1. Introduction
The creep-resistant alloy 2.25Cr-1Mo-0.25V was developed following the conventional low-alloyed CrMo steels in the early 1990s [
1,
2]. In comparison to the basic alloy 2.25Cr-1Mo, the V-modified alloy shows several advantages such as an increased resistance to hydrogen embrittlement, a better resistance to overlay disbonding and a good toughness in combination with high levels of mechanical properties at elevated temperatures [
3,
4,
5]. Since 1995, when the Italian company Nuovo Pignone was the first to fabricate a reactor from this V-modified alloy in Europe, it has often been used for heavy wall pressure vessels in power stations or in petroleum and chemical plants, for example in hydrocracking reactors [
1,
5,
6]. For this purpose, the 2.25Cr-1Mo-0.25V steels are commonly joined with submerged-arc welding (SAW). There, the V-modified grade entails some disadvantages compared to the conventional alloy 2.25Cr-1Mo. These are low levels of weld metal toughness directly after welding and a higher sensitivity to reheat cracking in the weld metal or in the heat affected zone (HAZ) of the base metal during the subsequent heat treatment [
6,
7].
To improve these disadvantages, it is of great importance to know how the numerous welding parameters during SAW affect the microstructure of the resulting weld metal and the HAZ in between the individual weld beads. One of the most important parameters during welding is the heat input, as it directly influences the cooling rate and in further consequence the microstructure and mechanical properties of the multi-layer weld metal and its multiple HAZs. A higher heat input during welding results in a larger weld pool and a longer cooling time, which in further consequence also changes the cooling conditions and the microstructure of the HAZs between the weld beads. Continuous cooling transformation (CCT) diagrams are an important tool for predicting microstructural transformations during heat treatment in steels, but are not applicable for the different areas of the HAZs of a multi-layer weld metal. During welding, usually very high cooling rates occur and each zone in the weld metal experiences different peak temperatures depending on its distance from the weld pool. Therefore, common CCT diagrams are not applicable for predicting the different phases occurring within the HAZ between the weld beads of a complex multi-layer weld metal. Compared to common heat treatments, the HAZs within the all-weld metal only experience very short austenitizing times of just a few seconds, which are too short to homogenize the microstructure leading to an irregular distribution of alloying elements. After this insufficient homogenization, the weld bead and the adjacent HAZ to the subjacent weld bead experience extremely high cooling rates of many hundreds of °C/s, depending on the welding procedure and the distance to the molten weld pool [
8]. Furthermore, weld metal commonly consists of numerous non-metallic inclusions which are formed in the liquid face and, in further consequence, influence the phase transformations during cooling [
8,
9]. For estimating the optimum welding conditions to adjust a certain microstructure in the multi-layer weld metal’s HAZs, the application of welding CCT diagrams is indispensable. Welding CCT diagram created by conducting dilatometer measurements on all-weld metal specimens, give an insight to the microstructural changes within the multiple HAZs in-between the weld beads of a multi-layer weld metal. This is of particular interest for the SAW process, which is often applied for thick walled vessels, where multi-layer welding is the preferred technique.
The literature only provides classical CCT diagrams for the base alloy 2.25Cr-1Mo [
10,
11,
12,
13,
14] and classical CCT diagrams for other Cr-Mo alloys with various chemical compositions or peak temperatures and cooling rates [
12,
14,
15,
16], as well as classical CCT diagrams for the V containing alloys T22 and T24 which differ from the alloy 2.25Cr-1Mo-0.25V in terms of their B, Ti and N content [
17]. Unfortunately, these CCT diagrams do not display the transformations taking place in the HAZs of SAW V-modified alloy 2.25Cr-1Mo-0.25V as elements like Cr, Mo and V strongly influence the transformation times and temperatures and the HAZs between the weld beads in a multi-layer weld metal experience a significantly different temperature profile than the temperature profiles used for classical heat treatments [
8,
9]. No welding CCT diagrams applicable to low Cr creep resistant all-weld metal which display the different HAZs between the individual weld beads are available to the authors’ best knowledge.
The microstructure which develops within the HAZ between the individual weld beads during SAW of a 2.25Cr-1Mo-0.25V multi-layer weld metal is of significant importance for the application under creep load. Yang et al. [
18,
19,
20] investigated the creep behavior of different sub-zones in a low alloyed CrMoV multi-pass weld metal and experienced a remarkable different creep behavior depending on the grain size and morphology of the tested region. According to their studies, the anisotropic creep behavior of the multi-pass weld metal is related to the coupling effect of an inhomogeneous microstructure due to the layered structure and the different HAZs in-between these layers and the loading direction during the creep tests [
18,
19,
20].
Concerning the 2.25Cr-1Mo-0.25V steels chemical composition, it has to be taken into account that higher levels of Cr and Mo have a transformation delaying effect and therefore lead to a shift of the bainite transformation curves to longer cooling times. Beside this effect, Cr, Mo and V cause a reduction in the martensite start temperature and consequently favor the bainitic microstructure. In addition, higher levels of Cr, Mo and V lead to a separation of the pearlite and bainite stage [
8,
9].
This paper intends to improve the understanding for the phase transformations in the V-modified 2.25Cr-1Mo-0.25V alloy during various heat treatments by providing a classic CCT diagram with a peak temperature of 1200 °C for the base alloy as well as welding CCT-diagrams suitable for the different HAZs between the weld beads in a SAW multi-layer weld metal. For this purpose, three different welding CCT diagrams with varying peak temperatures have been created:
850 °C peak temperature for the intercritically heated zone (ICHAZ)
1000 °C peak temperature for the fine grained heat affected zone (FGHAZ)
1400 °C peak temperature for the coarse grained heat affected zone (CGHAZ)
By creating welding CCT diagrams from weld metal specimens which correspond to the different regions in the HAZ between the individual weld beads, the authors provide a tool for the target-oriented adaption of the SAW parameters. The variation of welding voltage, welding current and travel speed, which strongly influence the cooling rate of the deposited weld bead, offers the possibility to purposefully adjust the microstructure of the HAZs within the multi-layer weld metals. Hereby, the amount of undesired microstructural constituents such as large quantities of martensite and ferrite which can have a negative effect on the weld metals mechanical properties and its behavior under creep conditions can be reduced.
2. Materials and Methods
The dilatometer specimens for the CCT diagrams of the weld metal were taken from a 2.25Cr-1Mo-0.25V multi-layer submerged arc all-weld metal which was built up with two weld beads per layer and 9 layers in total. The weld seam was prepared using two 24 mm thick 2.25Cr-1Mo-0.25V steel plates and a 2.25Cr-1Mo packing strip. The root gap was 22 mm and the bevel angle was 0°.
Table 1 shows the used welding parameters.
Table 2 represents the weld metals chemical composition.
Beside the listed elements in
Table 2 the weld metal also contains Phosphorus and Sulphur in small amounts (<110 ppm). No post weld heat treatment was applied.
Figure 1 shows a schematic drawing of the weld metals cross section displaying the layered structure consisting of two weld beads per layer. To ensure comparability by avoiding variations in the chemical composition, all dilatometer specimens were taken from the last two weld beads which were not thermally influenced by further weld beads. In addition, the dilatometer specimens were taken from the middle of the last two weld beads with adequate distance to the heat affected zone between the last two weld beads and to the HAZ to the base metal. The positions in the last weld beads where the dilatometer specimens were taken from are marked with dashed circles in
Figure 1.
The dilatometer samples for the CCT diagram of the 2.25Cr-1Mo-0.25V base metal were taken from a 24 mm thick steel plate with the chemical composition given in
Table 3.
Beside the listed elements in
Table 3, the base metal also contains Phosphorus and Sulphur in small amounts (≤60 ppm).
The cylindrical dilatometer specimens had a diameter of 5 mm and a length of 10 mm. The base areas were manually grinded plane parallel and perpendicular to the cylinder axis to ensure a good contact with the measurement device.
The continuous cooling curves were recorded using a quenching dilatometer DIL805 from TA Instruments (New Castle, DE, USA). During the dilatometry experiments the samples where heated up with 20 °C/s and cooled with varying cooling rates to simulate different t8/5-times (= time for cooling the dilatometer specimen from 800 to 500 °C) during welding and heat treatment, respectively. The gas used for cooling was He, as in contrast to N2, it enables fast cooling rates even at low temperatures. In the course of the dilatometer measurements, the relative length change of the cylindrical dilatometer specimens was detected.
For the welding CCT diagrams, the all-weld metal dilatometer specimens were cooled with ten to eleven different t8/5-times of 1/2/5/10/25/50/100/250/500/1000/(2500) s, respectively. The 15 t8/5-times applied for the base metal CCT diagram were 2/5/10/50/80/120/150/200/250/300/500/600/800/2000/5000 s.
To correctly portray the different HAZs between the individual weld beads of the multi-layer weld metal, three different CCT diagrams for the coarse grained HAZ (CGHAZ), the fine grained HAZ (FGHAZ) and the intercritical HAZ (ICHAZ) were generated. The three welding CCT diagrams exhibit the same cooling rates and only differ in terms of their peak temperatures. For the CCT diagram of the CGHAZ the peak temperature was 1400 °C and for the CCT diagram of the FGHAZ it was 1000 °C leading to an austenitic initial state. For the CCT diagram of the ICHAZ, a peak temperature of 850 °C within the intercritical region was chosen leading to an austenitic-ferritic initial microstructure before cooling.
Figure 2 shows a modified schematic drawing of a weld bead within a multi-layer weld metal and its adjacent HAZ to the subjacent weld bead as well as the corresponding peak temperatures according to G. Schulze [
8]. The peak temperatures chosen for the welding CCT diagrams shown in this study are highlighted in green, blue and red, respectively. It has to be noted that the Fe-Fe
3C diagram only gives an indication which phase transformations might take place, as it describes the thermodynamic equilibrium which is only reached at very low cooling rates and is therefore not suitable for the process of welding were usually rapid cooling takes place. Furthermore, it has to be taken into account that the investigated alloy contains a certain amount of alloying elements beside carbon which again influences the phase diagram.
For all three welding CCT diagrams, the dilatometer samples were heated to the peak temperature and then cooled without any dwell time in order to realistically represent the welding process. For the base metal CCT diagram, the dilatometer samples were heated with 20 °C/s to a peak temperature of 1200 °C and then cooled with various cooling rates after a dwell time of 5 min. The continuous cooling curves for the different CCT diagrams of the weld metal and the base metal CCT diagram were evaluated with the dilatometer TA-Instruments WIN TA-Software DIL 805 (New Castle, DE, USA).
After the dilatometer experiments all the samples were embedded in Polyfast parallel to the cylinder axis before they were automatically ground and polished with 3 µm and 1 µm diamond suspension using the polishing automat Struers Tegramin-30. The polished samples were treated with Nital etching solution (3% HNO3 in ethanol) for optical microscopic (OM) investigation with the optical microscope Zeiss Axio Imager M1m and scanning electron microscopy (SEM) using a Versa 3D DualBeam workstation from Thermo Fisher Scientific (former FEI) (Waltham, MA, USA).
To estimate the amount of ferrite in the dilatometer samples which experienced lower cooling rates, the OM pictures were analysed with Stream Motion image processing software from Olympus (Tokyo, Japan).
For each dilatometer specimen the hardness was tested using a Vickers hardness tester from EMCO-TEST Prüfmaschinen GmbH (Kuchl, Austria). Every specimen was tested three times with HV10 using a testing force of 98.10 N and a testing time of 15 s. The three Vickers hardness indents were made along the longitudinal axis of the dilatometer specimens cross sections. For the welding and base metal CCT diagrams the hardness mean value was taken.
The Ac
1 and Ac
3 temperatures of the all-weld metal and the base metal were determined experimentally by means of dilatometer measurements. For this purpose, the samples were heated up to 600 °C with a heating rate of 10 °C/s and then further heated up to 1000 °C with a very low heating rate of 0.008 °C/s according to ASTM A 1033-04 [
21]. No dwell time was applied and the cooling was performed with a cooling rate of 30 °C/s. The dilatometer measurement was repeated three times and the heating curves were evaluated by means of the dilatometer WIN TA-Software DIL 805DIL 805.
Author Contributions
Conceptualization: H.S. and R.S.; Investigation: H.S., B.L. and T.F.; Methodology: H.S., F.G., R.K., M.S.-N. and R.S.; Project administration: H.S. and R.S.; Supervision: F.G., R.K., M.S.-N. and R.S.; Visualization: H.S. and B.L.; Writing—original draft: H.S.; Writing—review and editing: B.L., T.F., F.G., R.K., M.S.-N. and R.S. All authors have read and agreed to the published version of the manuscript.
Funding
Funding of the Austrian BMK (846933) in the framework of the program “Production of the future” and the “BMK Professorship for Industry” is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hucińska, J. Advanced vanadium modified steels for high pressure hydrogen reactors. Adv. Mater. Sci. 2003, 4, 21–27. [Google Scholar]
- Detemple, I.; Hanus, F.; Luxenburger, G. Advanced steels for hydrogen reactors. Hydrocarb. Eng. 1999, 4, 1–8. [Google Scholar]
- Pereira, P.A.S.; Franco, C.S.G.; Guerra Filho, J.L.M.; dos Santos, D.S. Hydrogen effects on the microstructure of a 2.25Cr–1Mo–0.25 V steel welded joint. Int. J. Hydrog. Energy 2015, 40, 17136–17143. [Google Scholar] [CrossRef]
- Chauvy, C.; Pillot, S. Prevention of weld metal reheat cracking during CrMoV heavy reactors fabrication. In Proceedings of the ASME Pressure Vessels and Piping Conference, Prague, Czech Republic, 26–30 July 2009; pp. 243–251. [Google Scholar]
- Ichikawa, K.; Horii, Y.; Sueda, A.; Kobayashi, J. Thoughness and creep strength of modified 2.25Cr-1Mo steel weld metal. Weld. J.-Incl. Weld. Res. Suppl. 1995, 74, 230–238. [Google Scholar]
- Chovet, C.; Schmitt, J.-P. Additional recommendations for welding Cr-Mo-V steels for petrochemical applications. Weld. World 2011, 55, 31–38. [Google Scholar] [CrossRef]
- Dhooge, A.; Vinckier, A. Reheat cracking—A review of recent studies. Int. J. Press. Vessel. Pip. 1987, 27, 239–269. [Google Scholar] [CrossRef]
- Schulze, G. Die Metallurgie des Schweissens. Eisenwerkstoffe - nichteisenmetallische Werkstoffe, 4th ed.; Springer: Heidelberg, Germany; New York, NY, USA, 2010; ISBN 9783642031823. [Google Scholar]
- Bargel, H.-J.; Schulze, G. Werkstoffkunde, 12th ed.; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-3-662-48629-0. [Google Scholar]
- Depinoy, S. Microstructural Evolution of a 2.25Cr - 1 Mo Steel During Austenitization and Temper: Austenite Grain Growth, Carbide Precipitation Sequence and Effects on Mechanical Properties. Ph.D. Thesis, Ecole Nationale Supérieure des Mines de Paris, Paris, France, 2015. [Google Scholar]
- Deng, D.; Tong, Y.; Ma, N.; Murakawa, H. Prediction of the residual welding stress in 2.25Cr-1Mo steel by taking into account the effect of the solid-state phase transformations. Acta Metall. Sin. (Engl. Lett.) 2013, 26, 333–339. [Google Scholar] [CrossRef] [Green Version]
- Na, H.S.; Kim, B.H.; Lee, S.H.; Kang, C.H. Thermodynamic alloy design of high strength and toughness in 300 mm thick pressure vessel wall of 1.25Cr-0.5Mo steel. Metals 2018, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Badu, S.S.; Badeshia, H.K.D.H. Mechanism of the Transition from Bainite to Acicular Ferrite. Mater. Trans. JIM 1991, 32, 679–688. [Google Scholar]
- Tassa, O.; Bourges, P.; Pillot, S.; Mendes Rodrigues, M.; De Cesaris, A.; van Wortel, H. Development of Cr-Mo-V Ferritic Steels for High Temperature Hydroprocessing Rectors with Enhanced Behavior. EUR 2005, 21419, 1–100. [Google Scholar]
- Tartaglia, J.M.; Kuelz, A.N.; Thelander, V.H. The effects of alloying elements on the continuous cooling transformation behavior of 2¼Cr-1Mo steels. J. Mater. Eng. Perform. 2018, 27, 6349–6364. [Google Scholar] [CrossRef]
- Mayr, P.; Schlacher, C.; Siefert, J.A.; Parker, J.D. Microstructural features, mechanical properties and high temperature failures of ferritic to ferritic dissimilar welds. Int. Mater. Rev. 2019, 64, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Paddea, S.; Masuyama, F.; Shibli, A. T23 and T24 — New generation low alloyed steels. In Coal Power Plant Materials and Life Assessment; Woodhead Publishing: Cambridge, UK, 2014; pp. 87–106. [Google Scholar]
- Yang, B.; Xuan, F.-Z. Nonhomogeneous microstructure related creep damage of the CrMoV multi-pass weld metal. Mater. Sci. Eng. A 2019, 763, 138122. [Google Scholar] [CrossRef]
- Yang, B.; Xuan, F.-Z. Creep behavior of subzones in a CrMoV weldment characterized by the in-situ creep test with miniature specimens. Mater. Sci. Eng. A 2018, 723, 148–156. [Google Scholar] [CrossRef]
- Yang, B.; Xuan, F.-Z.; Liu, X.-P. Heterogeneous creep behavior of a CrMoV multi-pass weld metal. Mater. Sci. Eng. A 2017, 690, 6–15. [Google Scholar] [CrossRef]
- ASTM, A. 1033-04, Standard Practice for Quantitative Measurement and Reporting of Hypoeutectoid Carbon and Low-Alloy Steel Phase Transformations; ASTM International: West Conshohocken, PA, USA, 2004; pp. 1–14. [Google Scholar]
- Keehan, E.; Karlsson, L.; Andrén, H.-O.; Bhadeshia, H.K.D.H. Influence of carbon, manganese and nickel on microstructure and properties of strong steel weld metals: Part 3—Increased strength resulting from carbon additions. Sci. Technol. Weld. Join. 2006, 11, 19–24. [Google Scholar] [CrossRef]
- Keehan, E.; Karlsson, L.; Bhadeshia, H.K.D.H.; Thuvander, M. Three-dimensional analysis of coalesced bainite using focused ion beam tomography. Mater. Charact. 2008, 59, 877–882. [Google Scholar] [CrossRef]
- Pak, J.H.; Bhadeshia, H.K.D.H.; Karlsson, L.; Keehan, E. Coalesced bainite by isothermal transformation of reheated weld metal. Sci. Technol. Weld. Join. 2008, 13, 593–597. [Google Scholar] [CrossRef]
- Chang, L.C.; Bhadeshia, H.K.D.H. Microstructure of lower bainite formed at large undercoolings below bainite start temperature. Mater. Sci. Technol. 1996, 12, 233–236. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H.; Keehan, E.; Karlsson, L.; Andrén, H.-O. Coalesced Bainite. Trans. Indian Inst. Met. 2006, 59, 689–694. [Google Scholar]
Figure 1.
Schematic drawing of the upper part of the cross section of the investigated 2.25Cr-1Mo-0.25V SAW (submerged-arc welding) all-weld metal. The positions in the last two weld beads where the dilatometer-specimens were taken from are highlighted with dashed circles.
Figure 2.
Schematic drawing of the different areas within the HAZ between the weld beads in a multi-layer weld metal and the corresponding peak temperatures for low alloyed weld metal with low C-contents according to G. Schulze [
8]. The peak temperatures chosen for the CGHAZ, the FGHAZ and the ICHAZ welding CCT diagram are highlighted with green, blue and red lines.
Figure 3.
CCT diagram for the CGHAZ between the weld beads of a 2.25Cr-1Mo-0.25V multi-layer SAW all-weld metal with a peak temperature of 1400 °C.
Figure 4.
OM images of the microstructure of the dilatometer specimens heated to 1400 °C peak temperature and cooled with different t8/5-times of (a) 1 s; (b) 10 s; (c) 250 s; and (d) 1000 s at lower and higher magnification.
Figure 5.
SEM images of the microstructure of the dilatometer specimens heated to 1400 °C peak temperature and cooled with different t8/5-times of (a) 1 s; (b) 10 s; (c) 250 s; and (d) 1000 s showing martensite (M), lower bainite (BL), coalesced bainite (BC), upper bainite (BU) and ferrite (F).
Figure 6.
CCT diagram for the FGHAZ between the weld beads of a 2.25Cr-1Mo-0.25V multi-layer SAW all-weld metal with a peak temperature of 1000 °C.
Figure 7.
OM images of the microstructure of the dilatometer specimens heated to 1000 °C peak temperature and cooled with different t8/5-times of (a) 1 s; (b) 10 s; (c) 250 s; and (d) 1000 s at lower and higher magnification.
Figure 8.
SEM images of the microstructure of the dilatometer specimens heated to 1000 °C peak temperature and cooled with different t8/5-times of (a) 1 s; (b) 10 s; (c) 250 s and (d) 1000 s showing lower bainite (BL), coalesced bainite (BC), upper bainite (BU) and ferrite (F).
Figure 9.
CCT diagram for the ICHAZ between the weld beads of a 2.25Cr-1Mo-0.25V multi-layer SAW all-weld metal with a peak temperature of 850 °C.
Figure 10.
OM images of the microstructure of the dilatometer specimens heated to 850 °C peak temperature and cooled with different t8/5-times of (a) 1 s; (b) 10 s; (c) 250 s; and (d) 1000 s at lower and higher magnification.
Figure 11.
SEM images of the microstructure of the dilatometer specimens heated to 850 °C peak temperature and cooled with different t8/5-times of (a) 1 s; (b) 10 s; (c) 250 s; and (d) 1000 s showing lower bainite (BL), coalesced bainite (BC), upper bainite (BU) and ferrite (F).
Figure 12.
Vickers hardness and ferrite phase fraction as a function of the t8/5-times for the dilatometers specimens of the all-weld metal’s CGHAZ, FGHAZ and ICHAZ CCT diagram.
Figure 13.
CCT diagram for the base metal with a peak temperature of 1200 °C and a dwell time of 5 min.
Figure 14.
OM images of the microstructure of the dilatometer specimens from the base metal CCT diagram which were heated to 1200 °C for 5 min and cooled with different cooling t8/5-times of (a) 5 s; (b) 120 s; (c) 500 s; and (d) 5000 s at lower and higher magnifications, respectively.
Figure 15.
SEM images of the microstructure of the dilatometer specimens heated to 1200 °C for 5 min and cooled with different t8/5-times of (a) 5 s; (b) 120 s; (c) 500 s; and (d) 5000 s showing lower bainite (BL), coalesced bainite (BC) and upper bainite (BU).
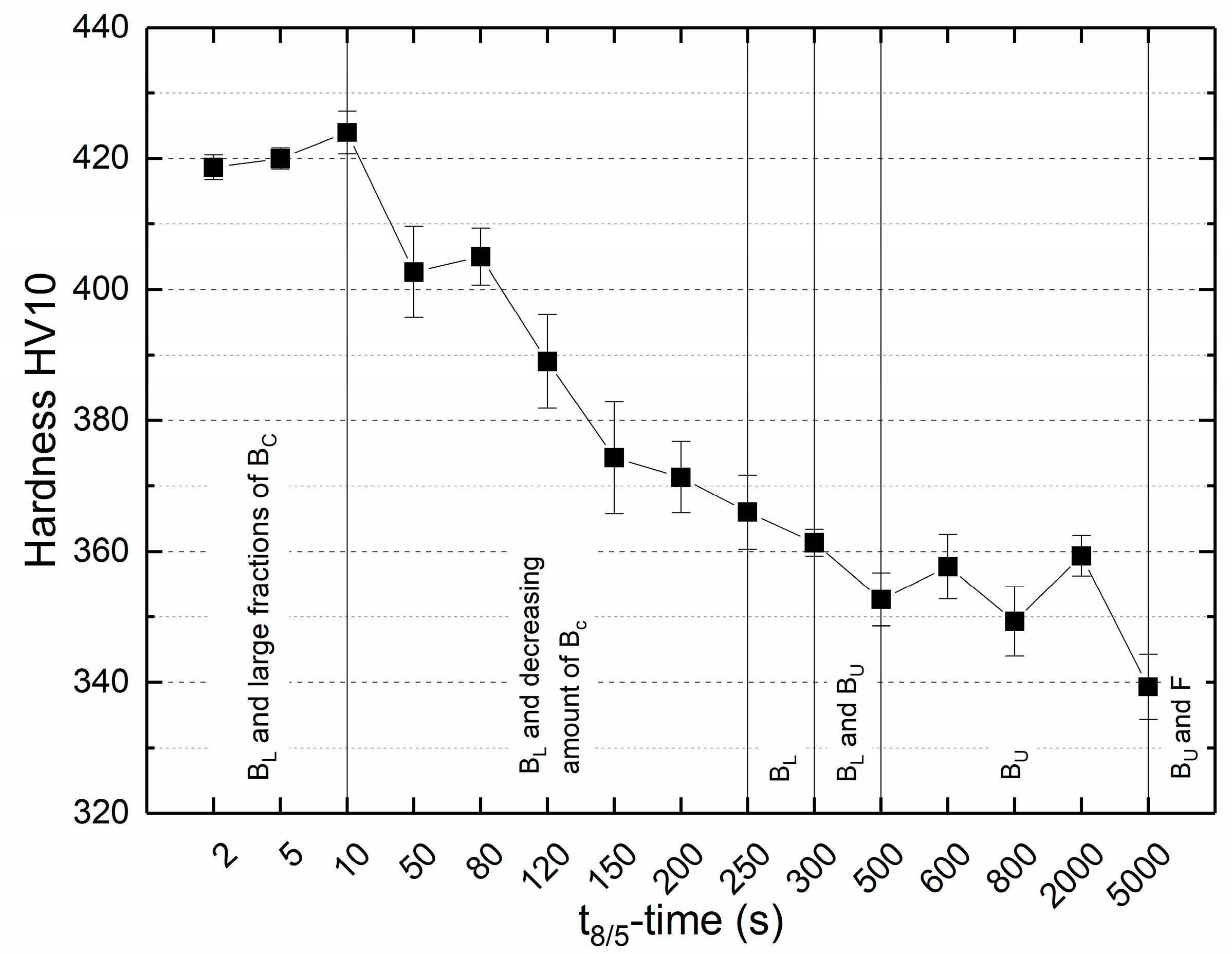
Figure 16.
Vickers hardness as a function of the t8/5-time and the observed corresponding microstructure constituents lower bainite (BL), coalesced bainite (BC), upper bainite (BU) and ferrite (F).
Table 1.
Overview of the Tandem-SAW (submerged-arc welding) conditions.
| Polarity | Heat Input | Current | Voltage | Interpass Temperature | Preheat Temperature | Welding Sequence | Welding Position |
|---|
| AC/AC | 20 kJ/cm | 550 A | 30 V | 230 °C | 200 °C | left ↔ right
alternating | PA |
Table 2.
Chemical composition in wt.% of the investigated 2.25Cr-1Mo-0.25V SAW weld metal.
| Fe | Cr | Mn | Mo | V | Si | C | N |
|---|
| Balance | 2.2 | 1.0 | 1.0 | 0.3 | 0.1 | 0.08 | 0.02 |
Table 3.
Chemical composition in wt.% of the investigated 2.25Cr-1Mo-0.25V base metal steel plate.
| Fe | Cr | Mn | Mo | V | Si | C |
|---|
| Balance | 2.3 | 0.6 | 1.1 | 0.3 | 0.08 | 0.14 |
Table 4.
Mean values for the Ac1 and Ac3 temperature of the 2.25Cr-1Mo-0.25V all-weld metal and the corresponding base metal determined by means of dilatometer measurements. All temperatures are given in °C.
| Base Metal | Weld Metal |
|---|
| Ac1 | Ac3 | Ac1 | Ac3 |
| 793 ± 2 | 910 ± 3 | 779 ± 3 | 926 ± 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).