCl | N/A |
| FC-P | Protected fatigue corrosion | 3.5 wt% NaCl | −1.3 V (vs. Ag/AgCl) |
| FC-A | Accelerated fatigue corrosion | 3.5 wt% NaCl | +0.4 V (vs. Ag/AgCl) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, S.; Russo, F.; Lemmi, A.M.; Benedetti, M.; Fontanari, V. Fatigue Corrosion Behavior of Friction Welded Dissimilar Joints in Different Testing Conditions. Metals 2020, 10, 1018. https://doi.org/10.3390/met10081018
Rossi S, Russo F, Lemmi AM, Benedetti M, Fontanari V. Fatigue Corrosion Behavior of Friction Welded Dissimilar Joints in Different Testing Conditions. Metals. 2020; 10(8):1018. https://doi.org/10.3390/met10081018
Chicago/Turabian StyleRossi, Stefano, Francesca Russo, Alberto Maria Lemmi, Matteo Benedetti, and Viglio Fontanari. 2020. "Fatigue Corrosion Behavior of Friction Welded Dissimilar Joints in Different Testing Conditions" Metals 10, no. 8: 1018. https://doi.org/10.3390/met10081018
APA StyleRossi, S., Russo, F., Lemmi, A. M., Benedetti, M., & Fontanari, V. (2020). Fatigue Corrosion Behavior of Friction Welded Dissimilar Joints in Different Testing Conditions. Metals, 10(8), 1018. https://doi.org/10.3390/met10081018








