Removal of Metallic Iron from Reduced Ilmenite by Aeration Leaching
Abstract
:1. Introduction
2. Materials and Methods
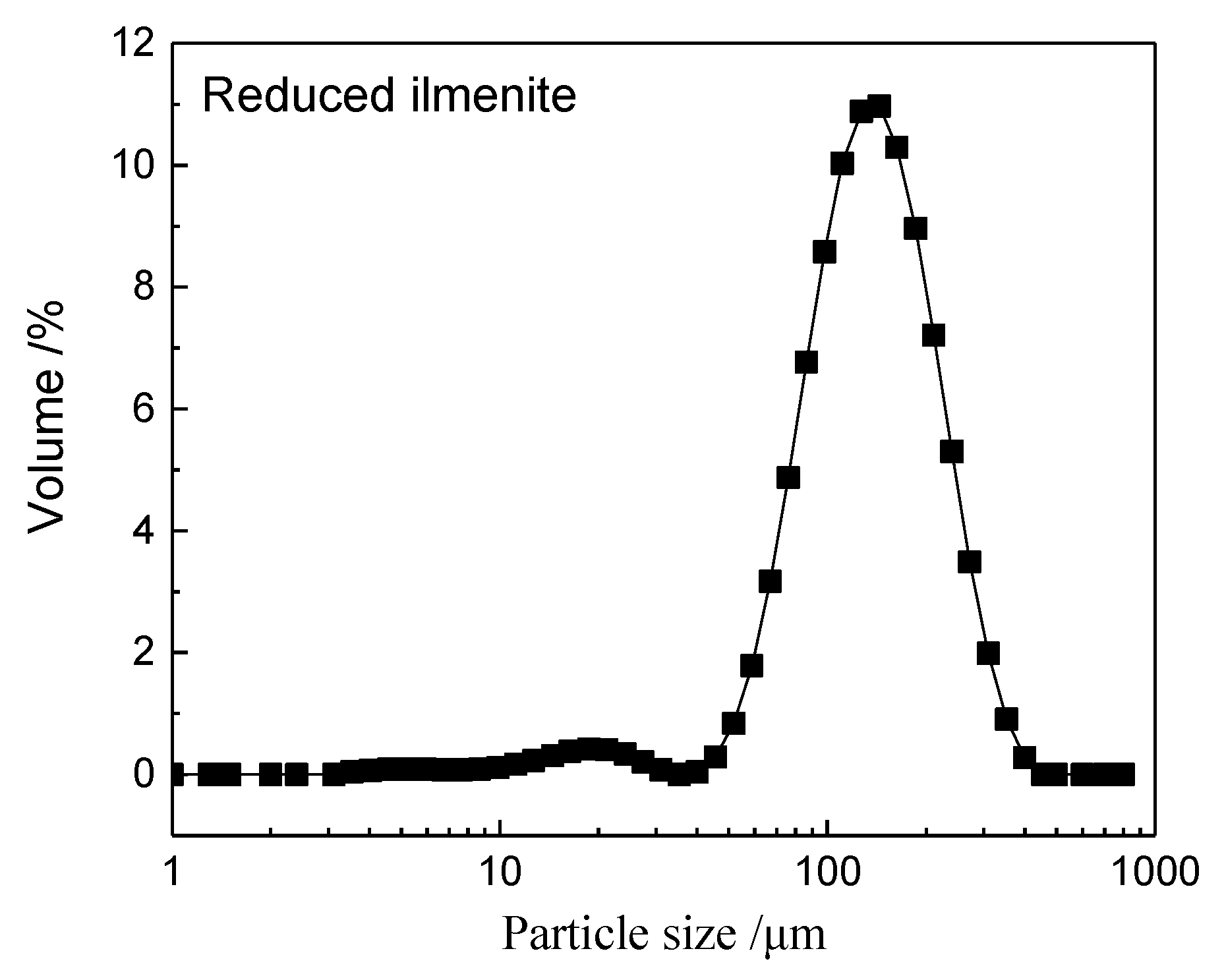
2.1. Materials
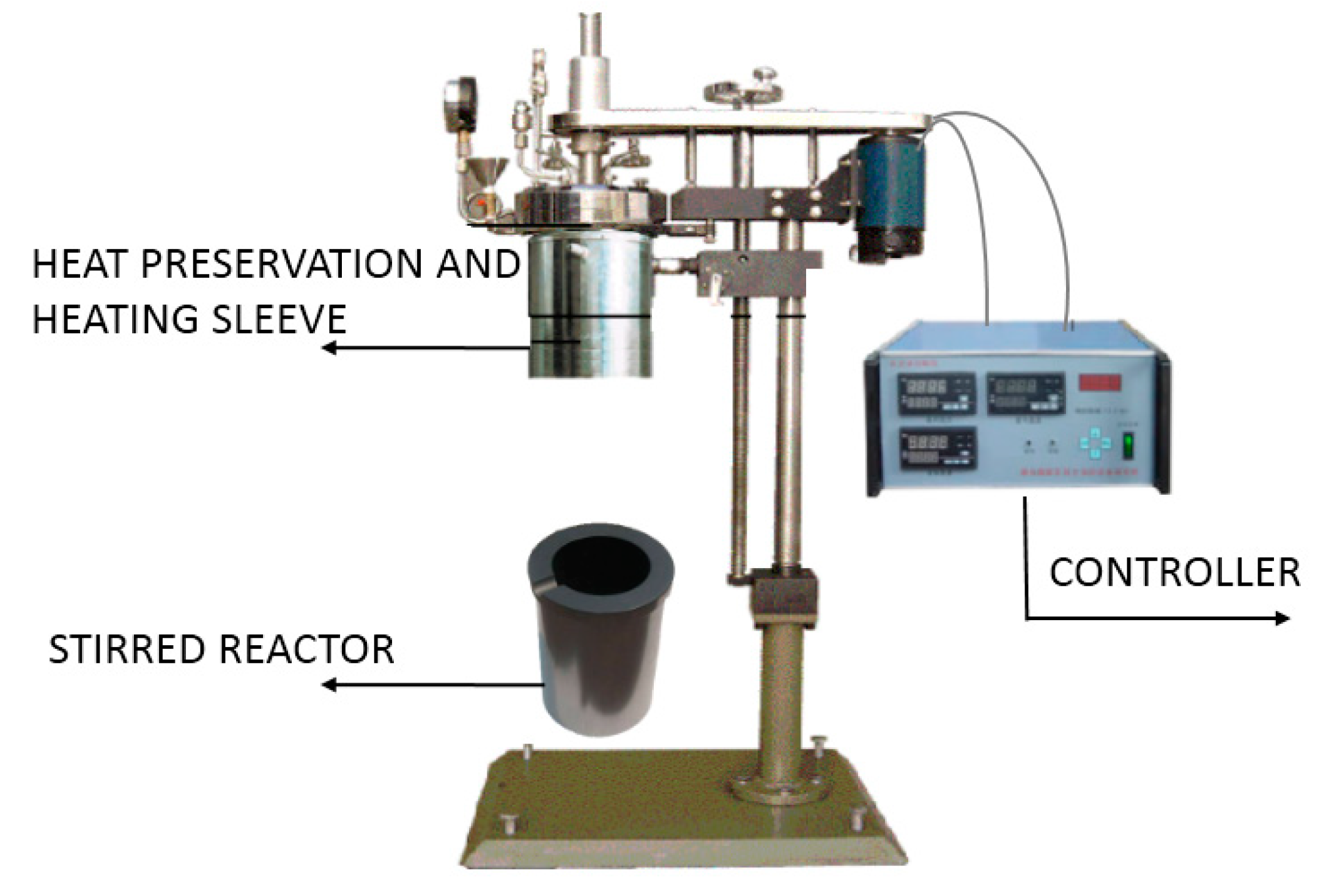
2.2. Aeration Conditions
3. Results and Discussion
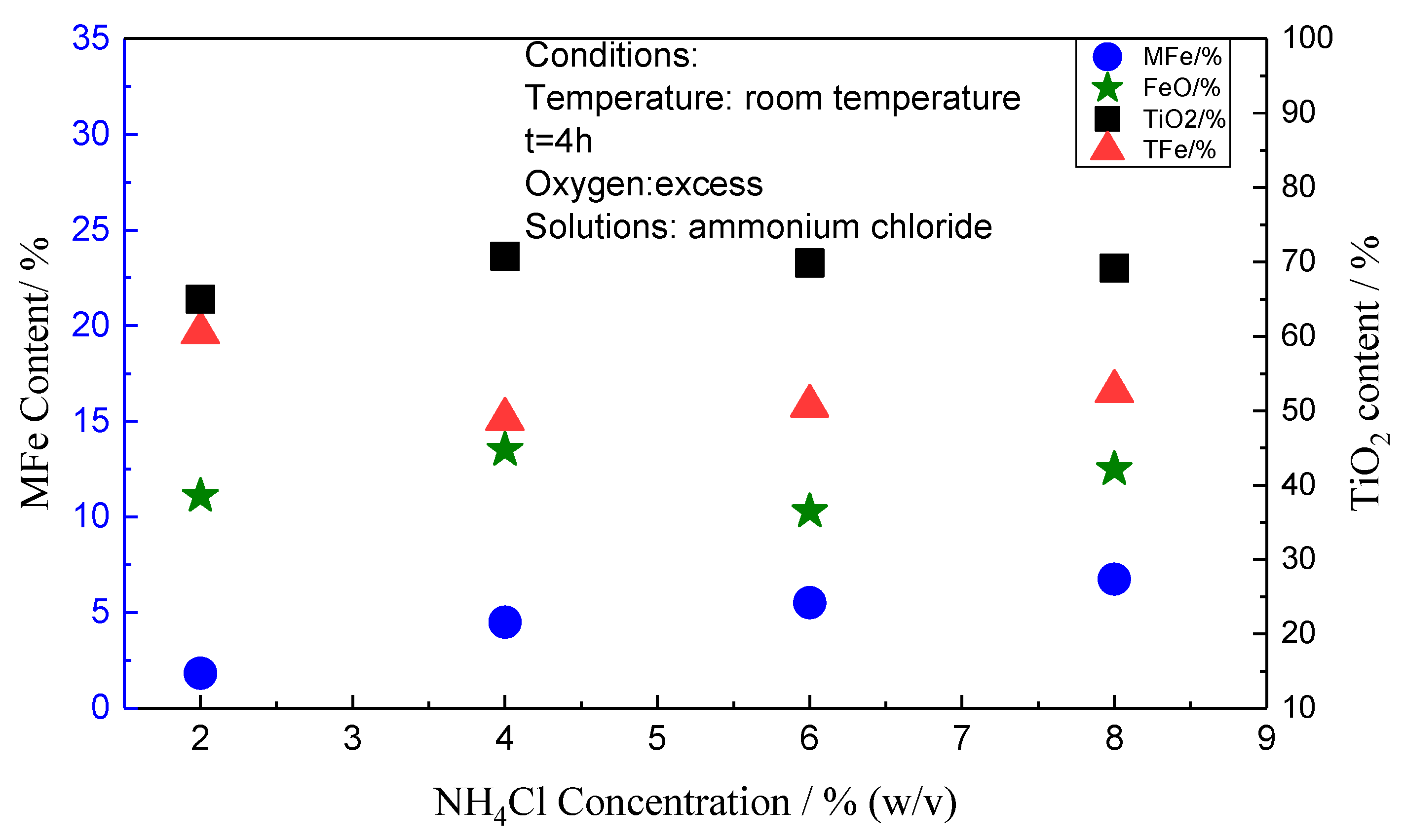
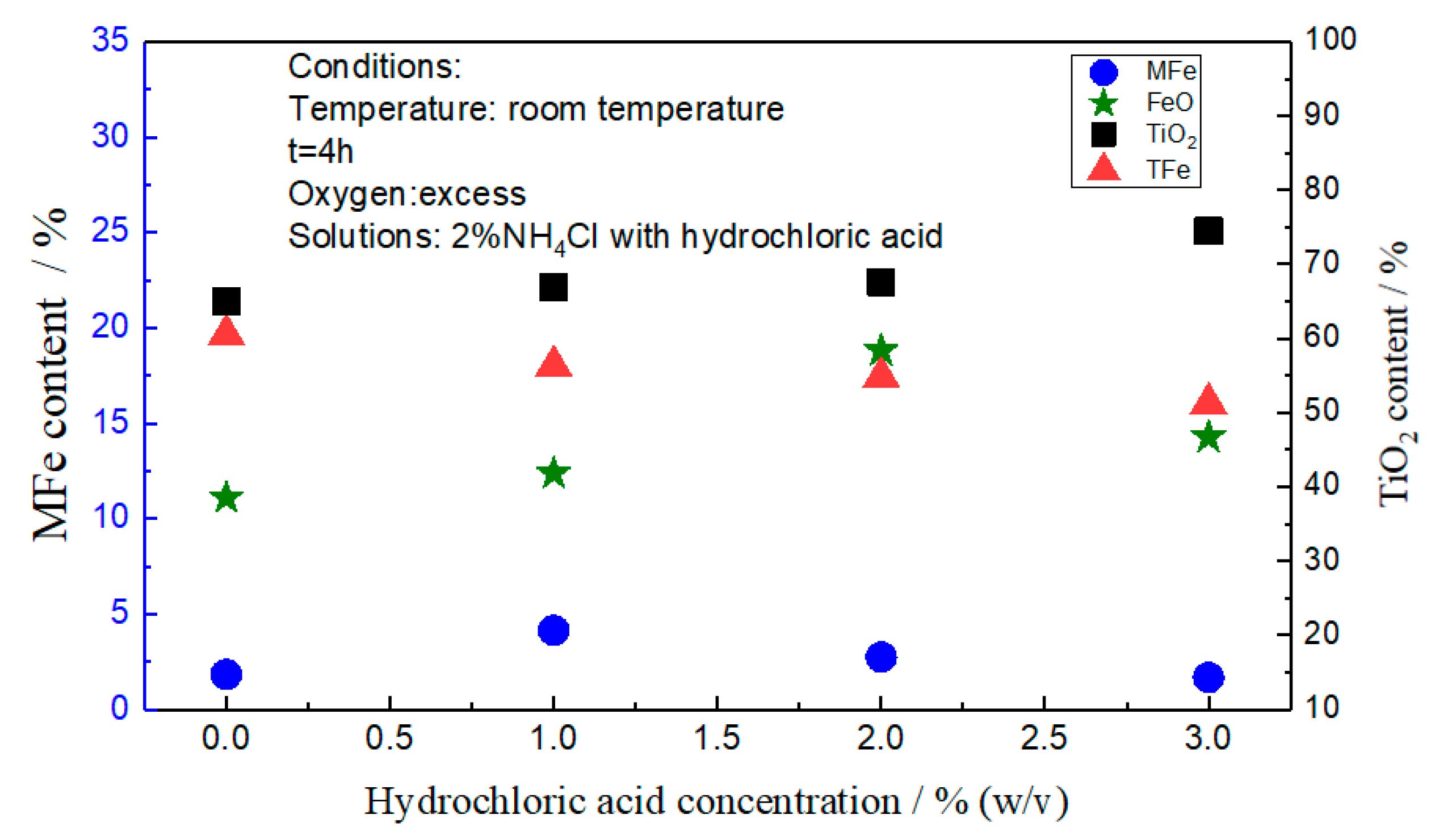
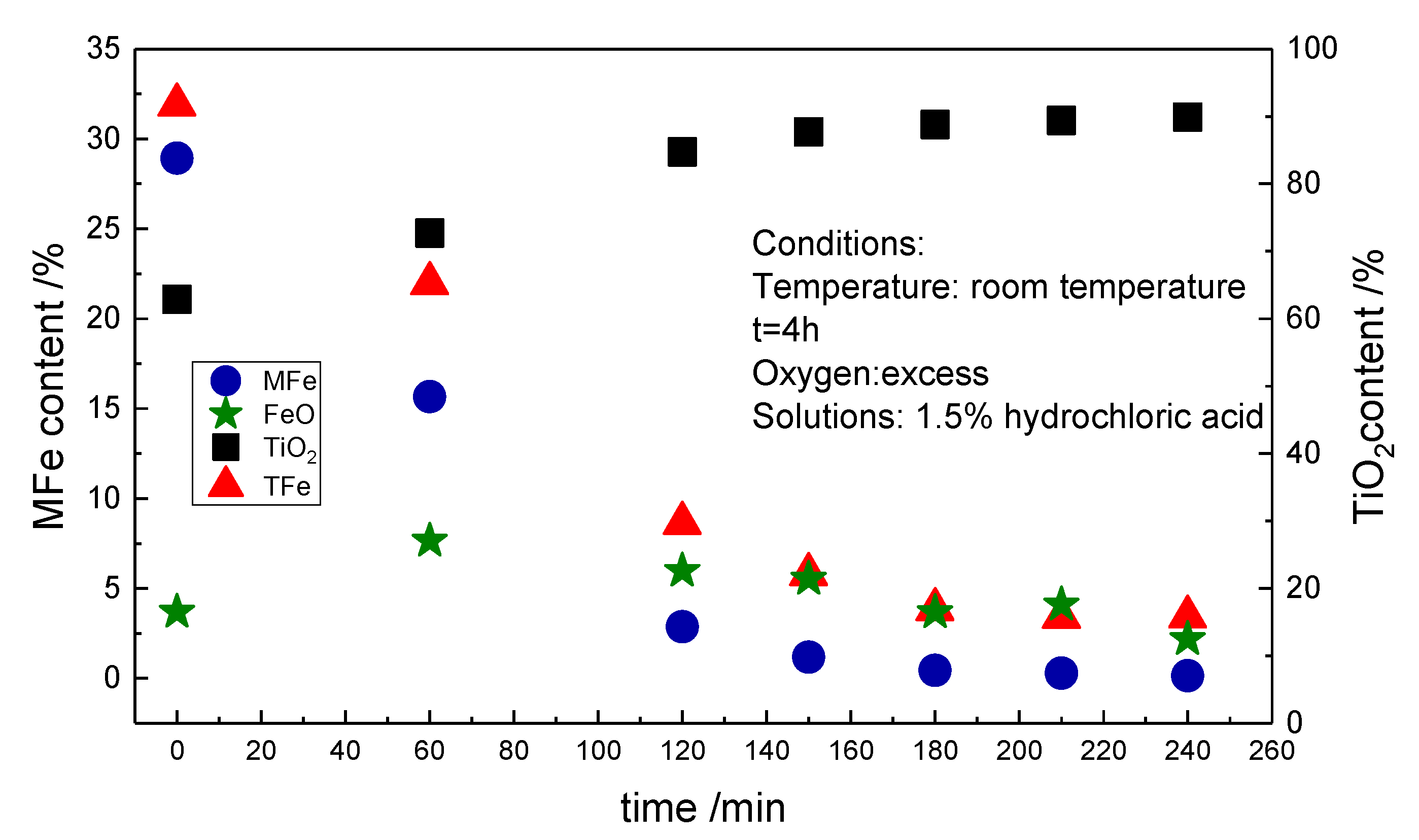
3.1. Effect of Solution Composition
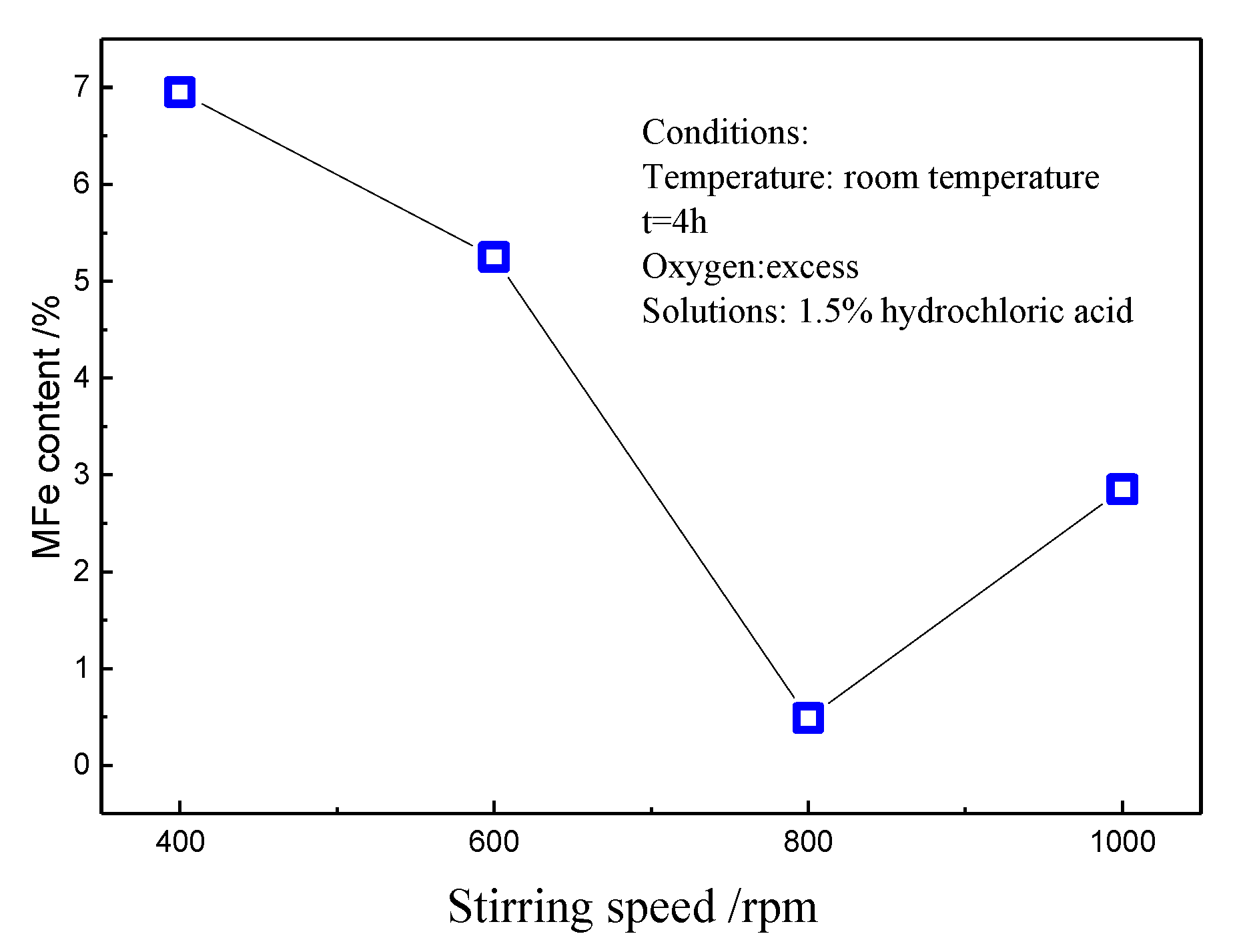
3.2. Effect of Stirring Speed
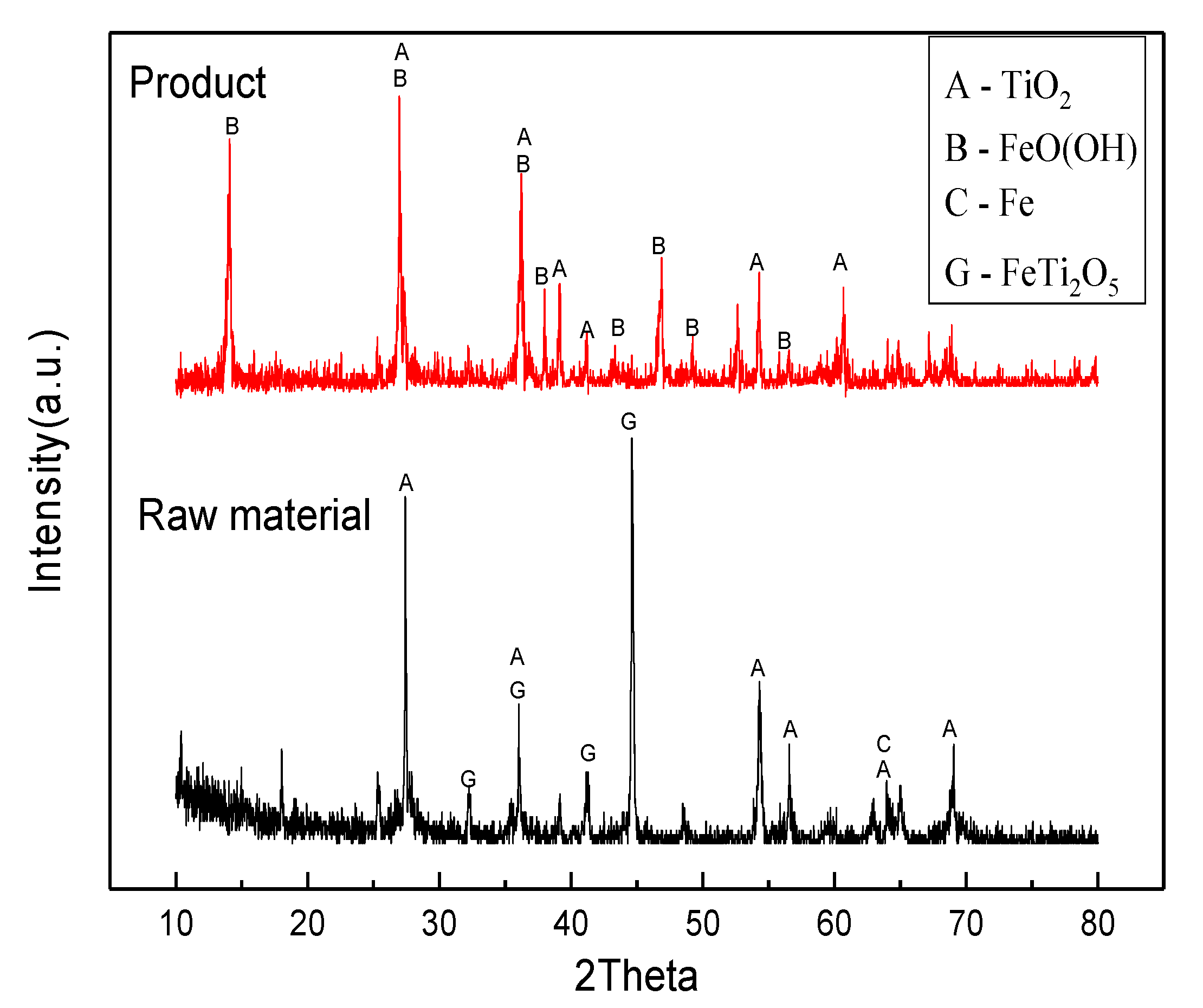
3.3. Phase Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, W.S.; Zhu, Z.W.; Cheng, C.Y. A literature review of titanium metallurgical processes. Hydrometallurgy 2011, 108, 177–188. [Google Scholar] [CrossRef]
- Lakshmanan, V.I.; Bhowmick, A.; Halim, M.A. Titanium dioxide: Production, properties, and applications. Chem. Phys. Res. J. 2014, 7, 37–42. [Google Scholar]
- Que, Y.; Weng, J.; Hu, L. Applications of titanium dioxide in perovskite solar cells. Prog. Chem. 2016, 28, 40–50. [Google Scholar]
- Guo, Y.F.; Liu, S.S.; Jiang, T.; Qiu, G.Z.; Chen, F. A process for producing synthetic rutile from Panzhihua titanium slag. Hydrometallurgy 2014, 134, 147–148. [Google Scholar] [CrossRef]
- Yaraghi, A.; Sapri, M.H.A.; Baharun, N.; Rezan, S.A. Aeration leaching of iron from nitrided Malaysian ilmenite reduced by polystyrene-Coal reductant. Procedia Chem. 2016, 19, 715. [Google Scholar] [CrossRef] [Green Version]
- Becher, R.G. Improved process for the beneficiation of ores containing contaminating iron. Aust. Patent 1963, 247, 110. [Google Scholar]
- Farrow, J.B.; Ritchie, I.M.; Mangono, P. The reaction between reduced ilmenite and oxygen in ammonium chloride solution. Hydrometallurgy 1987, 18, 21–38. [Google Scholar] [CrossRef]
- Hoecker, W. Process for the Production of Synthetic Rutile. U.S. Patent No. AU19940056301, 22 February 1994. [Google Scholar]
- Sekimoto, H.; Yahaba, S.; Chiba, S.; Yamaguchi, K. New separation technique of titanium and iron for titanium ore upgrading. In Proceedings of the 13th World Conference on Titanium, TMS (The Minerals, Metals & Materials Society), Warrendale, PA, USA, 2 May 2016. [Google Scholar]
- Bracanin, B.F.; Clements, R.J.; Davey, J.M. Direct reduction—the Western titanium process for the production of synthetic rutile, ferutil and sponge iron. Australas. Inst. Min. Metall. Pro. 1980, 275, 33–36. [Google Scholar]
- Fletcher, S.; Bruckard, W.J.; Calle, C.; Carey, K.C.; Horne, M.D.; Ruzbacky, R.; Sparrow, G.J. Soluble catalysts for the oxygen reduction reaction, and their application to Becher aeration. Ind. Eng. Chem. Res. 2019, 58, 10190–10198. [Google Scholar] [CrossRef]
- Jayasekera, S.; Marinovich, Y.; Avraamides, J.; Bailey, S.I. Pressure leaching of reduced ilmenite: Electrochemical aspects. Hydrometallurgy 1995, 39, 183–199. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Truong, T.N.; Duong, B.N. Impact of organic acid additions on the formation of precipitated ironcompounds. Acta Metall. Slovaca 2016, 22, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Truong, T.N.; Nguyen, T.T.; Duong, B.N. Acetic acid and sodium acetate mixtures as an aeration catalyst in the removal of metallic iron in reduced ilmenite. Acta Metall. Slovaca 2017, 23, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Bruckard, W.J.; Calle, C.; Fletcher, S.; Horne, M.D.; Sparrow, G.J.; Urban, A.J. The application of anthraquinone redox catalysts for accelerating the aeration step in the becher process. Hydrometallurgy 2004, 73, 111–121. [Google Scholar] [CrossRef]
- Guo, Y.F.; Liu, X.; Qiu, G.Z.; Jiang, T. Strengthening of metallic iron rust in reduced ilmenite. J. Cent. South Univ. Sci. Technol. 2012, 43, 797–802. [Google Scholar]
- Geetha, K.S.; Surender, G.D. ntensification of iron removal rate during oxygen leaching through gas-liquid mass transfer enhancement. Metall. Mater. Trans. B 2001, 3, 961–963. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Pei, G.S.; Lv, W.; Liu, S.L.; Lv, X.W.; Qiu, G.B. Preparation of synthetic rutile from reduced ilmenite through the aeration leaching process. Chem. Eng. Process. 2020, 147, 107774. [Google Scholar] [CrossRef]
- Li, X.; Xing, P.F.; Du, X.H.; Gao, S.B.; Chen, C. Influencing factors and kinetics analysis on the leaching of iron from boron carbide waste-scrap with ultrasound-assisted method. Ultrason. Sonochem. 2017, 38, 84–91. [Google Scholar] [CrossRef]
- Ma, J.Y.; Zhang, Y.F.; Qin, Y.; Wu, Z.K.; Wang, T.L.; Wang, C. The leaching kinetics of K-feldspar in sulfuric acid with the aid of ultrasound. Ultrason. Sonochem. 2017, 35, 304–312. [Google Scholar] [CrossRef]
- Feng, D.; Ren, Q.X.; Ru, H.Q.; Wang, W.; Ren, S.Y.; Jiang, Y.; Liu, B.Y.; Chang, S.X.; Zhang, C.P.; Yang, T. Iron removal from ultra-fine silicon carbide powders with ultrasound-assisted and its kinetics. Mater. Chem. Phys. 2020, 247, 122860. [Google Scholar] [CrossRef]
- Sun, D.L.; Wu, K.M.; Hu, J. Removal of iron from leaching solution of zinc ore by goethite process. Hydrometall. China 2015, 34, 68–71. [Google Scholar]
- Chen, X.; Chen, S.H.; Cai, S.M. Breakdown of the passive film on iron by Cl− in a acidic solution. Acta Phys. Chim. Sin. 1988, 4, 3823–3886. [Google Scholar]


| Component | TiO2 | MFe | FeO | TFe | CaO | MgO | Mn | Al2O3 | SiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Content | 62.88 | 28.93 | 3.69 | 31.90 | 0.15 | 0.23 | 1.89 | 1.55 | 1.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Li, M.; Zhou, L.; Zheng, M.; Zhang, T. Removal of Metallic Iron from Reduced Ilmenite by Aeration Leaching. Metals 2020, 10, 1020. https://doi.org/10.3390/met10081020
Zhao Q, Li M, Zhou L, Zheng M, Zhang T. Removal of Metallic Iron from Reduced Ilmenite by Aeration Leaching. Metals. 2020; 10(8):1020. https://doi.org/10.3390/met10081020
Chicago/Turabian StyleZhao, Qiuyue, Maoyuan Li, Lei Zhou, Mingzhao Zheng, and Ting’an Zhang. 2020. "Removal of Metallic Iron from Reduced Ilmenite by Aeration Leaching" Metals 10, no. 8: 1020. https://doi.org/10.3390/met10081020





