Effects of High-Temperature Tempering on Mechanical Properties and Microstructure of SA738 Gr.B Steel
Abstract
:1. Introduction
2. Material and Methods
3. Results
3.1. Mechanical Properties
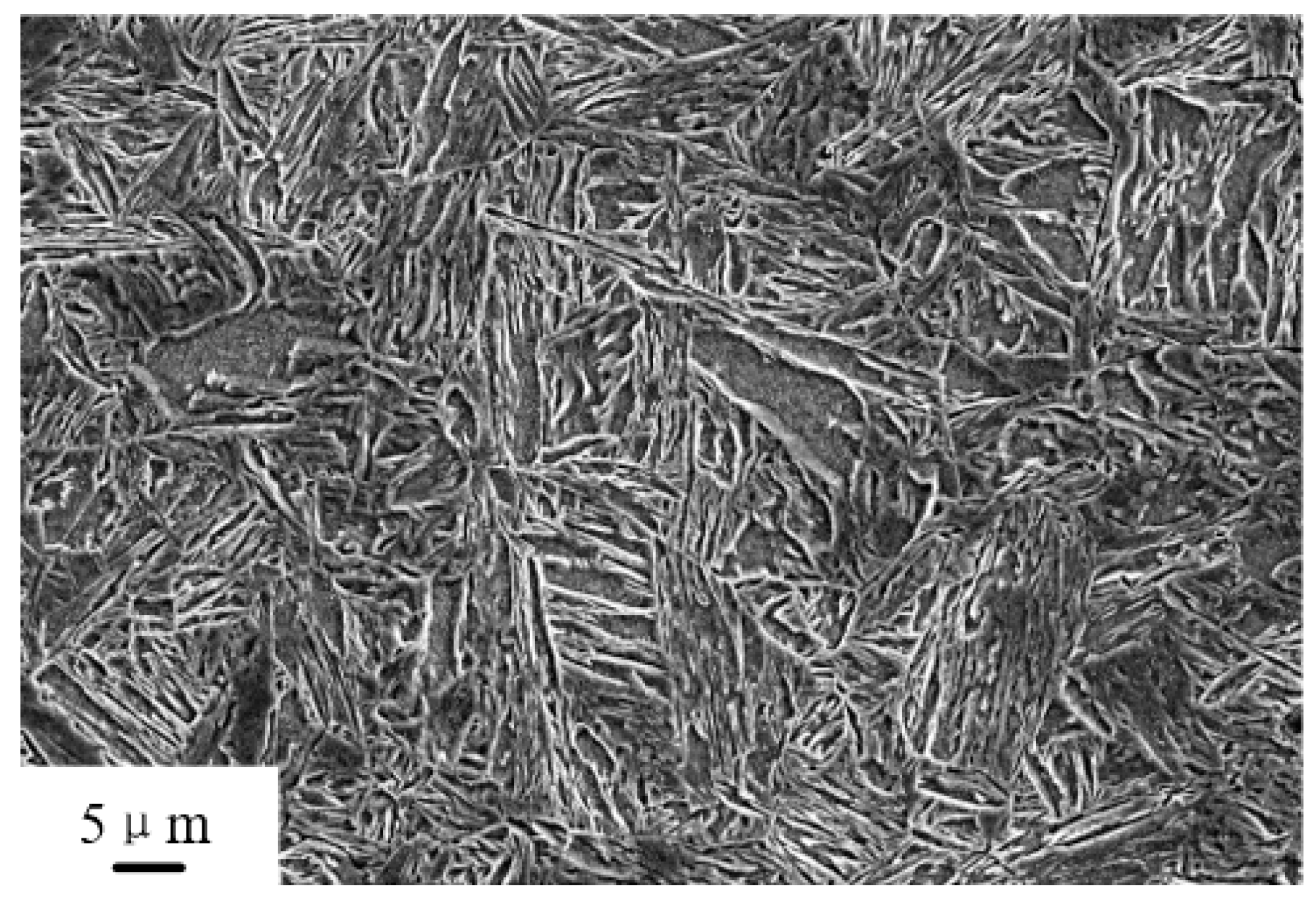
3.2. Microstructure of the Tempered Steel
4. Discussion
4.1. Three Different Tempering Temperature Ranges
4.2. Mechanism of Effect of Plate Martensite on Properties
4.3. Formation Mechanism of Plate Martensite
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Information Office of the State Council of the People’s Republic of China. China’s Nuclear Security; Information Office of the State Council of the People’s Republic of China: Beijing, China, 2019. [Google Scholar]
- Yang, J.; Wang, W.W.; Qiu, S.Z.; Tian, W.X.; Su, G.H.; Wu, Y.W. Simulation and analysis on 10-in. cold leg small break loca for ap1000. Ann. Nucl. Energy 2012, 46, 81–89. [Google Scholar] [CrossRef]
- Hung, Z.Y.; Ferng, Y.M.; Hsu, W.S.; Pei, B.S.; Chen, Y.S. Analysis of ap1000 containment passive cooling system during a loss-of-coolant accident. Ann. Nucl. Energy 2015, 85, 717–724. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, G.; Chen, N.; Lei, P.; Shen, Q. Investigation of mechanical properties and proton irradiation behaviors of sa-738 gr.b steel used as reactor containment. Nucl. Mater. Energy 2016, 8, 18–22. [Google Scholar] [CrossRef] [Green Version]
- ASME Boiler and Pressure Vessel Code. Materials Part A: Ferrous Materials Specifications; ASEM: New York, NY, USA, 2013; pp. 1365–1372. [Google Scholar]
- Han, Q.B.; Jiang, S.Y.; Sun, W.H. Study on heat treatment process of sa738 Gr. B steel plats for nuclear power plant. Shandong Metall. 2014, 3, 36–38. [Google Scholar]
- Sun, L.S. Microstructure and security analysis of steel used for nuclear power plant with heat treatment. Foundry Technol. 2015, 36, 356–358. [Google Scholar]
- Bi, Z.C.; Zhang, J.M.; Liu, X.D.; Jiang, S.Y.; Sun, W.H. Microstructure and mechanical properties of q and t heavy plate for nuclear power station purpose. J. Iron Steel Res. 2011, 23, 59–62. [Google Scholar]
- Zhang, Y.L.; Hui, H. Investigation of mechanical properties and ductile-brittle transition behaviors of sa738gr.b steel used as reactor containment. Key Eng. Mater. 2019, 795, 66–73. [Google Scholar] [CrossRef]
- ISO. Metallic Materials-Tensile Testing—Part 1: Method of Test at Room Temperature; GB/T 228-2010; ISO: Beijing, China, 2011. [Google Scholar]
- Abbaschian, R. Physical Metallurgy Principles, 4th ed.; Cengage: Boston, MA, USA, 2009. [Google Scholar]
- Callister, W.D., Jr. Fundamentals of Materials Science and Engineering; Wiley John & Sons: Hoboken, NJ, USA, 2001; Volume 34, p. 824. [Google Scholar]
- Xie, Z.J.; Ma, X.P.; Shang, C.J.; Wang, X.M.; Subramanian, S.V. Nano-sized precipitation and properties of a low carbon niobium micro-alloyed bainitic steel. Mater. Sci. Eng. A 2015, 641, 37–44. [Google Scholar] [CrossRef]
- Wang, S.C.; Hsieh, R.I.; Liou, H.Y.; Yang, J.R. The effects of rolling processes on the microstructure and mechanical properties of ultralow carbon bainitic steels. Mater. Sci. Eng. A 1992, 157, 29–36. [Google Scholar] [CrossRef]
- Takyama, S.; Ogura, T.; Fu, S.C.; McMahon, C.J. The Calculation of Transition Temperature Changes in Steels Due to Temper Embrittlement. Metall. Trans. A 1980, 11, 1513. [Google Scholar] [CrossRef]
- Jiang, Z.H.; Wang, P.; Li, D.Z.; L, Y.Y. Effects of tempering temperature on the microstructure and mechanical properties of granular bainite in 2.25Cr-1Mo-0.25V steel. Acta Metall. Sin. 2015, 51, 925–934. [Google Scholar]
- Lan, L.Y.; Qiu, C.L.; Zhao, D.W.; Gao, X.H.; Du, L.X. Effect of austenite grain size on isothermal bainite transformation in low carbon microalloyed steel. Mater. Sci. Technol. 2012, 27, 1657–1663. [Google Scholar] [CrossRef]
- Ji, Y.P.; Liu, Z.C.; Ren, H.P. Twin crystal substructure of martensite in steel. Trans. Mater. Heat Treat. 2013, 34, 162–165. [Google Scholar]
- Krauss, G.; Marder, A.R. The morphology of martensite in iron alloys. Metall. Trans. 1971, 2, 2343. [Google Scholar] [CrossRef]
- Huang, D.H.; Thomas, G. Structure and mechanical properties of tempered martensite and lower bainite in fe-ni-mn-c steels. Metall. Trans. 1971, 2, 1587–1598. [Google Scholar]
- Kelly, P.M.; Jostsons, A.; Blake, R.G. The orientation relationship between lath martensite and austenite in low carbon, low alloy steels. Acta Metall. Mater. 1990, 38, 1075–1081. [Google Scholar] [CrossRef]
- Callister, W.D., Jr.; Rethwisch, D.G. Material Science and Engineering—An Introduction; Wiley John & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ahn, Y.S.; Kim, H.D.; Byun, T.S.; Oh, Y.J.; Kim, G.M.; Hong, J.H. Application of intercritical heat treatment to improve toughness of sa508 cl.3 reactor pressure vessel steel. Nucl. Eng. Des. 1999, 194, 161–177. [Google Scholar] [CrossRef]
- Wang, R.G.; Luo, Z.H.; Sun, D.D.; Liu, H.Y.; Li, X.L.; Li, J.N. Study on process for quenching and tempering sa-738 Gr.B steel plate used for safety case of nuclear reactor. Angang Technol. 2014, 2, 33–36. [Google Scholar]









| Element | C | Mn | P | S | Si | Ni | Cr | Mo | Nb | V | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard | ≤0.2 | 0.9–1.5 | 0.008 | 0.005 | 0.15–0.55 | ≤0.6 | ≤0.3 | ≤0.3 | Total ≤ 0.08 | Bal. | ||
| Experimental | 0.14 | 1.55 | 0.008 | 0.001 | 0.25 | 0.55 | 0.23 | 0.28 | Total = 0.077 | Bal. | ||
| Temperature/°C | C | Mn | Ni | Cr | Mo | Ms |
|---|---|---|---|---|---|---|
| 690 | 0.52 | 3.80 | 1.15 | 0.36 | 0.19 | 161.6 |
| 710 | 0.42 | 3.23 | 1.03 | 0.34 | 0.21 | 175.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, S.; Zhao, C.; Song, M.; Jiang, Z. Effects of High-Temperature Tempering on Mechanical Properties and Microstructure of SA738 Gr.B Steel. Metals 2020, 10, 1207. https://doi.org/10.3390/met10091207
Li Y, Zhang S, Zhao C, Song M, Jiang Z. Effects of High-Temperature Tempering on Mechanical Properties and Microstructure of SA738 Gr.B Steel. Metals. 2020; 10(9):1207. https://doi.org/10.3390/met10091207
Chicago/Turabian StyleLi, Yanmei, Shuzhan Zhang, Chunyao Zhao, Minghui Song, and Zaiwei Jiang. 2020. "Effects of High-Temperature Tempering on Mechanical Properties and Microstructure of SA738 Gr.B Steel" Metals 10, no. 9: 1207. https://doi.org/10.3390/met10091207
APA StyleLi, Y., Zhang, S., Zhao, C., Song, M., & Jiang, Z. (2020). Effects of High-Temperature Tempering on Mechanical Properties and Microstructure of SA738 Gr.B Steel. Metals, 10(9), 1207. https://doi.org/10.3390/met10091207





