Effect of Fe Content on the As-Cast Microstructures of Ti–6Al–4V–xFe Alloys
Abstract
:1. Introduction
2. Model and Experiments
2.1. Phase Field Model
- (1)
- The diffusion coefficients of Al, V, and Fe in the solid phase and the liquid phase did not change in the simulation.
- (2)
- The temperature gradient and cooling rate in the whole process remained invariant.
2.2. Experiments
3. Results and Discussions
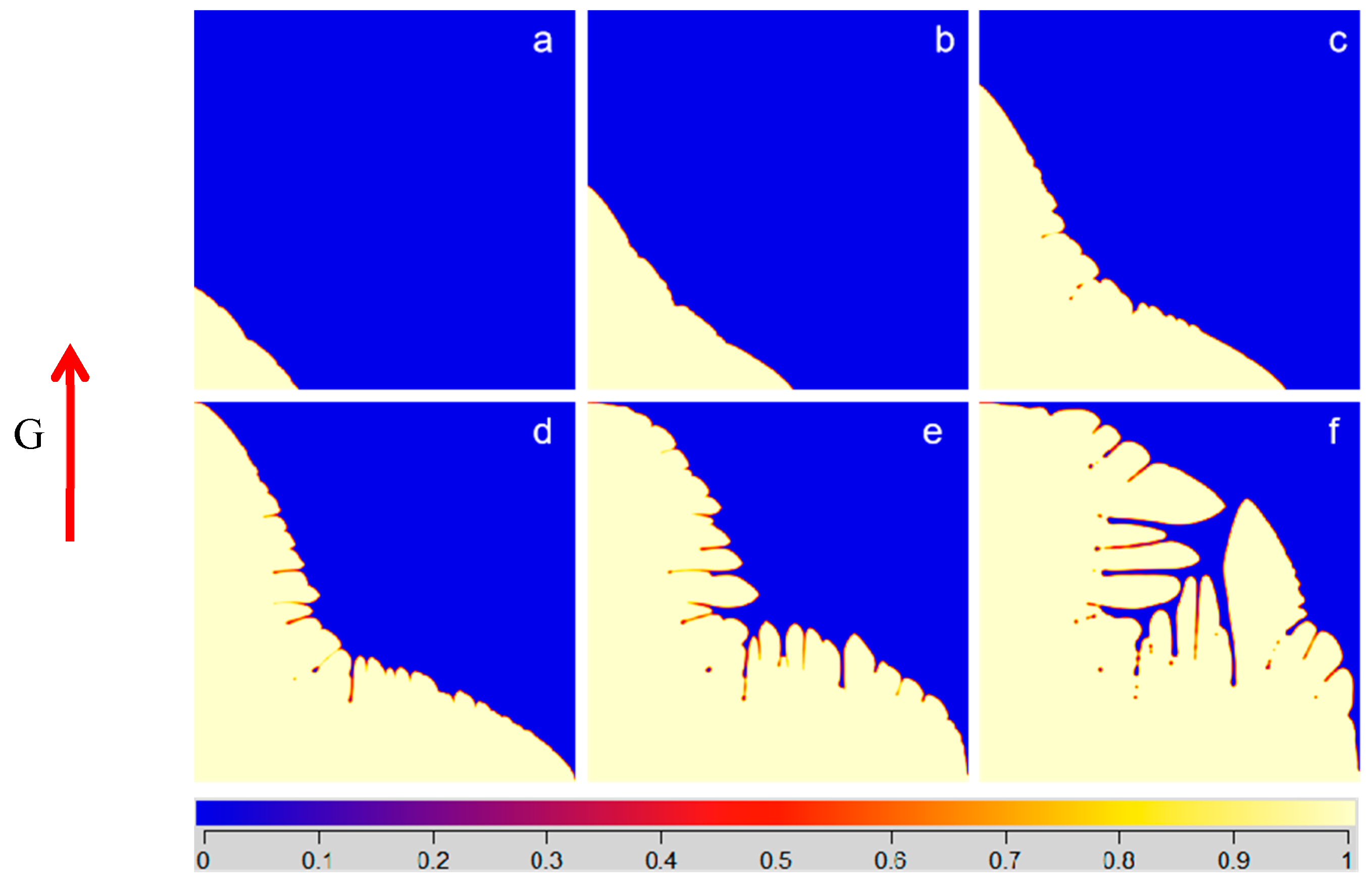
3.1. Effect of Fe Content on the Microstructure of Single Crystal
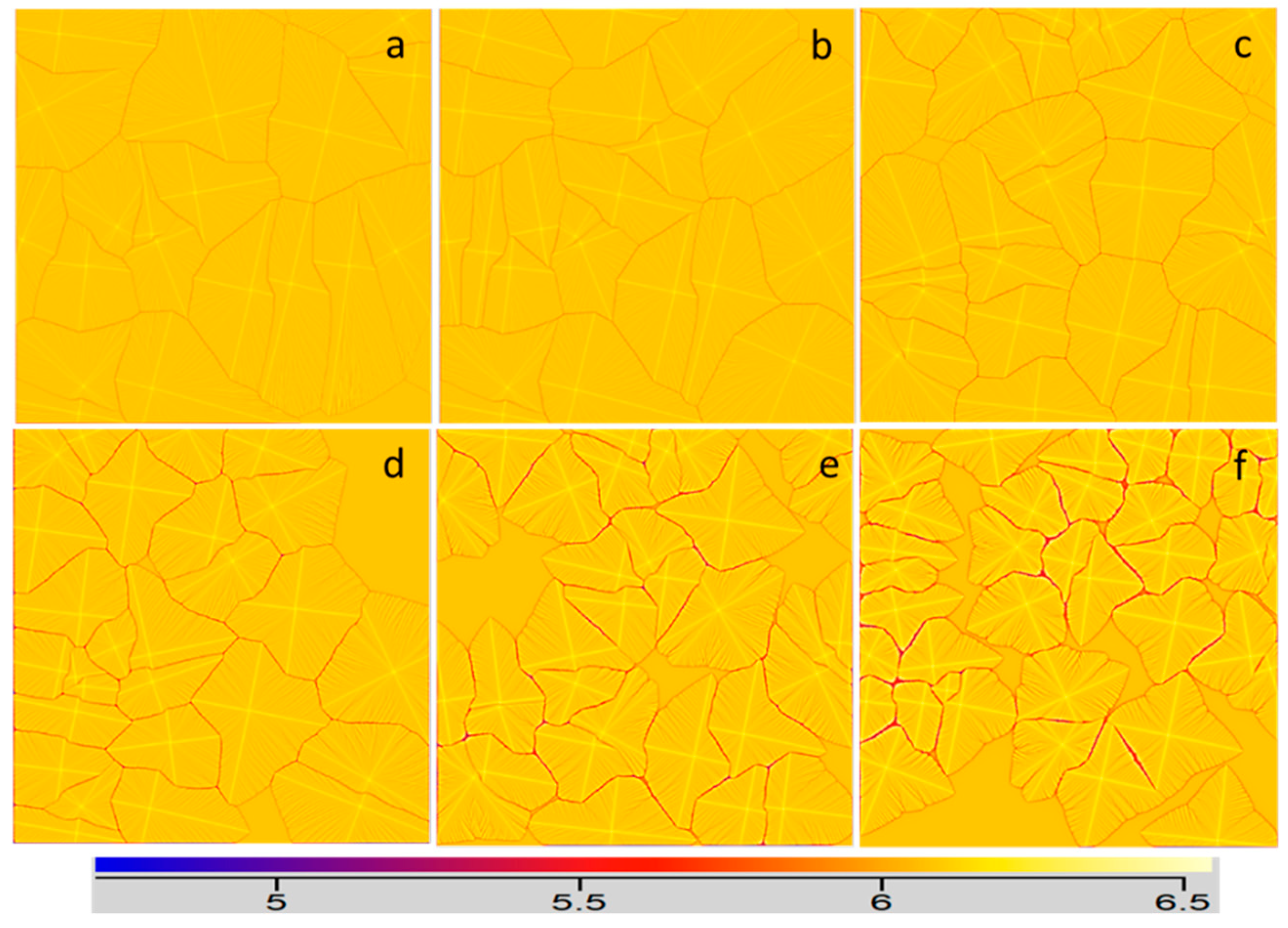
3.2. Effect of Fe Content on the Microstructure of Multiple Grains
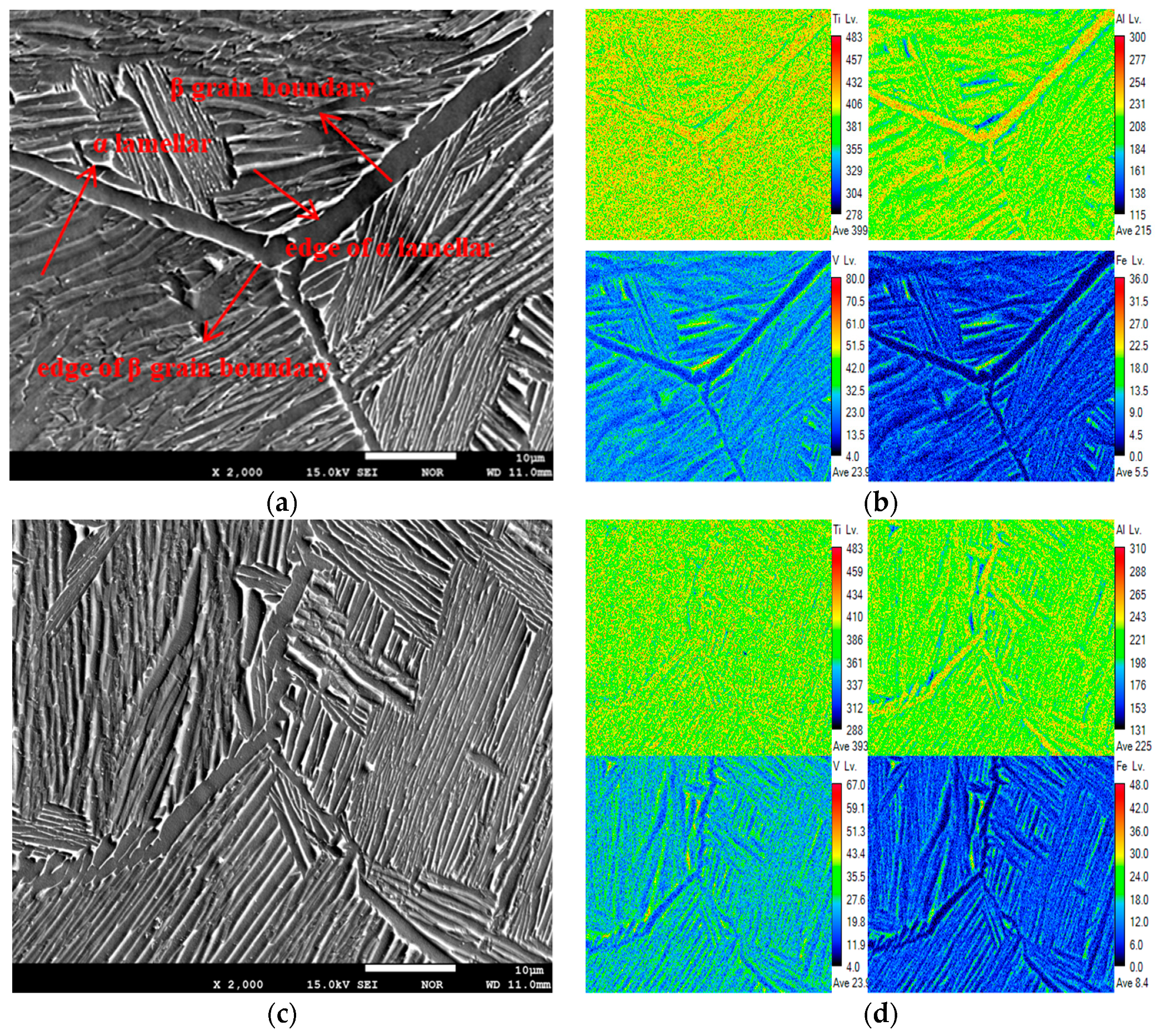
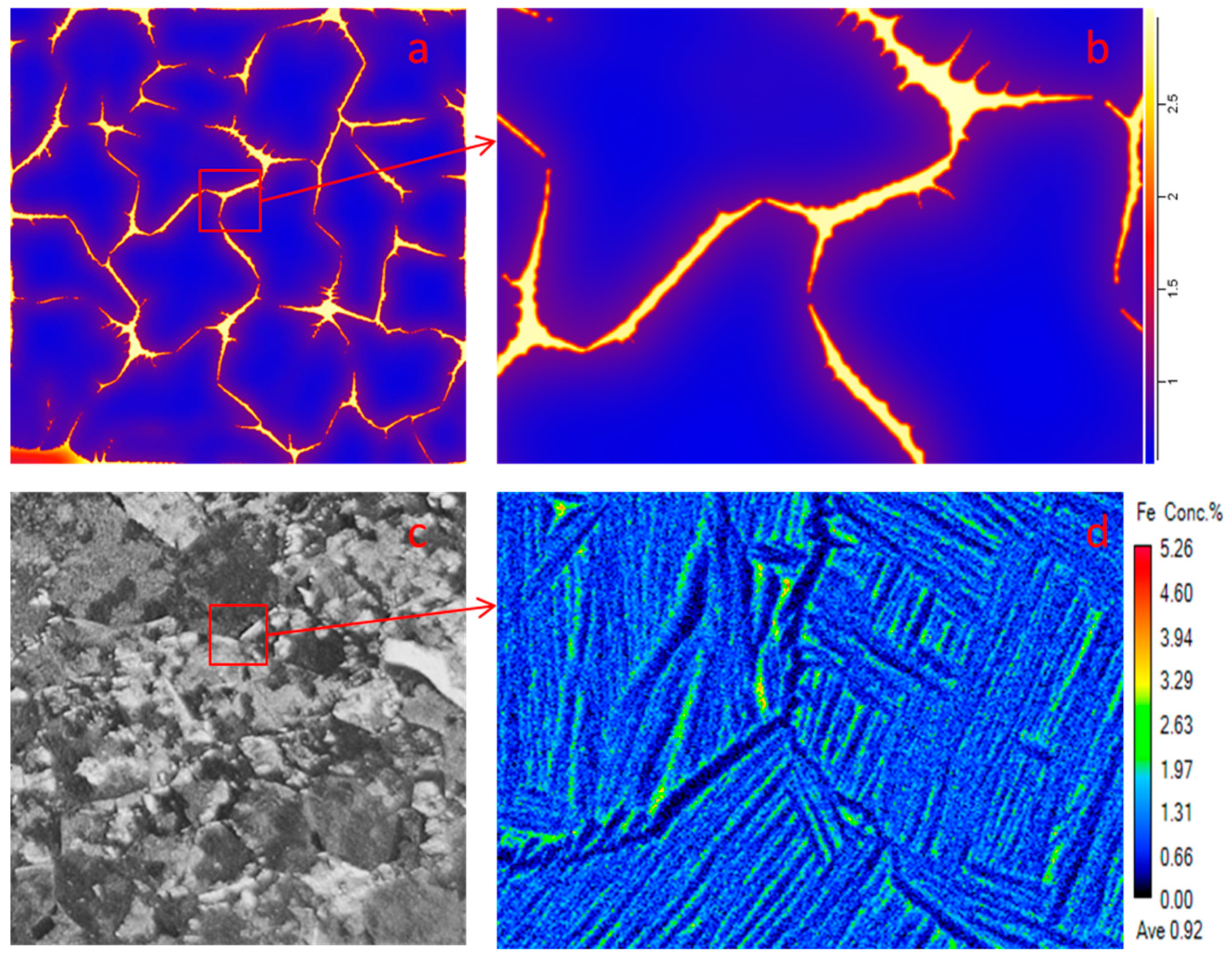
3.3. Element Distribution in Ti–6Al–4V–xFe Alloy
4. Conclusions
- (1)
- The segregation of Fe element at the grain boundary of Ti–6Al–4V–xFe alloys can inhibit the interface mobility, thus promoting the formation of a local supercooling zone and making the grains easier to grow into dendrites.
- (2)
- With the increase of Fe content, the grain size of the alloy decreased gradually. When there was no Fe in the alloy, the grain size was the largest (radius close to 2.29 mm), the grains were more uniform, and the shape of the grain was close to circular. The grain size decreased gradually with an increase in the Fe content and the overall decrease trend slowed down. When the Fe content was 0.9, the average grain radius was the smallest, which was about 1.03 mm.
- (3)
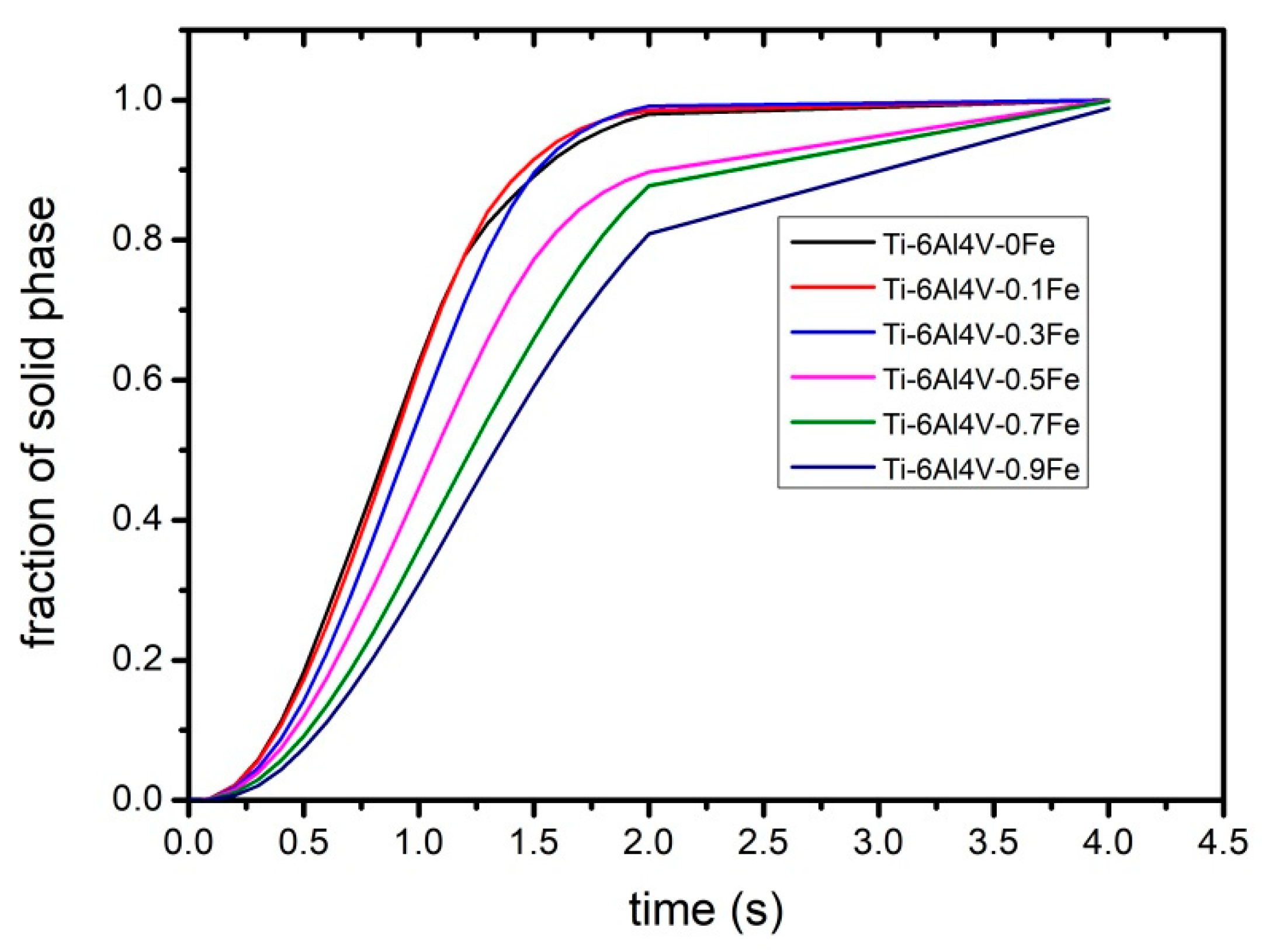
- With the increase in Fe content, the distance of diffusion layer δn increased in the liquid phase. Within a certain range, there was a linear relationship between them.
- (4)
- The segregation of Fe was more obvious than that of Al and V. With the increase in Fe content, the segregation of Fe increased, but there was less of an effect on Al and V.
Author Contributions
Funding
Conflicts of Interest
References
- Allen, P. Titanium alloy development. Adv. Mater. Process. 1996, 150, 35–37. [Google Scholar]
- Lütjering, G.; Williams, J.C. Titanium; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Xin-ping, Z.; Si-rong, Y.; Zhen-ming, H.; Qiu-hua, H. Mechanical properties of new type ti-fe-mo-mn-nb-zr titanium alloy. Chin. J. Nonferrous Met. 2002, 12, 78–82. [Google Scholar]
- Hotta, S.; Yamada, K.; Murakami, T.; Narushima, T.; Iguchi, Y.; Ouchi, C. Beta. Grain refinement due to small amounts of yttrium addition in.Alpha.+.Beta. Type titanium alloy, sp-700. ISIJ Int. 2006, 46, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Kudo, T.; Murakami, S.; Itsumi, Y. Influence of microstructure on formability in ti-fe alloy. J. Mater. Process. Technol. 2010, 60, 33–36. [Google Scholar]
- Bermingham, M.J.; Mcdonald, S.D.; Stjohn, D.H.; Dargusch, M.S. Segregation and grain refinement in cast titanium alloys. J. Mater. Res. 2009, 24, 1529–1535. [Google Scholar] [CrossRef]
- Ehtemam-Haghighi, S.; Liu, Y.; Cao, G.; Zhang, L.-C. Phase transition, microstructural evolution and mechanical properties of ti-nb-fe alloys induced by fe addition. Mater. Des. 2016, 97, 279–286. [Google Scholar] [CrossRef]
- Boettinger, W.J.; Warren, J.A.; Beckermann, C.; Karma, A. Phase-field simulation of solidification. Ann. Rev. Mater. Res. 2002, 32, 163–194. [Google Scholar] [CrossRef]
- Gyoon Kim, S.; Tae Kim, W.; Suzuki, T.; Ode, M. Phase-field modeling of eutectic solidification. J. Cryst. Growth 2004, 261, 135–158. [Google Scholar] [CrossRef]
- Suzuki, T.; Ode, M.; Kim, S.G.; Kim, W.T. Phase-field model of dendritic growth. J. Cryst. Growth 2002, 237, 125–131. [Google Scholar] [CrossRef]
- Kermanpur, A.; Jafari, M.; Vaghayenegar, M. Electromagnetic-thermal coupled simulation of levitation melting of metals. J. Mater. Process. Technol. 2011, 211, 222–229. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; He, H.; Huangfu, Y.; Zhu, J. Electromagnetic-thermal-deformed-fluid-coupled simulation for levitation melting of titanium. IEEE Trans. Magn. 2016, 52, 1–4. [Google Scholar] [CrossRef]
- Kundin, J.; Kumar, R.; Schlieter, A.; Choudhary, M.A.; Gemming, T.; Kühn, U.; Eckert, J.; Emmerich, H. Phase-field modeling of eutectic ti–fe alloy solidification. Comput. Mater. Sci. 2012, 63, 319–328. [Google Scholar] [CrossRef]
- Gong, X.; Chou, K. Phase-field modeling of microstructure evolution in electron beam additive manufacturing. JOM J. Miner. Met. Mater. Soc. 2015, 67, 1176–1182. [Google Scholar] [CrossRef]
- Sahoo, S.; Chou, K. Phase-field simulation of microstructure evolution of ti–6al–4v in electron beam additive manufacturing process. Addit. Manuf. 2016, 9, 14–24. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J. Phase field simulation of dendritic solidification of ti-6al-4v during additive manufacturing process. JOM 2018, 70, 2392–2399. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Ohshida, S.; Nonaka, K.; Yoshida, Y.; Fujita, F.E. Diffusion of iron in β ti-fe alloys. Scr. Mater. 1996, 34, 949–953. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Tang, B.; Kou, H.; Segurado, J.; Cui, Y. Computational study of atomic mobility for bcc phase in ti–al–fe system. Calphad 2014, 46, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Tiley, J.S.; Shiveley, A.R.; Pilchak, A.L.; Shade, P.A.; Groeber, M.A. 3d reconstruction of prior β grains in friction stir–processed ti–6al–4v. J. Microsc. 2014, 255, 71–77. [Google Scholar] [CrossRef]
- Böttger, B.; Eiken, J.; Apel, M. Phase-field simulation of microstructure formation in technical castings—A self-consistent homoenthalpic approach to the micro–macro problem. J. Comput. Phys. 2009, 228, 6784–6795. [Google Scholar] [CrossRef]
- Eiken, J.; Bottger, B.; Steinbach, I. Multiphase-field approach for multicomponent alloys with extrapolation scheme for numerical application. Phys. Rev. EStat. NonlinearSoft Matter Phys. 2006, 73, 066122. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, X.; Su, Y.; Wu, S.; Li, B.; Fu, H. Phase-field simulation of structure evolution at high growth velocities during directional solidification of ti55al45 alloy. Intermetallics 2005, 13, 275–279. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Dendrite growth at the limit of stability: Tip radius and spacing. Acta Metall. 1981, 29, 11–20. [Google Scholar] [CrossRef]








| Physical Parameters | Ti–6Al–4V–xFe |
|---|---|
| Interface energy σ (J/cm2) | 2 10−5 |
| Al Liquid diffusion coefficient Dl (cm2/s) | 1.5 10−5 |
| Al Solid diffusion coefficient Ds (cm2/s) | 1.3 10−7 |
| V Liquid diffusion coefficient Dl (cm2/s) | 5 10−5 |
| V Solid diffusion coefficient Ds (cm2/s) | 6.9 10−7 |
| Fe Liquid diffusion coefficient Dl (cm2/s) | 1 10−4 |
| Fe Solid diffusion coefficient Ds (cm2/s) | 2 10−5 |
| Molar volume V (cm3/mol) | 11.2 |
| Calculated temperature T (K) | 1950 |
| Anisotropic strength η | 0.05 |
| Alloys | Al (wt%) | V (wt%) | Fe (wt%) | O (wt%) |
|---|---|---|---|---|
| Ti–6Al–4V | 5.98 | 4.10 | 0.03 | 0.083 |
| Ti–6Al–4V–0.1Fe | 5.92 | 4.05 | 0.13 | 0.110 |
| Ti–6Al–4V–0.3Fe | 5.99 | 4.09 | 0.33 | 0.084 |
| Ti–6Al–4V–0.5Fe | 5.95 | 4.07 | 0.52 | 0.076 |
| Ti–6Al–4V–0.7Fe | 5.92 | 4.02 | 0.73 | 0.081 |
| Ti–6Al–4V–0.9Fe | 5.99 | 4.10 | 0.91 | 0.033 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, L.; Hu, R.; Gu, Y.; Zhou, D.; Chen, F.; Zhou, L.; Chang, H. Effect of Fe Content on the As-Cast Microstructures of Ti–6Al–4V–xFe Alloys. Metals 2020, 10, 989. https://doi.org/10.3390/met10080989
Ding L, Hu R, Gu Y, Zhou D, Chen F, Zhou L, Chang H. Effect of Fe Content on the As-Cast Microstructures of Ti–6Al–4V–xFe Alloys. Metals. 2020; 10(8):989. https://doi.org/10.3390/met10080989
Chicago/Turabian StyleDing, Ling, Rui Hu, Yulei Gu, Danying Zhou, Fuwen Chen, Lian Zhou, and Hui Chang. 2020. "Effect of Fe Content on the As-Cast Microstructures of Ti–6Al–4V–xFe Alloys" Metals 10, no. 8: 989. https://doi.org/10.3390/met10080989
APA StyleDing, L., Hu, R., Gu, Y., Zhou, D., Chen, F., Zhou, L., & Chang, H. (2020). Effect of Fe Content on the As-Cast Microstructures of Ti–6Al–4V–xFe Alloys. Metals, 10(8), 989. https://doi.org/10.3390/met10080989





