Microorganisms and Plants in the Recovery of Metals from the Printed Circuit Boards of Computers and Cell Phones: A Mini Review
Abstract
:1. Introduction
2. The Printed Circuit Boards of Computers and Cell Phones
3. Microorganisms in the Recovery of Metals from the Printed Circuit Boards of Computers and Cell Phones
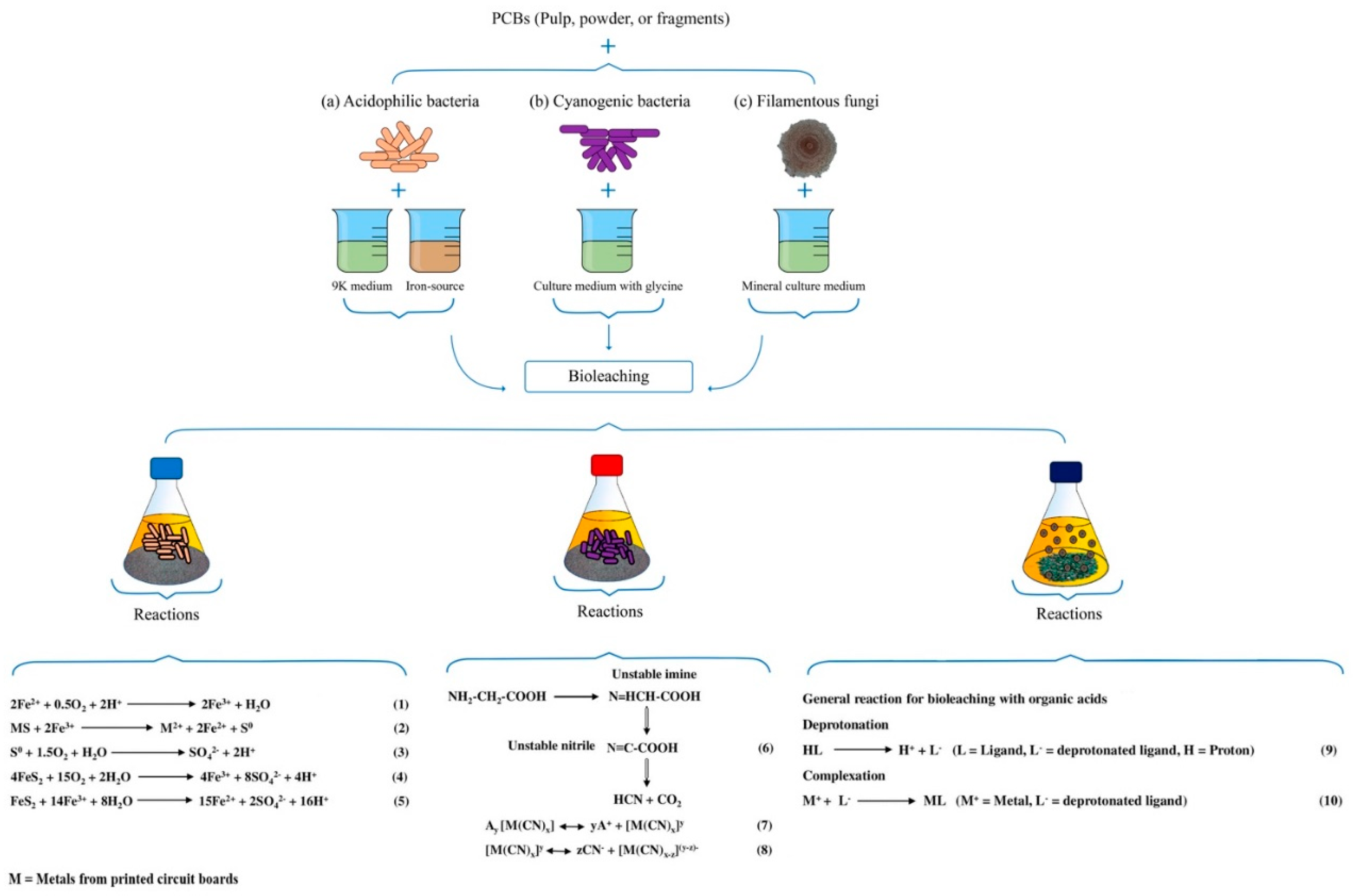
| Prokaryote (Bacteria or Archaea) | Prokaryote Type | PCBs Type | Recovered Metals * | Bioleaching Time | Bioleaching Type | Original Conditions | Optimized Conditions | References |
|---|---|---|---|---|---|---|---|---|
| Acidithiobacillus ferroxidans | Acidophilic | Computers | 82% Cu, and 18% Cu | 6 days | One-step | Absence of ferrous ions in the culture medium. | Addition of ferrous ion (7 g·L−1). | [52] |
| Acidithiobacillus ferroxidans | Acidophilic | Computers | 99% Cu | 9 days | One-step | Two sieve fractions (1.0–3.0 and 0.5–1.0 mm). In a concentration range of PCBs from 0.7% to 1.9% w/v. | Decrease of the sieve fraction (0.5–1.0 mm) and decrease of the concentration of the PCBs (0.7% w/v). | [53] |
| Acidithiobacillus thiooxidans | Acidophilic | Computers | 75% Cu | 9 days | One-step | Two sieve fractions (1.0–3.0 and 0.5–1.0 mm). In a concentration range of PCBs from 0.7% to 1.9% w/v | Decrease of the sieve fraction (0.5–1.0 mm) and decrease of the concentration of the PCBs (0.7% w/v). | [53] |
| Leptospirillum ferriphilum-dominated consortium | Acidophilic | Computers | 60% Cu, 76% Zn, and 71% Ni | 10–15 days | One-step | Sabouraud dextrose broth (SDB1) supplemented with 45 g·L−1 FeSO4·7H2O and PCBs (1 g). | SDB1 supplemented with 45 g·L−1 FeSO4·7H2O and simultaneous addition to the medium of the inoculum (10% v/v of activated inoculum) and PCBs (1 g) | [51] |
| Leptospirillum ferriphilum-dominated consortium | Acidophilic | Computers | 85% Cu, 97% Zn, and 93% Ni | 6–8 days | Two-step | Biologically generated Fe3+ iron (0.80%) containing medium, free from cells and 1 g PCBs. | Addition of the PCBs to the culture medium after complete oxidation of Fe2+ to Fe3+. Biologically generated Fe3+ iron (0.80%) containing medium with viable inoculum and 1 g PCBs. | [51] |
| Consortium formed by Acidithiobacillus caldus, Leptospirillum ferriphilum, Sulfobacillus thermosulphidooxans, and Ferroplasma sp. | Acidophilic | Computers | 97% Cu | 10 days | One-step | Bioleaching carried out in three 50 L bioreactors (stirred tank) in batch mode and then carried out in continuous mode (pulp density 1% w/v). | The bioleaching experiment carried out in a continuous mode with a slurry flow rate of 10 L per day for 5 days (feeding stopped for the next 5 days to adapt the microorganisms, pulp density 1% w/v). | [77] |
| Acidithiobacillus ferrooxidans | Acidophilic | Computers | 95% Cu, and 75% Zn | 9 days | One-step | Initial conditions (30–50 °C, solid concentration of 5% w/v, and 10 g·L−1 of Fe2+). | Optimization of culture conditions (30 °C, solid concentration of 5% w/v, bacterial inoculum, and 10 g·L−1 of Fe2+). | [64] |
| Leptospirillum ferrooxidans | Acidophilic | Computers | 40% Cu, and 20% Zn | 4 days | One-step | Initial conditions (30–50 °C, solid concentration of 5% w/v, 10 g·L−1 of Fe2+). | Optimization of culture conditions (30 °C, solid concentration of 5% w/v, bacterial inoculum, 10 g·L−1 of Fe2+). | [64] |
| Acidiphilium acidophilum | Acidophilic | Computers | 100% Cu | 2.5 h | Indirect bioleaching | Growth of the bacteria for 10 days in 9 K medium with glucose and sulfur. | Immersion of the PCBs in the culture medium in which the bacteria grew (without cells) + hydrogen peroxide (30%). | [72] |
| Consortium formed by Acidithiobacillus ferrooxidans, Ferroplasma acidiphilum and Leptospirillum ferriphilum | Acidophilic | Computers | 81% Cu | 5 days | One-step | No addition of graphite. | Addition of graphite (0.5 g). | [56] |
| Acidithiobacillus ferrooxidans | Acidophilic | Computers | 32% Cu | 7 days | One-step | Carried out in a range of inoculum concentrations (10–40% w/v), pH level (1.6–2.4), and pulp densities (1–4% w/v). | Optimization of culture conditions (pH 2, pulp density 1% w/v, and 40% v/v bacterial inoculum). | [63] |
| Pseudomonas balearica SAE1 | Cyanogenic | Computers | 74% Au, and 42% Ag | 7 days | Two-step | Initial conditions (5% v/v bacterial inoculum, and 30 °C, without PCBs). | Optimization of culture conditions (pH 8.6, pulp density 0.5% w/v, 6.8 g·L−1 glycine, and 31.2 °C ). | [62] |
| Pseudomonas chlororaphis | Cyanogenic | Cell phones | 52% Cu, 8% Au, and 12% Ag | 4 days | One-step | Carried out with different temperatures (15, 25, and 35 °C), pH 7 and 10, and different amino acids (glycine, methionine, and glycine + methionine). | Optimization of culture conditions (pH 7, 4.4 g·L−1 glycine + 2 g·L−1 methionine, and 25 °C). | [78] |
| Chromobacterium violaceum | Cyanogenic | Cell phones | 25% Cu, and 11% Au | 8 days | One-step | No addition of hydrogen peroxide. | Addition of hydrogen peroxide. | [49] |
| Chromobacterium violaceum | Cyanogenic | Cell phones | 38% Cu, and 11% Au | 8 days | One-step | No addition of small amounts of metal ions. | Addition of small amounts of metal ions (Na+, Mg2+, Pb2+ and Fe2+). | [79] |
| Acidithiobacillus ferrooxidans | Acidophilic | Cell phones | 100% Cu | 21 days | One-step | Bacteria inoculated into 9 K medium at volume doses of 10%, 20%, 50%, and 100% (v/v) at 20–22 °C and pH 2.5. | The highest recovery of copper obtained with 100% dose. | [50] |
| Leptospirillum ferriphilum-dominated consortium | Acidophilic | Cell phones | 87% Cu, 86% Zn, and 82% Ni | 10–15 days | One-step | SDB1 medium supplemented with 45 g·L−1 FeSO4·7H2O and PCBs (1 g). | SDB1 medium supplemented with 45 g·L−1 FeSO4·7H2O and simultaneous addition to the medium of the inoculum (10% v/v of activated inoculum) and PCBs (1 g) | [51] |
| Leptospirillum ferriphilum-dominated consortium | Acidophilic | Cell phones | 100% Cu, 99% Zn, and 85% Ni | 6-8 days | Two-step | Biologically generated Fe3+ iron (0.80%) containing medium, free from cells and 1 g PCBs. | Addition of the PCBs to the culture medium after complete oxidation of Fe2+ to Fe3+. Biologically generated Fe3+ iron (0.80%) containing medium with viable inoculum and 1 g PCBs. | [51] |
| Acidithiobacillus ferrooxidans | Acidophilic | Cell phones | 100% Cu, and 100% Ni | 24 days | One-step | Previous experiments on the adaptation of bacterial cultures to PCBs. | Adapted bacterial cultures. | [59] |
| Bacillus megaterium | Cyanogenic | Cell phones | 72% Cu, and 3% Au | Nm | One-step | Carried out with different concentrations of glycine (0.5, 2.4, 5.3, 8, and 10 g·L−1), different pH level (7, 7.6, 8.5, 9.4, and 10), and different pulp densities (2, 5.7, 8.1, 16.4, and 20 g·L−1). | Optimization of culture conditions (pH 10, 10 g·L−1 glycine, and pulp density 0.8% w/v). | [60] |
| Leptospirillum sp. | Acidophilic | Cell phones | 100% Cu | 6–8 days | One-step | Conducted with pure Fe oxidizers in Fe supplemented (9 g·L−1 FeSO4(H2O)7), pure Fe 9 K), medium, non-supplemented (0 g·L−1, pure Fe 0 K) medium and pure sulfur (S) oxidizers supplemented with 3 g·L−1 of elemental sulfur (Pure S 3 g·L−1 S0). | In pure Fe 0 K and pure Fe 9 K. | [66] |
| Acidithiobacillus ferrooxidans | Acidophilic | Cell phones | 94% Cu, 91% Ni, 91% Zn, 10% Pb, 75% Cr, and 54% Cd | 9 days | One-step | Without pre-treatment. | Mechanical activation pre-treatment. | [61] |
| Acidithiobacillus ferrooxidans | Acidophilic | Cell phones | 5–8% Cu | 10 days | One-step | Without magnetic separation of PCBs. | Magnetic separation of the PCBs. | [57] |
| Consortium formed by Acidithiobacillus ferrooxidans, Leptospirillum ferriphilum, Acidithiobacillus caldus, Acidithiobacillus thioxidans, Sulphobacillus sp. and Ferroplasma sp. | Acidophilic | Cell phones | 98–99% Cu | 12 days | One-step | Carried out with different pulp densities (7%, 10%, and 15% w/v). | Optimization of culture conditions, (pulp density 7%, 10%, and 15% w/v). | [66] |
| Acidiphilium acidophilum | Acidophilic | Cell phones | 2.4 mg Cu L−1, and 1.3 mg Zn L−1 | 15 days | One-step | Without adding lemon juice and citric acid. | Addition of lemon juice and citric acid. | [54] |
| Acidithiobacillus ferrooxidans. | Acidophilic | Cell phones | 95–100% Cu | 2 days | Two-step | Biologically generated Fe3+ iron containing medium, free from cells and 2625 g PCBs. | Addition of the PCBs to the culture medium after complete oxidation of Fe2+ to Fe3+. Biologically generated Fe3+ iron containing medium with viable inoculum and 2625 g PCBs. | [38] |
| Pseudomonas putida and Bacillus megaterium | Cyanogenic | Cell phones | 84% Au | 34 h | One-step | Nm | Optimization of culture conditions (pH 10, and pulp density 0.5% w/v). | [67] |
| Pseudomonas aeruginosa B3 | Cyanogenic | Cell phones | 57% Cu, 4 % Zn, and 5% Ni | 8–10 days | One-step | Nm | Optimization of culture conditions (adapted bacterial cultures, and pulp density 0.5% w/v) | [70] |
| Acidithiobacillus ferrivorans | Acidophilic | Mix of PCBs of computers and cell phones | 94% Cu | 10 days | One-step | Carried out with different pulp densities (0.5%, 1%, 2.5%, 5% w/v). | Optimization of culture conditions (pH 1.0–1.6, and pulp density 1% w/v). | [68] |
| Acidithiobacillus thiooxidans | Acidophilic | Mix of PCBs of computers and cell phones | 89% Cu | 10 days | One-step | Carried out with different pulp densities (0.5%, 1%, 2.5%, 5% w/v). | Optimization of culture conditions (pH 1.0–1.6, and pulp density 1% w/v). | [68] |
| Pseudomonas putida | Cyanogenic | Mix of PCBs of computers and cell phones | 44% Au | 2 days | Two-Step | Carried out with different pulp densities (0.5%, 1%, 2.55, 5% w/v) and different concentrations of glycine (5, 7.5, 10 g·L−1). | Optimization of culture conditions (pH 7.3–8.6, pulp density 1% w/v, and 10 g·L−1 glycine). | [68] |
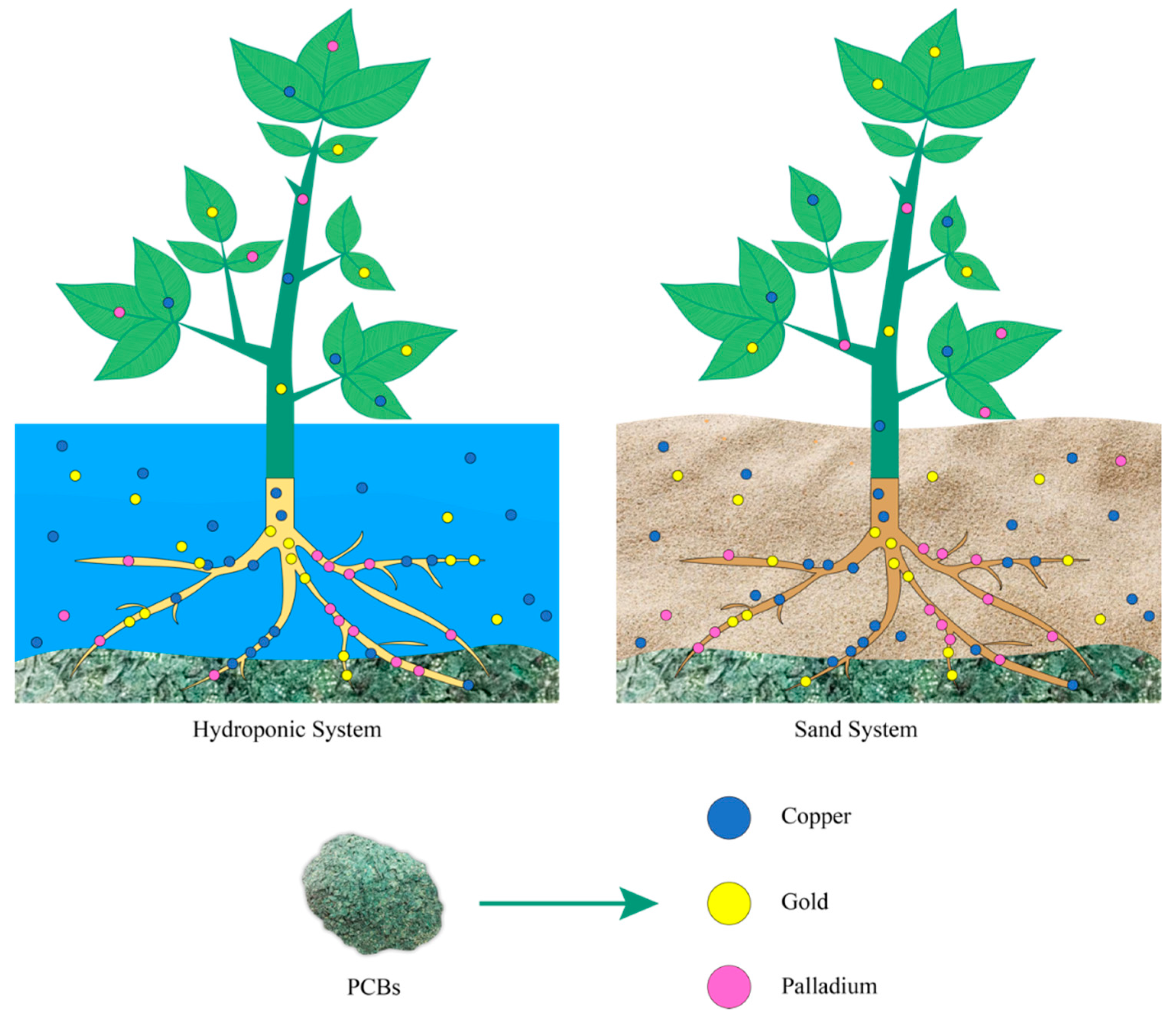
4. Plants in the Recovery of Metals from Printed Circuit Boards of Computers and Cell Phones
| Plant | System Type | PCBs Type | Recovered Metals | Growing Time in the Greenhouse | Mechanism | References |
|---|---|---|---|---|---|---|
| Medicago sativa L. | Sand | Computers | 2% Pd, and 0.01% Cu | 20 days | Phytoextraction | [98] |
| Lolium perenne L. | Sand | Computers | 0.08% Pd, and 0.2% Cu | 20 days | Phytoextraction | [98] |
| Triticum sp. | Hydroponic | Computers | 25% Au | 20 days | Phytoextraction | [83] |
| Sinapis alba L | Hydroponic | Cell phones | 6% Cu, 0.7% Au, and 3% Pd | 20 days | Phytoextraction | [99] |
| Helianthus annuus L. | Hydroponic | Cell phones | 7% Cu, 0.6% Au, and 5% Pd | 20 days | Phytoextraction | [99] |
| Lens culinaris | Hydroponic | Cell phones | 46% Au, and 47% Pd | 30 days | Phytoextraction | [100] |
5. Prospects and Recommendations for the Bio-Recovery of Metals from the PCBs of Computers and Cell Phones
5.1. Microorganisms
5.2. Plants
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Widmer, R.; Oswald-Krapf, H.; Sinha-Khetriwal, D.; Schnellmann, M.; Bönia, H. Global perspectives on e-waste. Environ. Impact Assess. Rev. 2005, 25, 436–458. [Google Scholar] [CrossRef]
- Sinha-Khetriwal, D.; Kraeuchi, P.; Widmer, R. Producer responsibility for e-waste management: Key issues for consideration—Learning from the Swiss experience. J. Environ. Manag. 2009, 1, 153–165. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical recovery of metals from electronic waste: A review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Ongondo, F.O.; Williams, I.D.; Cherrett, T.J. How are WEEE doing? A global review of the management of electrical and electronic wastes. Waste Manag. 2011, 31, 714–730. [Google Scholar] [CrossRef]
- Babu, B.R.; Parande, A.K.; Basha, C.A. Electrical and electronic waste: A global environmental problem. Waste Manag. Res. 2007, 25, 307–318. [Google Scholar]
- Pramila, S.; Fulekar, M.H.; Bhawana, P. E-Waste—A Challenge for tomorrow. Res. J. Recent. Sci. 2012, 1, 86–93. [Google Scholar]
- Rajarao, R.; Sahajwalla, V.; Cayumil, R.; Park, M.; Khanna, R. Novel approach for processing hazardous electronic waste. Procedia Environ. Sci. 2014, 21, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Dave, W.R.; Sodha, A.B.; Tipre, D.R. Chapter 8—Microbial Processes for Treatment of E-waste Printed Circuit Boards and Their Mechanisms for Metal(s) Solubilization. In Microbes for Sustainable Development and Bioremediation, 1st ed.; Chandra, R., Sobti, R.C., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–13. [Google Scholar]
- Song, Q.; Li, J. Environmental effects of heavy metals derived from the e-waste recycling activities in China: A systematic review. Waste Manag. 2014, 34, 2587–2594. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Zeng, X.; Li, J. Environmental pollution of electronic waste recycling in India: A critical review. Enviro. Pollut. 2016, 211, 259–270. [Google Scholar] [CrossRef]
- Kumar Ramasamy, R.; Congeevaram, S.; Thamaraiselvi, K. Evaluation of isolated fungal strain from e-waste recycling facility for effective sorption of toxic heavy metal Pb (II) ions and fungal protein molecular characterization- a mycoremediation approach. Asian J. Exp. Biol. Sci. 2011, 2, 342–347. [Google Scholar]
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. J. Hazard. Mater. 2019, 362, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.D.; Varma, A. A review on impact of e-waste on soil microbial community and ecosystem function. Pollution 2019, 5, 761–774. [Google Scholar]
- Işıldar, A.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Electronic waste as a secondary source of critical metals: Management and recovery technologies. Resour. Conserv. Recycl. 2018, 135, 296–312. [Google Scholar] [CrossRef]
- Dev, S.; Sachan, A.; Dehghani, F.; Ghosh, T.; Briggs, B.; Aggarwal, S. Mechanisms of biological recovery of rare-earth elements from industrial and electronic wastes: A review. Chem. Eng. J. 2020, 397, 124596. [Google Scholar] [CrossRef]
- Gonzalez-Baez, A.; Pantoja Munoz, L.; Garelick, H.; Purchase, D. Characterisation of rare earth elements in waste printed circuit boards (WPCBs) and their bioleaching potential. In Proceedings of the 16th International Conference on Environmental Science and Technology, Rhodes, Greece, 4–7 September 2019. CEST2019_00201. [Google Scholar]
- Argumedo-Delira, R.; Díaz-Martínez, M.E. Recuperación Microbiana de Metales a partir de Fuentes Secundarias. In Biotecnología Microbiana: Aplicaciones Energéticas, Ambientales y Alternas; Argumedo-Delira, R., Sánchez Viveros, G., Alarcón, A., García-Meza, J.V., Eds.; Universidad Veracruzana: Xalapa Veracruz, México, 2018; pp. 369–390. [Google Scholar]
- Dodson, J.R.; Hunt, A.J.; Parker, H.L.; Yang, Y.; Clark, J.H. Elemental sustainability: Towards the total recovery of scarce metals. Chem. Eng. Proc. Process Intensif. 2012, 51, 69–78. [Google Scholar] [CrossRef]
- Gramatyka, P.; Nowosielski, R.; Sakiewicz, P. Recycling of waste electrical and electronic equipment. JAMME 2007, 20, 535–538. [Google Scholar]
- Khaliq, A.; Rhamdhani, M.K.; Brooks, G.; Masood, S. Metal extraction processes for electronic waste and existing industrial routes: A review and Australian perspective. Resources 2014, 3, 152–179. [Google Scholar] [CrossRef] [Green Version]
- Tuncuk, A.; Stazi, V.; Akcil, A.; Yazici, E.Y.; Deveci, H. Aqueous metal recovery techniques from e-scrap: Hydrometallurgy in recycling. Miner. Eng. 2012, 25, 28–37. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Keerthanan, S.; Vithanage, M. 2-Urban mining of E-Waste: Treasure Hunting for Precious Nanometals. In Handbook of Electronic Waste Management; Prasad, M.N.V., Vithanage, M., Borthakur, A., Eds.; Elsevier: Oxford, UK, 2020; pp. 19–54. [Google Scholar]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.-H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S.; Mousavi, S.M. Optimal electronic waste combination for maximal recovery of Cu-Ni-Fe by Acidithiobacillus ferrooxidans. J. Clean. Prod. 2019, 240, 118077. [Google Scholar] [CrossRef]
- Li, J.; Shrivastava, P.; Gao, Z.; Zhang, H.-C. Printed circuit board recycling: A state-of-the-art survey. IEEE Trans. Electron. Packag. 2004, 27, 33–42. [Google Scholar]
- Sethurajan, M.; van Hullebusch, E.D. Leaching and selective recovery of Cu from printed circuit boards. Metals 2019, 9, 1034. [Google Scholar] [CrossRef] [Green Version]
- Yamane, L.H.; de Moraes, V.T.; Espinosa, D.C.R.; Tenório, J.A.S. Recycling of WEEE: Characterization of spent printed circuit boards from mobile phones and computers. Waste Manag. 2011, 31, 2553–2558. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Xu, M.; Lin, C.S.K.; Hui, C.-W.; McKay, G. Waste printed circuit board recycling techniques and product utilization. J. Hazard. Mater. 2015, 283, 234–243. [Google Scholar] [CrossRef]
- Goodship, V.; Stevels, A.; Huisman, J. Waste Electrical and Electronic Equipment (WEEE) Handbook; Elsevier: Cambridge, UK, 2012; pp. 291–292. [Google Scholar]
- Meng, L.; Guo, L.; Guo, Z. Separation of metals from metal-rich particles of crushed waste printed circuit boards by low-pressure filtration. Waste Manag. 2019, 84, 227–234. [Google Scholar] [CrossRef]
- Oguchi, M.; Murakami, S.; Sakanakura, H.; Kida, A.; Kameya, T. A preliminary categorization of end-of-life electrical and electronic equipment as secondary metal resources. Waste Manag. 2011, 31, 2150–2160. [Google Scholar] [CrossRef]
- Pant, D.; Joshi, D.; Upreti, M.K.; Kotnala, R.K. Chemical and biological extraction of metals present in E waste: A hybrid technology. Waste Manag. 2012, 32, 979–990. [Google Scholar] [CrossRef]
- Godlewska-Żyłkiewicz, B. Microorganisms in inorganic chemical analysis. Anal. Bioanal. Chem. 2006, 384, 114–123. [Google Scholar] [CrossRef]
- Brandl, H.; Faramarzi, M.A. Microbe-metal-interactions for the biotechnological treatment of metal-containing solid waste. China Particuol. 2006, 4, 93–97. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Li, J. Mechano-microbial systems: An ecofriendly approach for copper bioleaching from waste printed circuit board. Waste Manag. Res. 2019, 37, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Benzal, E.; Solé, M.; Lao, C.; Gamisans, X.; Dorado, A.D. Elemental copper recovery from e-wastes mediated with a two-step bioleaching process. Waste Biomass Valori. 2020. [Google Scholar] [CrossRef]
- Faramarzi, M.A.; Manzari, M.M.; Brandl, H. Bioleaching of metals from wastes and low-grade sources by HCN-forming microorganisms. Hydrometallurgy 2020, 191, 105228. [Google Scholar] [CrossRef]
- Asghari, I.; Mousavi, S.M.; Amiri, F.; Tavassoli, S. Bioleaching of spent refinery catalysts: A review. J. Ind. Eng. Chem. 2013, 19, 1069–1081. [Google Scholar] [CrossRef]
- Jadhav, U.; Sua, C.; Hocheng, H. Leaching of metals from printed circuit board powder by an Aspergillus niger culture supernatant and hydrogen peroxide. RSC Adv. 2016, 6, 43442–43452. [Google Scholar] [CrossRef]
- Faraji, F.; Golmohammadzadeh, R.; Rashchi, F.; Alimardani, N. Fungal bioleaching of WPCBs using Aspergillus niger: Observation, optimization and kinetics. J. Environ. Manag. 2018, 217, 775–787. [Google Scholar] [CrossRef]
- Xia, M.; Bao, P.; Zhang, S.; Liu, A.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Extraction and characterization of extracellular polymeric substances from a mixed fungal culture during the adaptation process with waste printed circuit boards. Environ. Sci. Pollut. Res. 2019, 26, 22137–22146. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Arias, J.E.; Argumedo-Delira, R.; Alarcón, A.; Mendoza-López, M.R.; García-Barradas, O.; Cruz-Sánchez, J.S.; Ferrera-Cerrato, R.; Jiménez-Fernández, M. Bioleaching of gold, copper and nickel from waste mobile phone PCBs and computer goldfinger motherboards by two Aspergillus niger strains. Braz. J. Microbiol. 2015, 46, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Argumedo-Delira, R.; Gómez-Martínez, M.J.; Soto, B.J. Gold bioleaching from printed circuit boards of mobile phones by Aspergillus niger in a culture without agitation and with glucose as a carbon source. Metals 2019, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Martínez, M.E.; Argumedo-Delira, R.; Sánchez-Viveros, G.; Alarcón, A.; Mendoza-López, M.R. Microbial bioleaching of Ag, Au and Cu from printed circuit boards of mobile phone. Curr. Microbiol. 2019, 76, 536–544. [Google Scholar] [CrossRef]
- Arab, B.; Hassanpour, F.; Arshadi, M.; Yaghmaei, S.; Hamedi, H. Optimized bioleaching of copper by indigenous cyanogenic bacteria isolated from the landfill of e-waste. J. Environ. 2020, 261, 110124. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Bai, J.; Feng, Y.; Zhang, C.; Wang, J.; Yuan, W.; KaiminShih, K. Chapter 9—Biotechnological Initiatives in E-waste Management: Recycling and Business Opportunities. In Electronic Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Eds.; Elsevier: Oxford, UK, 2019; pp. 201–223. [Google Scholar]
- Chi, T.D.; Lee, J.C.; Pandey, B.D.; Yoo, K.; Jeong, J. Bioleaching of gold and copper from waste mobile phone PCBs by using a cyanogenic bacterium. Miner. Eng. 2011, 24, 1219–1222. [Google Scholar] [CrossRef]
- Willner, J.; Fornalczyk, A. Extraction of metals from electronic waste by bacterial leaching. Environ. Prot. Eng. 2013, 39, 197–207. [Google Scholar]
- Shah, M.B.; Tipre, D.R.; Dave, S.R. Chemical and biological processes for multi-metal extraction from waste printed circuit boards of computers and mobile phones. Waste Manag. Res. 2014, 32, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Cho, K.; Kim, D.; Kim, D. Microbial recovery of copper from printed circuit boards of waste computer by Acidithiobacillus ferrooxidans. J. Environ. Sci. Heal. A 2004, 39, 2973–2982. [Google Scholar] [CrossRef]
- Wang, J.; Bai, J.; Xu, J.; Liang, B. Bioleaching of metals from printed wire boards by Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans and their mixture. J. Hazard. Mater. 2009, 172, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Hait, S. Extraction of Cu and Zn from High-grade Printed Circuit Board Scraps by Conventional and Hybrid Bioleaching. In Advances in Waste Management; Kalamdhad, A., Singh, J., Dhamodharan, K., Eds.; Springer: Singapore, 2019; pp. 511–523. [Google Scholar]
- Garg, H.; Nagar, N.; Dash, A.; Gahan, C.S. Efficiency assessment of pure Fe oxidizing microorganisms in iron supplemented and non-supplemented medium and pure S oxidizing microorganisms for bioleaching of mobile phone printed circuit boards. Biosci. Biotech. Res. Comm. 2019, 12, 425–434. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, Q.; Kamali, A.R.; Sand, W.; Yang, H. Effect of graphite on copper bioleaching from waste printed circuit boards. Minerals 2020, 10, 79. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, L.M.; Rosario, C.G.A.; de Carvalho, M.A.; Espinosa, D.C.R.; Tenório, J.A.S. Copper recovery from printed circuit boards from smartphones through bioleaching. In Proceedings of the TMS 2019 148th, Annual Meeting & Exhibition Supplemental Proceedings, San Antonio, TX, USA, 10–14 March 2019; The Minerals, Metals & Materials Society, Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 837–844. [Google Scholar]
- Gu, W.; Bai, J.; Lu, L.; Zhuang, X.; Zhao, J.; Yuan, W.; Zhang, C.; Wang, J. Improved bioleaching efficiency of metals from waste printed circuit boards by mechanical activation. Waste Manag. 2019, 98, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, M.; Mousavi, S.M. Multi-objective optimization of heavy metals bioleaching from discarded mobile phone PCBs: Simultaneous Cu and Ni recovery using Acidithiobacillus ferrooxidans. Sep. Purif. Technol. 2015, 147, 210–219. [Google Scholar] [CrossRef]
- Arshadi, M.; Mousavi, S.M.; Rasoulnia, P. Enhancement of simultaneous gold and copper recovery from discarded mobile phone PCBs using Bacillus megaterium: RSM based optimization of effective factors and evaluation of their interactions. Waste Manag. 2016, 57, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Bas, A.D.; Yazici, E.Y.; Deveci, H. Bacteria-assisted Leaching of Waste Computer Printed Circuit Boards. In Proceedings of the XIII International Mineral Processing Symposium (IMPS), Bodrum, Turkey, 10–12 October 2013; Özdag, H., Bozkurt, V., Ipek, H., Bilir, K., Eds.; Department of Mining Engineering Eskisehir Osmangazi University: Eskisehir, Turkey, 2012; pp. 435–441. [Google Scholar]
- Kumar, A.; Saini, H.S.; Kumar, S. Enhancement of gold and silver recovery from discarded computer printed circuit boards by Pseudomonas balearica SAE1 using response surface methodology (RSM). 3 Biotech. 2018, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, M.; Gurumurthy, K. Enhanced bioleaching of copper from circuit boards of computer waste by Acidithiobacillus ferroxidans. Environ. Chem. Lett. 2019, 17, 1873–1879. [Google Scholar] [CrossRef]
- Becci, A.; Amato, A.; Fonti, V.; Karaj, D.; Beolchini, F. An innovative biotechnology for metal recovery from printed circuit boards. Resour. Conserv. Recycl. 2020, 153, 104549. [Google Scholar] [CrossRef]
- Natarajan, G.; Ting, Y.-P. Pretreatment of e-waste and mutation of alkali-tolerant cyanogenic bacteria promote gold biorecovery. Bioresour. Technol. 2014, 152, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Garg, H.; Nagar, N.; Ellamparuthy, G.; Angadi, S.I.; Gahan, C.S. Bench scale microbial catalysed leaching of mobile phone PCBs with an increasing pulp density. Heliyon 2019, 5, e02883. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, H.; Yang, W.; Wu, Z.; Liu, W.; Yang, C. Bioleaching assisted foam fractionation for recovery of gold from the printed circuit boards of discarded cellphone. Waste Manag. 2020, 101, 200–209. [Google Scholar] [CrossRef]
- Işıldar, A.; van de Vossenberg, J.; Rene, E.R.; van Hullebusch, E.D.; Lens, P.N.L. Two-step bioleaching of copper and gold from discarded printed circuit boards (PCB). Waste Manag. 2016, 57, 149–157. [Google Scholar] [CrossRef]
- Khatri, B.R.; Sodha, A.B.; Shah, M.B.; Tipre, D.R.; Dave, S.R. Chemical and microbial leaching of base metals from obsolete cell-phone printed circuit boards. Sustain. Environ. Res. 2018, 28, 333–339. [Google Scholar] [CrossRef]
- Khatri, B.R.; Tipre, D.R.; Dave, S.R. Isolation and identification of HCN producing Pseudomonas spp. and their application in metal extraction from waste cell phone PCBs. IJRAR 2018, 5, 237–241. [Google Scholar]
- Adhapure, N.N.; Dhakephalkar, P.K.; Dhakephalkar, A.P.; Tembhurkar, V.R.; Rajgure, A.V.; Deshmukh, A.M. Use of large pieces of printed circuit boards for bioleaching to avoid precipitate contamination problem and to simplify overall metal recovery. MethodsX 2014, 1, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Chandane, P.; Jori, C.; Chaudhari, H.; Bhapkar, S.; Deshmukh, S.; Umesh Jadhav, U. Bioleaching of copper from large printed circuit boards for synthesis of organic-inorganic hybrid. Environ. Sci. Pollut. Res. 2020, 27, 5797–5808. [Google Scholar] [CrossRef] [PubMed]
- Knowles, C.J. Microorganisms and cyanide. Bacteriol. Rev. 1976, 40, 652–680. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, A.; Hait, S. Efficacy of Metal Extraction from Discarded Printed Circuit Board Using Aspergillus tubingensis. In Bioresource Utilization and Bioprocess; Ghosh, S., Sen, R., Chanakya, H., Pariatamby, A., Eds.; Springer: Singapore, 2020; pp. 167–175. [Google Scholar]
- Arinanda, M.; van Haute, Q.; Lambert, F.; Gaydardzhiev, S. Effects of operational parameters on the bio-assisted leaching of metals from pyrolized printed circuit boards. Miner. Eng. 2019, 134, 16–22. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S.; Mousavi, S.M. Study of plastics elimination in bioleaching of electronic waste using Acidithiobacillus ferrooxidans. Int. J. Environ. Sci. Technol. 2019, 16, 7113–7126. [Google Scholar] [CrossRef]
- Akbari, S.; Ahmadi, A. Recovery of copper from a mixture of printed circuit boards (PCBs) and sulphidic tailings using bioleaching and solvent extraction processes. Chem. Eng. Process. 2019, 142, 107584. [Google Scholar] [CrossRef]
- Ruan, J.; Zhu, X.; Qian, Y.; Hu, J. A new strain for recovering precious metals from waste printed circuit boards. Waste Manag. 2014, 34, 901–907. [Google Scholar] [CrossRef]
- Tran, C.D.; Lee, J.C.; Pandey, B.D.; Jeon, J.; Yoo, K.; Huynh, T.H. Bacterial cyanide generation in presence of metal ions (Na+, Mg2+, Fe2+, Pb2+) and gold bioleaching from waste PCBs. J. Chem. Eng. Jpn. 2011, 44, 692–701. [Google Scholar] [CrossRef]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Arshadi, M.; Nili, S.; Yaghmaei, S. Ni and Cu recovery by bioleaching from the printed circuit boards of mobile phones in non-conventional medium. J. Environ. 2019, 250, 109502. [Google Scholar] [CrossRef]
- Trivedi, A.; Hait, S. Bioleaching of selected metals from e-waste using pure and mixed cultures of Aspergillus species. In Measurement, Analysis and Remediation of Environmental Pollutants. Energy, Environment, and Sustainability; Gupta, T., Singh, S., Rajput, P., Agarwal, A., Eds.; Springer: Singapore, 2020; pp. 271–280. [Google Scholar]
- Díaz-Martínez, M.E. Plantas y Microorganismos Útiles en la Recuperación de Metales Provenientes de Residuos Electrónicos. Ph.D. Thesis, Universidad Veracruzana, Xalapa Veracruz, México, February 2019. [Google Scholar]
- Awasthi, A.K.; Li, J. Sustainable bioprospecting of electronic waste. Trends Biotechnol. 2019, 37, 677–680. [Google Scholar] [CrossRef]
- Peng, H.; Reid, M.S.; Le, X.C. Consumption of rice and fish in an electronic waste recycling area contributes significantly to total daily intake of mercury. J. Environ. Sci. 2015, 38, 83–86. [Google Scholar] [CrossRef]
- Tang, W.; Cheng, J.; Zhao, W.; Wang, W. Mercury levels and estimated total daily intakes for children and adults from an electronic waste recycling area in Taizhou, China: Key role of rice and fish consumption. J. Environ. Sci. 2015, 34, 107–115. [Google Scholar] [CrossRef]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Phytomining: A review. Miner. Eng. 2009, 22, 1007–1019. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Díaz-Martínez, M.E.; Argumedo-Delira, R.; Sánchez-Viveros, G.; Alarcón, A.; Trejo-Téllez, L.I. Lead phytoextraction from printed circuit computer boards by Lolium perenne L. and Medicago sativa L. Int. J. Phytoremediat. 2018, 20, 432–439. [Google Scholar] [CrossRef]
- Bloom, A.J. Continuous and Steady-State Nutrient Absorption by Intact Plants. In Application of Continuous and Steady State Methods to Root Biology; Torrey, J.G., Winship, L.J., Eds.; Dordrecht Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; pp. 147–163. [Google Scholar]
- AlShrouf, A. Hydroponics, aeroponic and aquaponic as compared with conventional farming. Am. Sci. Res. J. Eng. Technol. Sci. 2017, 27, 247–255. [Google Scholar]
- Ullah, A.; Heng, S.; Munis, M.F.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Pan, F.; Meng, Q.; Luo, S.; Shen, J.; Chen, B.; Khan, K.Y.; Japenga, J.; Ma, X.; Yang, X.; Feng, Y. Enhanced Cd extraction of oilseed rape (Brassica napus) by plant growth promoting bacteria isolated from Cd hyperaccumulator Sedum alfredii Hance. Int. J. Phytorem. 2017, 19, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, Y.; Rafiq, M.T.; Khan, K.Y.; Pan, F.; Yang, X.; Feng, Y. Improvement of cadmium uptake and accumulation in Sedum alfredii by endophytic bacteria Sphingomonas SaMR12: Effects on plant growth and root exudates. Chemosphere 2014, 117, 367–373. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zhang, X.; Dong, L.; Zhang, J.; Wei, Y.; Feng, Y.; Lu, L. Improved plant growth and Zn accumulation in grains of rice (Oryza sativa L.) by inoculation of endophytic microbes isolated from a Zn Hyperaccumulator, Sedum alfredii H. J. Agric. Food. Chem. 2014, 62, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Domínguez, G. Fitoextracción de Au, Ag, Cu y Pd por Medicago sativa L. y acumulación y lixiviación de Au y Cu por microorganismos promotores de crecimiento vegetal (MPCV) a partir de PCI de computadoras. Bachelor’s Thesis, Universidad Veracruzana, Xalapa Veracruz, México, September 2016. [Google Scholar]
- Viveros-Díaz, R. Medicago sativa L. y Lolium perenne L. en la Fitoextracción de Au, Ag, Cu y Pd a Partir de Placas de Circuito Impreso de Computadoras. Bachelor’s Thesis, Universidad Veracruzana, Xalapa Veracruz, México, December 2015. [Google Scholar]
- Portilla-Sangabriel, M. Fitoextracción de Au, Ag, Cu y Pd por Sinapis alba L. (Mostaza roja), Brassica juncea (Mostaza India) y Helianthus annuus L. (Girasol), a partir de radiografías y tarjetas de circuito impreso de teléfonos celulares. Bachelor’s Thesis, Universidad Veracruzana, Xalapa Veracruz, México, September 2016. [Google Scholar]
- Domínguez-Capitaine, B.P. Fitoextracción de Metales de Placas de Circuito Impreso de Teléfonos Celulares por Lens Culinaris y Brassica Juncea en un Cultivo Hidropónico. Bachelor’s Thesis, Universidad Veracruzana, Xalapa Veracruz, México, October 2019. [Google Scholar]
- Habibi, A.; Kourdestani, S.S.; Hadadi, M. Biohydrometallurgy as an environmentally friendly approach in metals recovery from electrical waste: A review. Waste Manag. Res. 2020, 38, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.; Ahmed, T.; Awual, M.R.; Rahman, A.; Sultana, M.; Aziz, A.A.; Monir, M.U.; Teo, S.H.; Hasan, M. Advances in sustainable approaches to recover metals from e-waste-A review. J. Clean. Prod. 2020, 244, 118815. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, S. Chapter 7-Bioleaching of Electronic Waste Using Extreme Acidophiles Electronic. In Electronic Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Eds.; Elsevier: Oxford, UK, 2019; pp. 153–174. [Google Scholar]
- Qiu, R.; Lin, M.; Ruan, J.; Fu, Y.; Hu, J.; Deng, M.; Qiu, R. Recovering full metallic resources from waste printed circuit boards: A refined review. J. Clean. Prod. 2020, 244, 118690. [Google Scholar] [CrossRef]
- Gopikrishnan, V.; Vignesh, A.; Radhakrishnan, M.; Joseph, J.; Shanmugasundaram, T.; Doble, M.; Balagurunathan, R. Chapter 10—Microbial Leaching of Heavy Metals from E-waste: Opportunities and Challenges. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Rathinam, N.K., Sani, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 189–216. [Google Scholar]
- Rienzie, R.; Perera, A.T.D.; Adassooriya, N.M. Chapter 6—Biorecovery of Precious Metal Nanoparticles from Waste Electrical and Electronic Equipments. In Electronic Waste Management and Treatment Technology; Prasad, M.N.V., Vithanage, M., Eds.; Elsevier: Oxford, UK, 2019; pp. 133–152. [Google Scholar]
- Awasthi, A.K.; Hasan, M.; Mishra, Y.K.; Pandey, A.K.; Tiwary, B.N.; Kuhad, R.C.; Gupta, V.K.; Thakur, V.K. Environmentally sound system for E-waste: Biotechnological perspectives. CRBIOT 2019, 1, 58–64. [Google Scholar] [CrossRef]


| Type of PCBs | Metals (wt %) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Fe | Pb | Ni | Al | Pd | Au | Ag | ||
| Computers | 20 | 7 | 1.5 | 1 | 5 | 0.011 | 0.025 | 0.100 | [3,27] |
| Cell Phones | 25 | 5 | 0.8 | 0.5 | 1 | 0.221 | 0.035 | 0.134 | [27,31,32] |
| Fungus | Fungus Type | PCBs Type | Recovered Metals * | Bioleaching Time | Bioleaching Type | Bioleaching Conditions | References |
|---|---|---|---|---|---|---|---|
| Trichoderma viride | Filamentous | Computers | 1% Pd, and 10% Au | 30 days | One-step | pH 5, 1 g PCBs of 0.5 × 0.5 cm2, and inoculation with mycelium. | [83] |
| Trichoderma atroviride | Filamentous | Computers | 1% Pd, and 13% Au | 30 days | One-step | pH 5, 1 g PCBs of 0.5 × 0.5 cm2, and inoculation with mycelium. | [83] |
| Aspergillus tubingensi | Filamentous | Computers | 34% Cu, 54% Zn, and 8% Ni | 33 days | One-step | pH 5, pulp density 0.25–1% w/v, and inoculation with spores. | [74] |
| Aspergillus niger | Filamentous | Computers | 7% Cu, 32% Ni, and 79% Zn | 33 days | One-step | Pulp density 0.1% w/v. | [82] |
| Consortium formed by Aspergillus niger and Aspergillus tubingensi | Filamentous | Computers | 76% Cu, 36% Ni, and 63% Zn | 33 days | One-step | Pulp density 0.1% w/v, and inoculation with spores. | [82] |
| Aspergillus niger MXPE6 | Filamentous | Cell phones | 5% Cu, 42% Au, and 0.8% Ni | 14 days | One-step | pH 4.4, and 0.2 g PCBs of 1 × 1 cm2. | [44] |
| Consortium formed by Aspergillus niger MXPE6 and Aspergillus niger MX7 | Filamentous | Cell phones | 56% Au, | 16 days | One-step | pH 5, 11 g PCBs of 0.0594 mm, 5 L bioreactors in batch mode, and inoculation with mycelium. | [45] |
| Aspergillus niger MXPE6 | Filamentous | Cell phones | 54% Ag | 35 days | One-step | pH 4.4, 11 g PCBs of 0.594 mm, and 5 L bioreactors in batch mode. | [46] |
| Aspergillus niger MX5 | Filamentous | Cell phones | 3% Cu, and 0.3% Au | 35 days | One-step | pH 4.4, 0.5 g PCBs of 0.594 mm, and inoculation with mycelium. | [46] |
| Aspergillus niger MX7 | Filamentous | Cell phones | 0.5% Cu, and 0.5% Au | 35 days | One-step | pH 4.4, 0.5 g PCBs of 0.594 mm, and inoculation with mycelium. | [46] |
| Candida orthopsilosis | Yeast | Cell phones | 1% Cu | 35 days | One-step | pH 4.4, 0.5 g PCBs of 0.594 mm, and inoculation with mycelium. | [46] |
| Penicillium simplicissimum | Filamentous | Cell phones | 90% Cu, and 89% Ni | Nm | One-step | Four kinds of carbon sources (i.e., sucrose, cheese whey, sugar, and sugar cane molasses). | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argumedo-Delira, R.; Díaz-Martínez, M.E.; Gómez-Martínez, M.J. Microorganisms and Plants in the Recovery of Metals from the Printed Circuit Boards of Computers and Cell Phones: A Mini Review. Metals 2020, 10, 1120. https://doi.org/10.3390/met10091120
Argumedo-Delira R, Díaz-Martínez ME, Gómez-Martínez MJ. Microorganisms and Plants in the Recovery of Metals from the Printed Circuit Boards of Computers and Cell Phones: A Mini Review. Metals. 2020; 10(9):1120. https://doi.org/10.3390/met10091120
Chicago/Turabian StyleArgumedo-Delira, Rosalba, María Esther Díaz-Martínez, and Mario Javier Gómez-Martínez. 2020. "Microorganisms and Plants in the Recovery of Metals from the Printed Circuit Boards of Computers and Cell Phones: A Mini Review" Metals 10, no. 9: 1120. https://doi.org/10.3390/met10091120





