Effects of Mold Materials on the Interfacial Reaction between Magnesium Alloy and Ceramic Shell Mold during Investment Casting
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Shell Mold Preparation
2.2. Melting and Casting Experiments
2.3. Evaluation of Microstructure and Mold-Metal Reaction
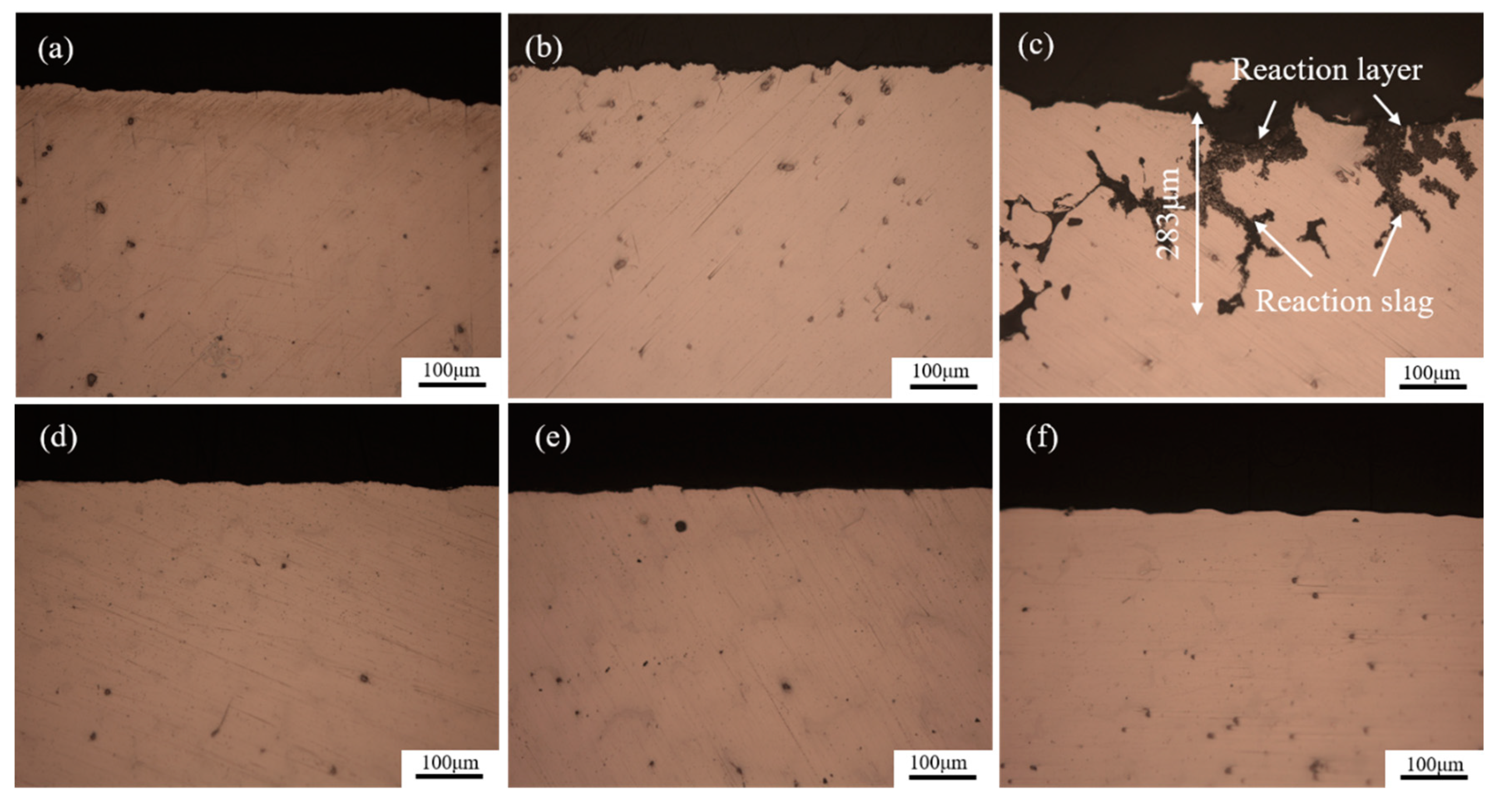
3. Results and Discussions
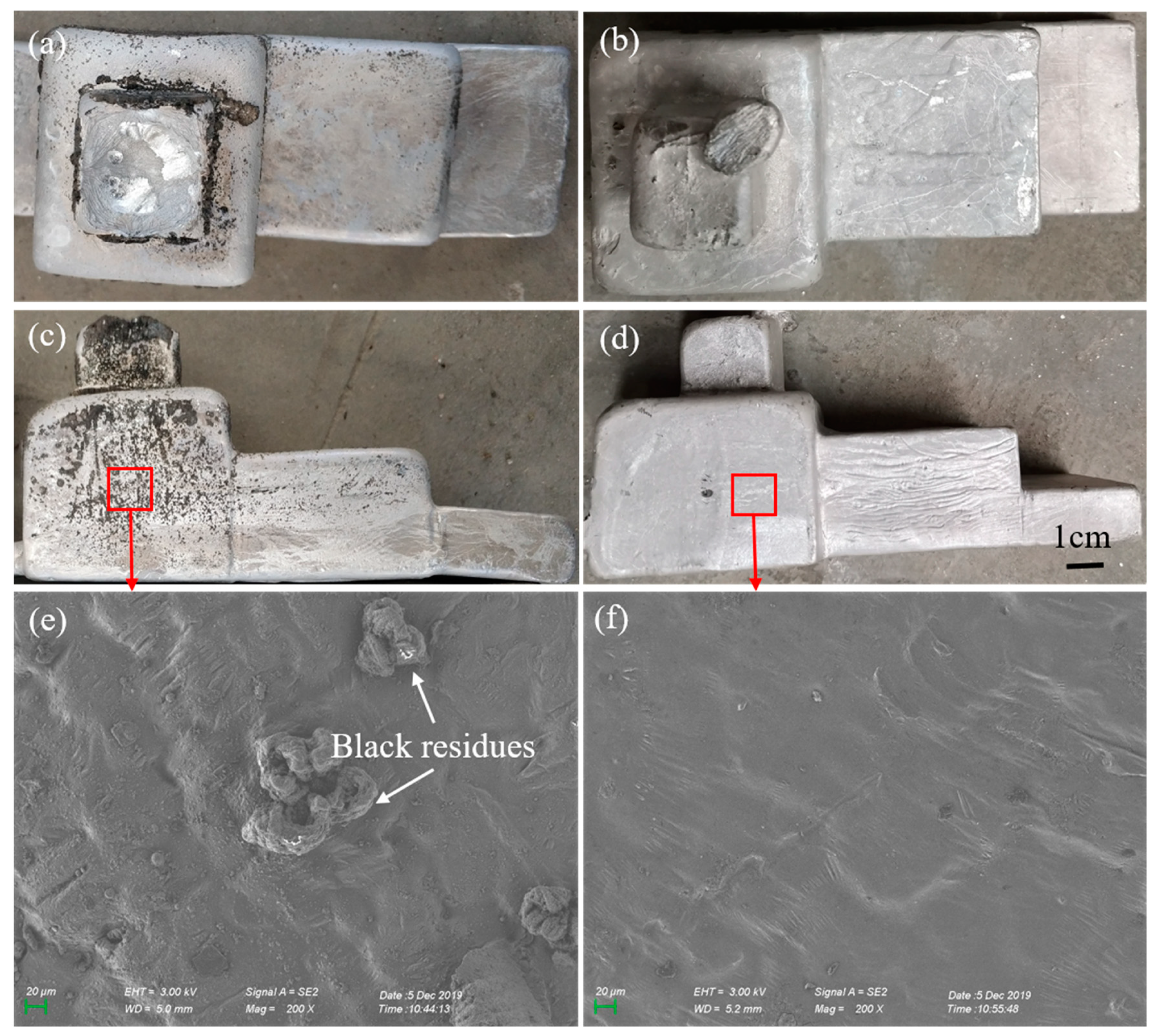
3.1. Surface Structure of the Investment Castings
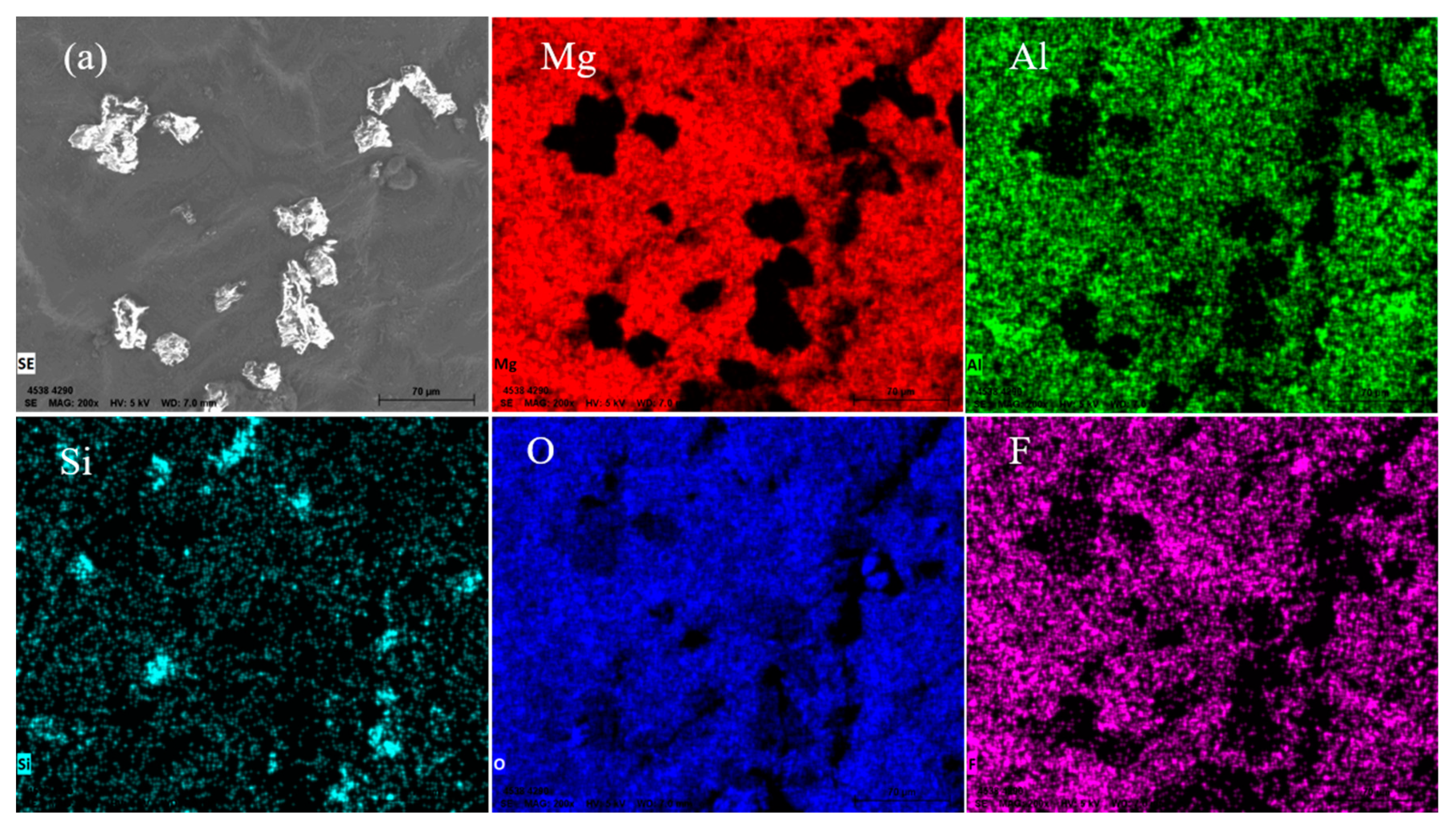
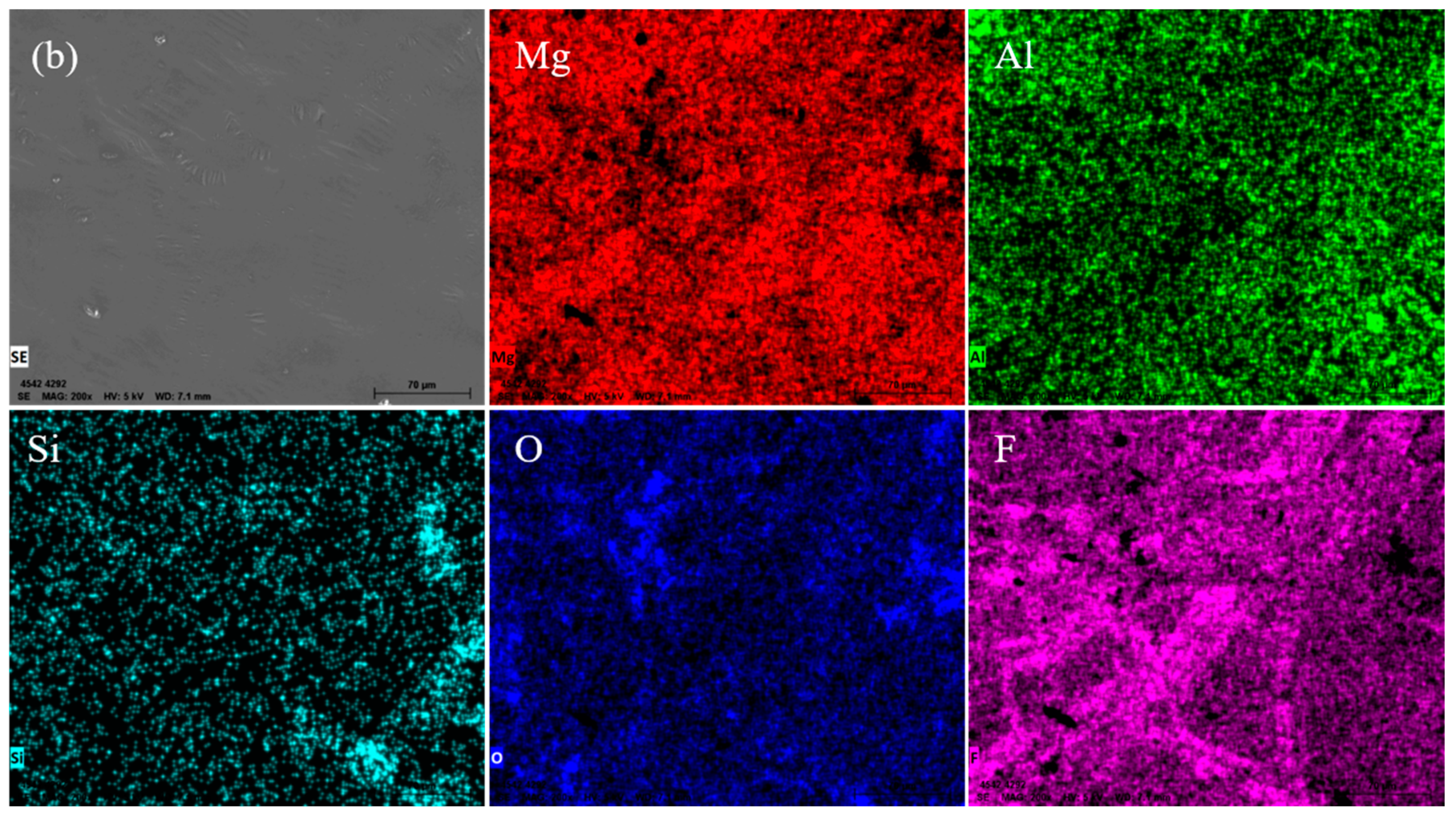
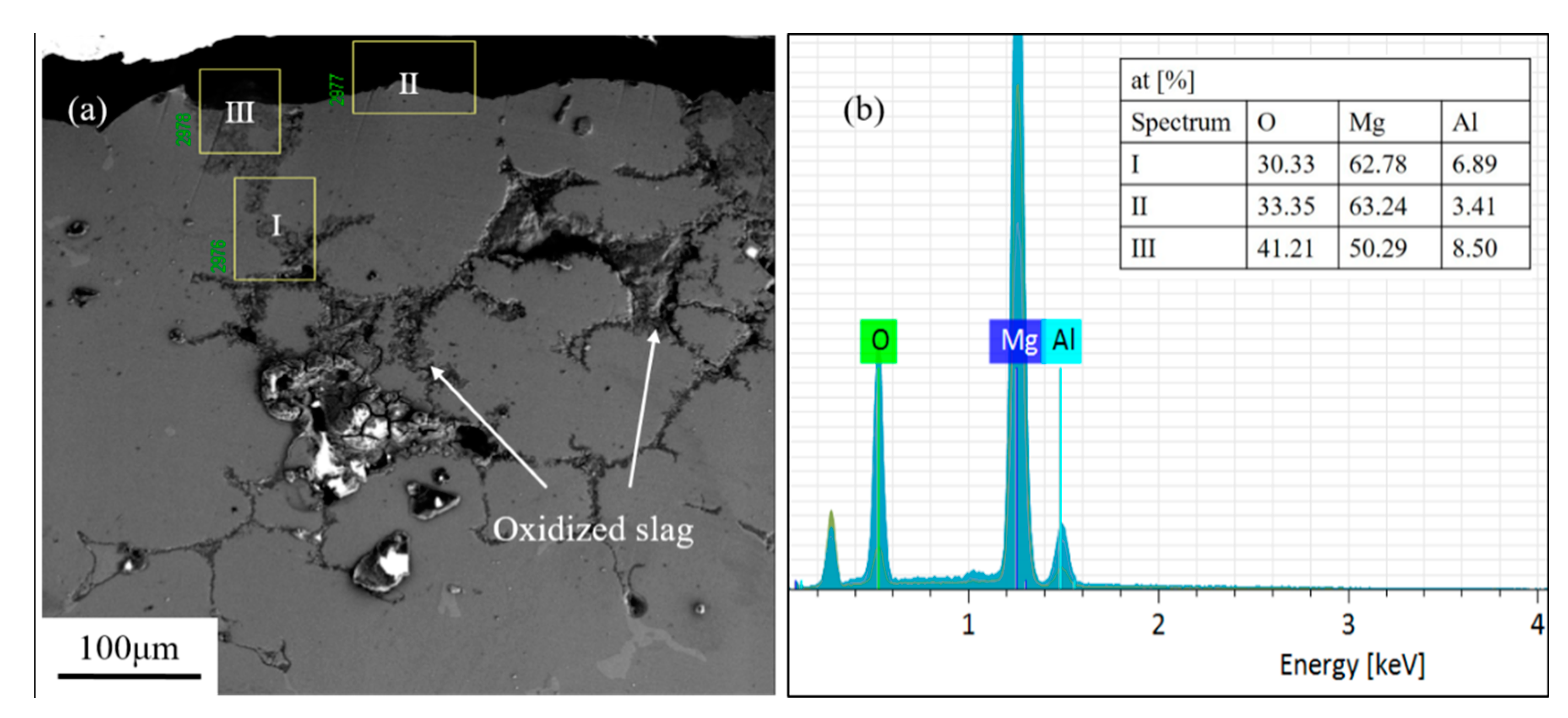
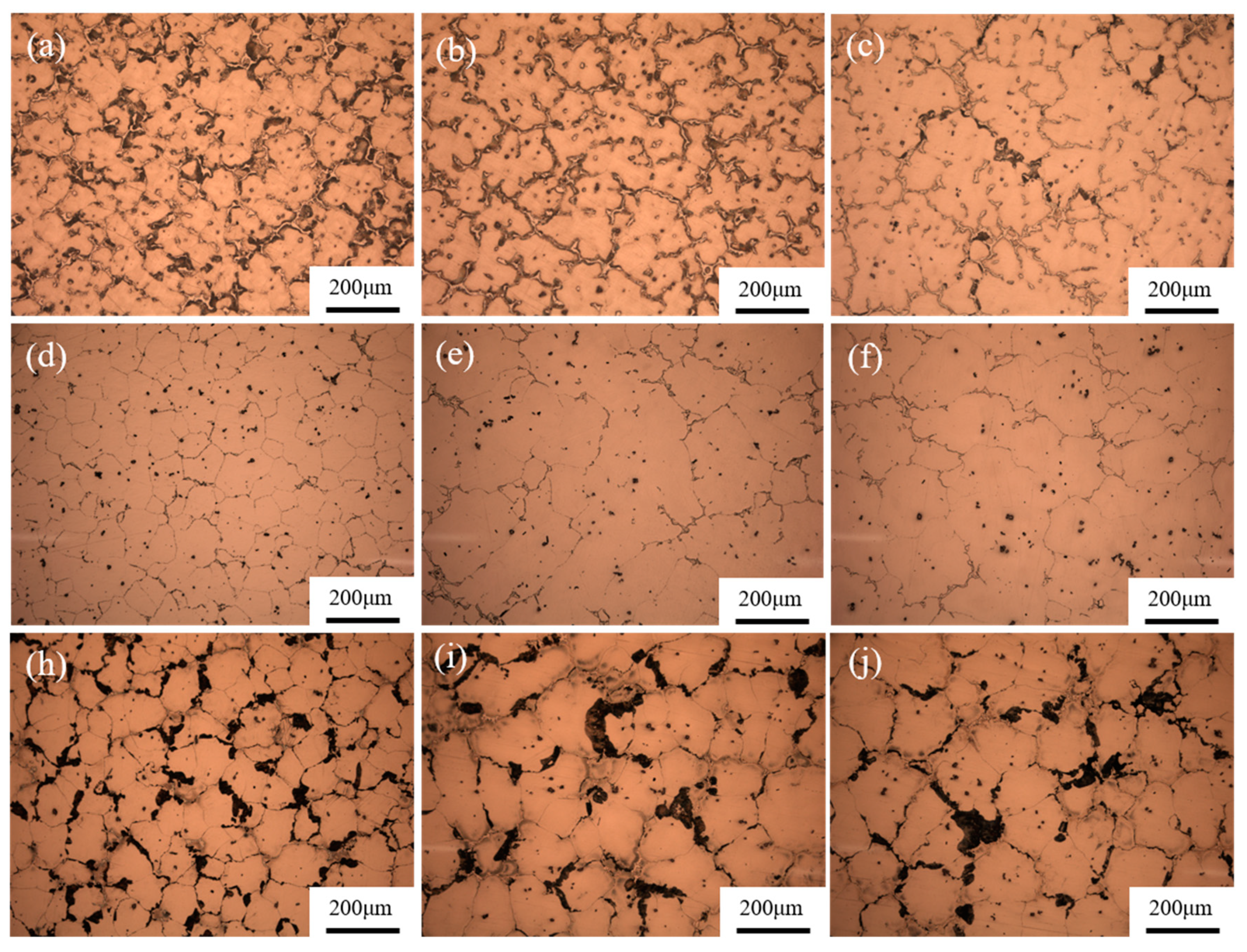
3.2. Section Structure of the Investment Castings
3.3. Microstructure and Mechanical Properties
4. Conclusions
- (1)
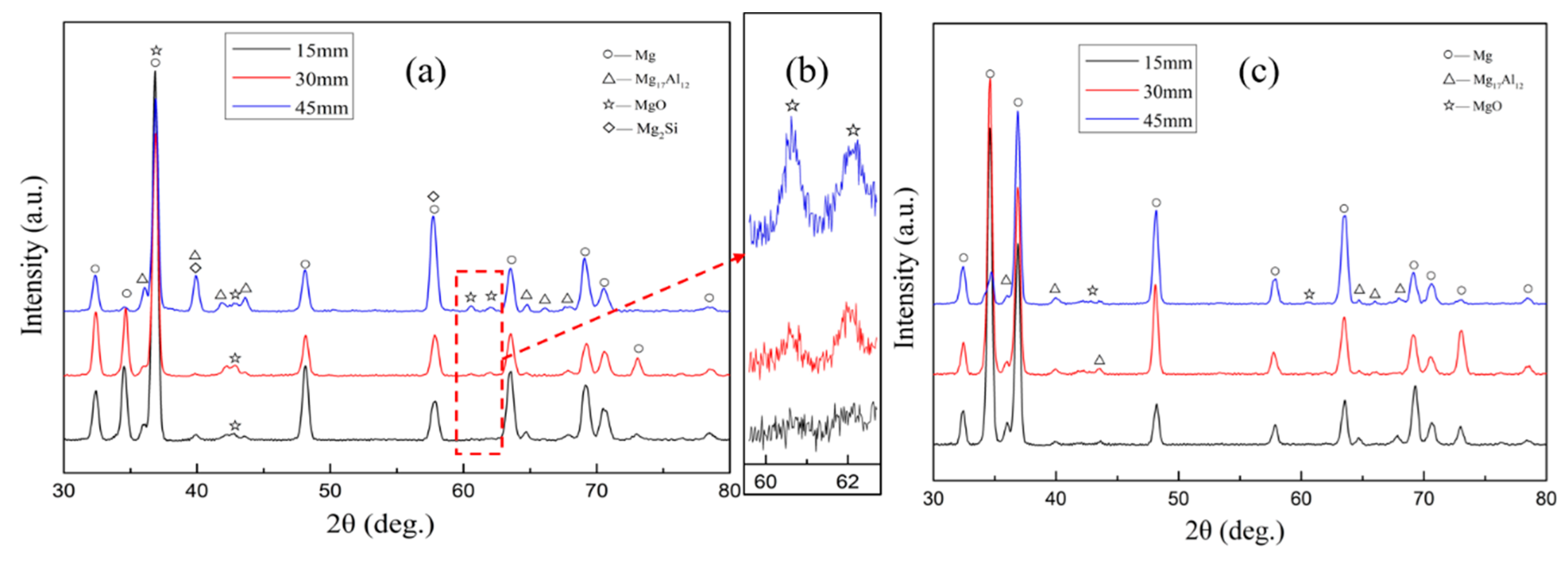
- Surface layer materials have a significant effect on the interfacial reaction between the AZ91D alloy investment casting and the ceramic shell mold. The chemical stability of the Al2O3 mold is better than that of the ZrSiO4 mold.
- (2)
- A serious reaction occurred on the casting surface of the ZrSiO4 mold; the main substance reacting with the magnesium alloy is silica. Dense black residues are formed on the casting surface and they mainly contain the reaction products MgO and Mg2Si. The interfacial reaction equation is: 4Mg + SiO2 = Mg2Si + 2MgO.
- (3)
- Wall thickness has a great influence on the interfacial reaction between the magnesium alloy and the ZrSiO4 mold. The interfacial reaction become more severe as the wall thickness increases. However, for the Al2O3 mold the sample thickness has no obvious effect on the mold-metal interface.
- (4)
- The Al2O3 mold has a good chemical stability to the magnesium molten alloy, leading to a high surface quality investment casting with a smooth and light surface. The main component of the surface film was MgO and Mg2F, and the reaction layer was 1.5 μm thin on average.
- (5)
- The casting thickness have a great influence on the microstructures and mechanical properties of AZ91D investment casting. The casting at 15 mm thickness has the smallest grain size and highest mechanical properties, with a 235 MPa UTS and 5.4% EL.
Author Contributions
Funding
Conflicts of Interest
References
- Jia, G.; Du, J. Foreign metal ions to control the morphology of Solution-Liquid-Solid reaction. Cryst. Growth Des. 2018, 18, 7489–7495. [Google Scholar] [CrossRef]
- Jia, G.; Du, J. Solution–Liquid–Solid growth of CuInTe2 and CuInSexTe2−x semiconductor nanowires. Inorg. Chem. 2018, 57, 14961–14966. [Google Scholar] [CrossRef]
- Jia, G.; Du, J. Catalyst-Assisted Solution–Liquid–Solid synthesis of CdS/CuInSe2 and CuInTe2/CuInSe2 nanorod heterostructures. Inorg. Chem. 2018, 58, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.M.; Chua, C.K.; Lee, C.W.; Feng, C.; Totong, K. Rapid prototyping and tooling techniques: A review of applications for rapid investment casting. Int. J. Adv. Manuf. Technol. 2005, 25, 308–320. [Google Scholar] [CrossRef]
- Pattnaik, S.; Karunakar, D.B.; Jha, P.K. Developments in investment casting process—A review. J. Mater. Process. Technol. 2012, 212, 2332–2348. [Google Scholar] [CrossRef]
- Jiayi, W.; Santosh, R.S.; Paul, C.L.; Guha, M. Design and Topology Optimization of 3D-Printed Wax Patterns for Rapid Investment Casting. Procedia Manuf. 2019, 34, 683–694. [Google Scholar]
- Hassan, J.; Mohd, H.I.; Ali, O. A Review of Ceramic Shell Investment Casting of Magnesium Alloys and Mold-Metal Reaction Suppression. Mater. Manuf. Process. 2013, 28, 843–856. [Google Scholar]
- Gurutze, A.; Iñaki, H.; Jukka, V.; Cingi, C.; Devenyi, S.; Townsend, J.; Mahmood, S.; Wendt, A.; Weiss, K.; Ben-Dov, A. Development of Investment-Casting Process of Mg-Alloys for Aerospace Applications. Adv. Eng. Mater. 2007, 9, 751–756. [Google Scholar]
- Zhan, Z.; Guy, M. Effect of inhibitor gas on mould-magnesium reactions in investment casting. Magnes. Technol. 2004, 197–202. [Google Scholar]
- Idris, M.H.; Clegg, A.J. The processing and evaluation of an investment-cast magnesium–base alloy. Trans. Am. Foundrymen’s Soc. 1996, 104, 237–244. [Google Scholar]
- Sin, S.L.; Dubé, D. Influence of process parameters on fluidity of investment-cast AZ91D magnesium alloy. Mater. Sci. Eng. A 2004, 386, 34–42. [Google Scholar] [CrossRef]
- Hassan, J.; Mohd, H.I.; Ali, O.; Farahany, S. In situ melting and solidification assessment of AZ91D granules by computer-aided thermal analysis during investment casting process. Mater. Des. 2013, 50, 181–190. [Google Scholar]
- Sin, S.L.; Dubé, D.; Tremblay, R. Interfacial reactions between AZ91D magnesium alloy and plaster mould material during investment casting. Mater. Sci. Technol. 2006, 22, 1456–1463. [Google Scholar] [CrossRef]
- Sin, S.L.; Dubé, D.; Tremblay, R. An investigation on microstructural and mechanical properties of solid mould investment casting of AZ91D magnesium alloy. Mater. Charact. 2008, 59, 178–187. [Google Scholar] [CrossRef]
- Shaekwang, K.; Taewhan, H.; Youngjig, K. Evaluation of Thermal Stability of Mold Materials for Magnesium Investment Casting. Mater. Trans. 2001, 42, 539–542. [Google Scholar]
- Kim, S.K.; Youn, J.I.; Kim, Y.J. Rotating cylinder manufacturing method and investment casting of SiCp/AZ91 HP magnesium composites. Mater. Sci. Technol. 2000, 16, 769–775. [Google Scholar] [CrossRef]
- Shaekwang, K.; Myounggyun, K.; Taewhan, H. Investment Casting of AZ91HP Magnesium Alloy. Met. Mater. 2000, 6, 275–279. [Google Scholar]
- Jafari, H.; Idris, M.H.; Ourdjini, A.; Kadir, M.R.A. An investigation on interfacial reaction between in-situ melted AZ91D magnesium alloy and ceramic shell mold during investment casting process. Mater. Chem. Phys. 2013, 138, 672–681. [Google Scholar] [CrossRef]
- Hassan, J.; Mohd, H.I.; Ali, O. An alternative approach in ceramic shell investment casting of AZ91D magnesium alloy: In situ melting technique. J. Mater. Process. Technol. 2014, 214, 988–997. [Google Scholar]
- Cingi, C. Mold-Metal Reactions in Magnesium Investment Castings; ProQuest Dissertations Publishing: Ann Arbor, MI, USA, 2006; pp. 37–42. [Google Scholar]
- Freitag, L.; Schafföner, S.; Lippert, N.; Fabauer, C.; Aneziris, C.G.; Legner, C.; Klotz, U.E. Silica-free investment casting molds based on calcium zirconate. Ceramics International. Ceram. Int. 2017, 43, 6807–6814. [Google Scholar] [CrossRef]
- Vyas, A.V.; Ayar, V.S.; Sutaria, M.P. Investigation on reactive wetting during investment casting of magnesium alloy AZ91. Mater. Today Proc. 2020, 2, 1–6. [Google Scholar] [CrossRef]
- Lopes, V.; Puga, H.; Barbosa, J.; Teixeira, J.C. Effect of yttria mould coating on the investment casting of AZ91D-1wt % CaO magnesium alloy. Int. J. Met. 2019, 14, 98–107. [Google Scholar]
- Gomes, F.; Puga, H.; Barbosa, J.; Ribeiro, C.S. Effect of melting pressure and superheating on chemical composition and contamination of yttria-coated ceramic crucible induction melted titanium alloys. J. Mater. Sci. 2011, 46, 4922–4936. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Jiang, B.; Xu, Y. Investment Casting Manual; Machinery Industry Press: Beijing, China, 2000; pp. 3–5. [Google Scholar]
- Dong, H.; Jin, Z.; Jinfeng, H.; Yong, L.; Guangyu, H. A review on ignition mechanisms and characteristics of magnesium alloys. J. Magnes. Alloy. 2020, 8, 329–344. [Google Scholar]
- Cashion, S.P.; Ricketts, N.J.; Hayes, P.C. Characterisation of protective surface films formed on molten magnesium protected by air/SF6 atmospheres. J. Light Met. 2002, 2, 37–42. [Google Scholar] [CrossRef]
- Pettersen, G.; Øvrelid, E.; Tranell, G.; Fenstad, J.; Gjestland, H. Characterisation of the surface films formed on molten magnesium in different protective atmospheres. Mater. Sci. Eng. A 2002, 332, 285–294. [Google Scholar] [CrossRef]
- Zeng, R.; Chiu, Y.; Jones, I.P. Characterisation of nano-sized Al–Mn–(Mg) particles in AZ91 and their effect on Mg17Al12 precipitation. J. Alloy. Compd. 2013, 579, 34–38. [Google Scholar] [CrossRef]
- Easton, M.; Abbott, T.; Cáceres, C. The effect of microstructural features and defects on the ductility of high-pressure die cast AS21, AM60 and AZ91. Mater. Sci. Forum 2003, 147, 419–422. [Google Scholar] [CrossRef]
- Nawrocki, J.; Motyka, M.; Szeliga, D.; Ziaja, W.; Cygan, R.; Sieniawski, J. Effect of cooling rate on macro-and microstructure of thin-walled nickel superalloy precision castings. J. Manuf. Process. 2020, 49, 153–161. [Google Scholar] [CrossRef]
- Jia, G.; Wang, C.; Yang, P.; Liu, J.; Zhang, W.; Li, R.; Zhang, S.; Du, J. Sulfur-free synthesis of size tunable rickardite (Cu3−xTe2) spheroids and planar squares. R. Soc. Open Sci. 2019, 6, 181602. [Google Scholar] [CrossRef] [Green Version]
- Jia, G.; Liu, B.; Wang, K.; Wang, C.; Yang, P.; Liu, J.; Zhang, W.; Li, R.; Zhang, S.; Du, J. CuInTe2 Nanocrystals: Shape and Size Control, Formation Mechanism and Application, and Use as Photovoltaics. Nanomaterials 2019, 9, 409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Liu, B.; Yang, P.; Liu, J.; Zhang, W.; Wang, C.; Lv, H.; Jia, G.; Li, R.; Zhang, S. One-pot synthesis of branched CuInSe2 nanowires based on solution-liquid-solid method and their implementation in photovoltaic devices. J. Cryst. Growth 2019, 523, 125152. [Google Scholar] [CrossRef]
- Jia, G.; Wang, K.; Liu, B.; Yang, P.; Liu, J.; Zhang, W.; Li, R.; Wang, C.; Zhanag, S.; Du, J. Cation exchange synthesis of CuInxGa1−xSe2 nanowires and their implementation in photovoltaic devices. RSC Adv. 2019, 9, 35780. [Google Scholar] [CrossRef] [Green Version]
- Zheng, N.C.; Li, X.F.; Yan, S.; Wang, Q.; Qiao, R.; Hu, J.H.; Fan, J.J.; Cao, G.Q.; Shao, G.S. Nano-porous hollow Li0.5La0.5TiO3 spheres and electronic structure modulation for ultra-fast H2S detection. J. Mater. Chem. A 2020, 8, 2376–2386. [Google Scholar] [CrossRef]
- Flemings, M.C. Solidification Processing; Metallurgical Transactions: New York, NY, USA, 1974. [Google Scholar]




| Mold Type | Coat No. | Slurry | Stucco | Dry Time (h) | |
|---|---|---|---|---|---|
| Oxide | Binder | ||||
| ZrSiO4 mold | 1 | zircon (325 mesh) | Colloidal silica | zircon powder (120 mesh) | 12 |
| 2–6 | mullite (120 mesh) | Colloidal silica | mullite (40–80 mesh) | 8 | |
| 7 | mullite (120 mesh) | Colloidal silica | 8 | ||
| Al2O3 mold | 1 | corundum (325 mesh) | alkaline zirconium sol | corundum powder (120 mesh) | 12 |
| 2–6 | mullite (120 mesh) | Colloidal silica | mullite (40–80 mesh) | 8 | |
| 7 | mullite (120 mesh) | Colloidal silica | 8 | ||
| Al | Zn | Mn | Fe | Si | Cu | Ni | Mg |
|---|---|---|---|---|---|---|---|
| 8.95 | 0.54 | 0.33 | <0.001 | <0.01 | <0.001 | <0.001 | Bal. |
| Thickness | UTS/MPa | YS/MPa | δ/% |
|---|---|---|---|
| 15 mm | 235 | 167 | 5.4 |
| 30 mm | 214 | 147 | 5.6 |
| 45 mm | 194 | 121 | 4.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Y.; Liu, J.; Du, J.; Zhang, W.; Xiao, Y.; Zhang, S.; Yang, P. Effects of Mold Materials on the Interfacial Reaction between Magnesium Alloy and Ceramic Shell Mold during Investment Casting. Metals 2020, 10, 991. https://doi.org/10.3390/met10080991
Hao Y, Liu J, Du J, Zhang W, Xiao Y, Zhang S, Yang P. Effects of Mold Materials on the Interfacial Reaction between Magnesium Alloy and Ceramic Shell Mold during Investment Casting. Metals. 2020; 10(8):991. https://doi.org/10.3390/met10080991
Chicago/Turabian StyleHao, Yiwei, Jinxue Liu, Jiang Du, Wenjie Zhang, Yang Xiao, Shaojun Zhang, and Peixu Yang. 2020. "Effects of Mold Materials on the Interfacial Reaction between Magnesium Alloy and Ceramic Shell Mold during Investment Casting" Metals 10, no. 8: 991. https://doi.org/10.3390/met10080991





