Effects of Ce on Microstructure and Mechanical Properties of LDX2101 Duplex Stainless Steel
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Production
2.2. Microstructure Observation
2.3. Mechanical Properties
3. Results and Discussion
3.1. Thermodynamic Analysis of Inclusions Containing Ce
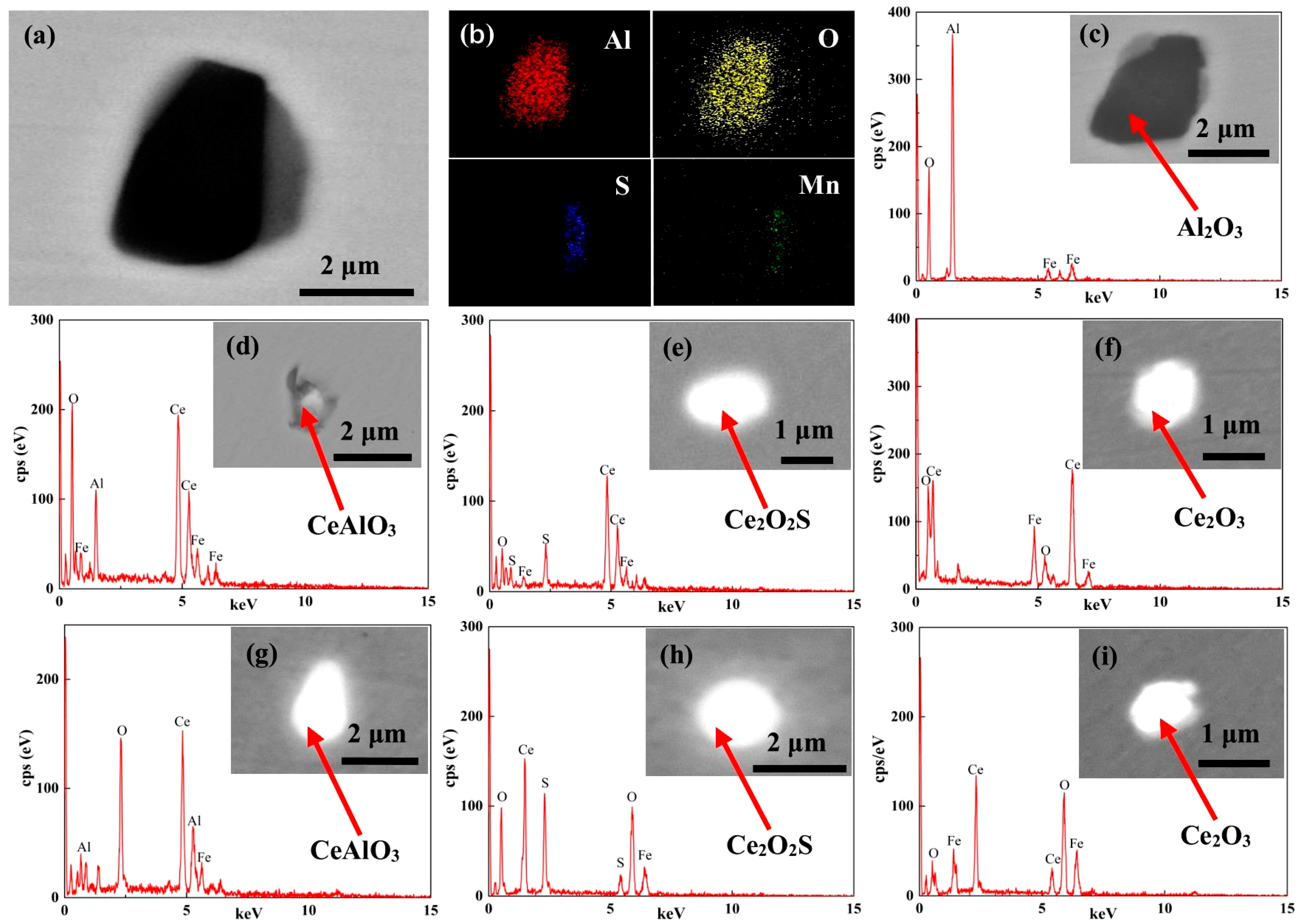
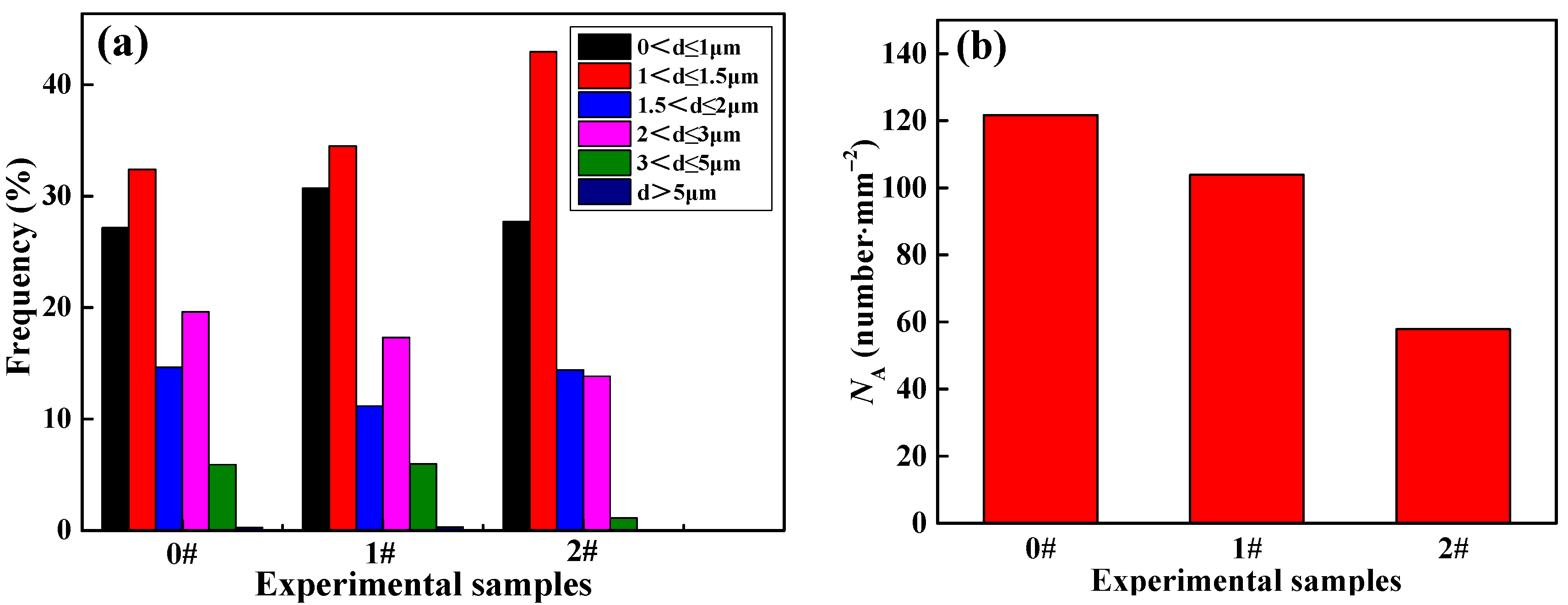
3.2. Effect of Ce on Inclusions
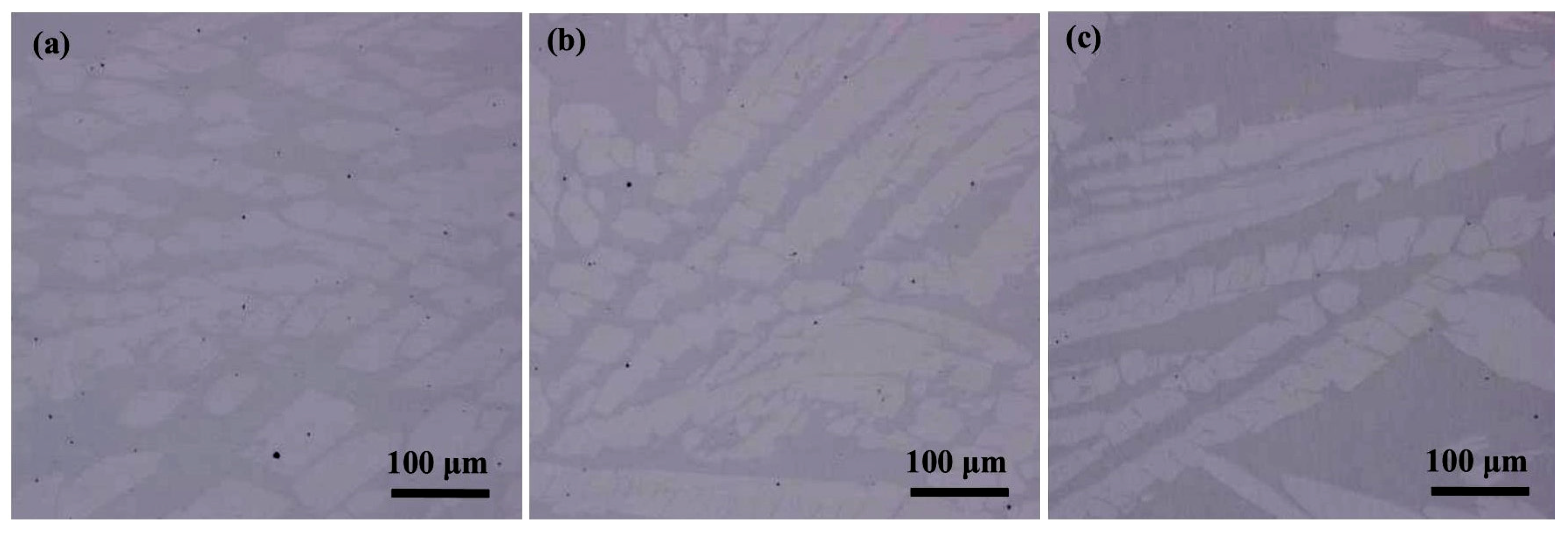
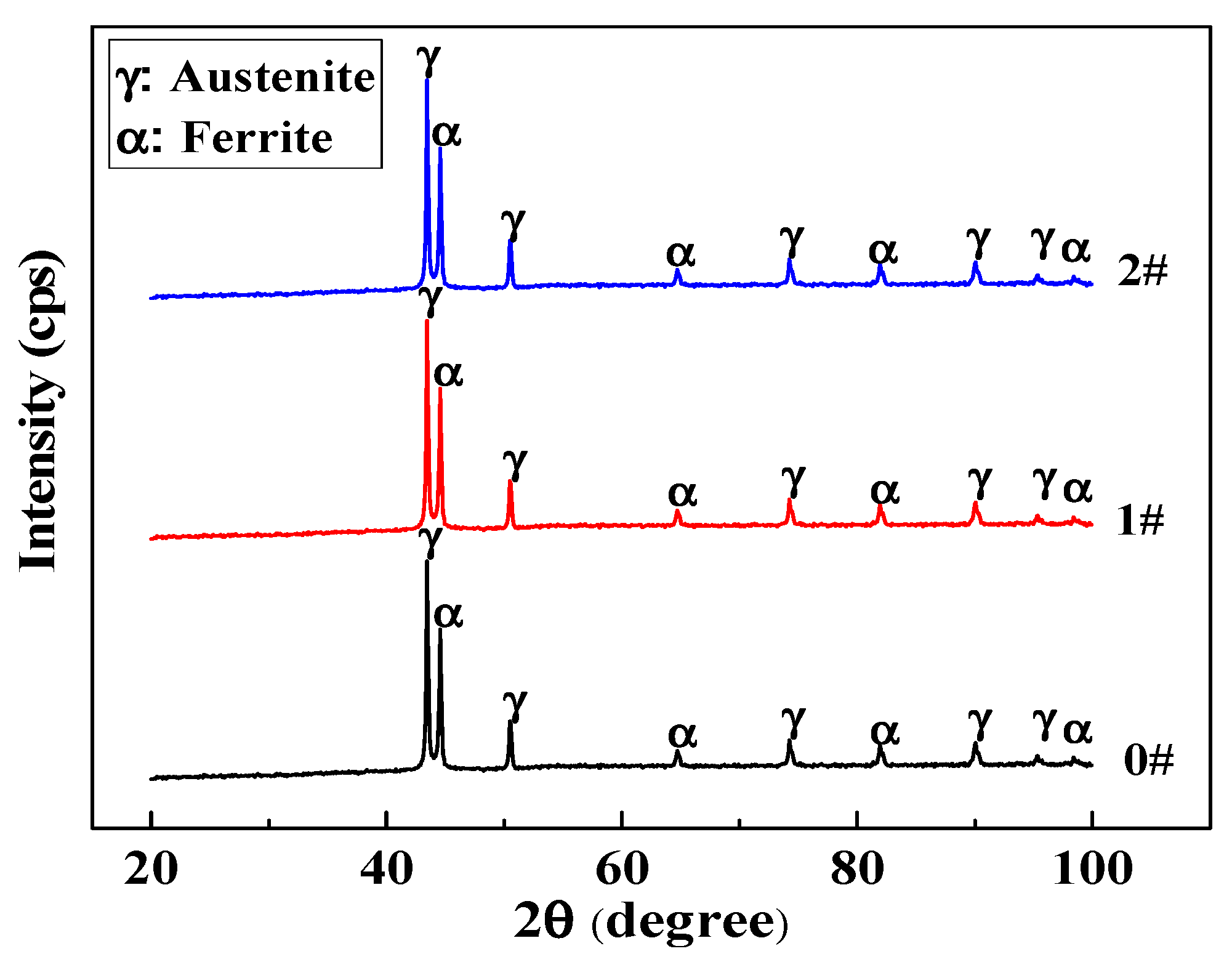
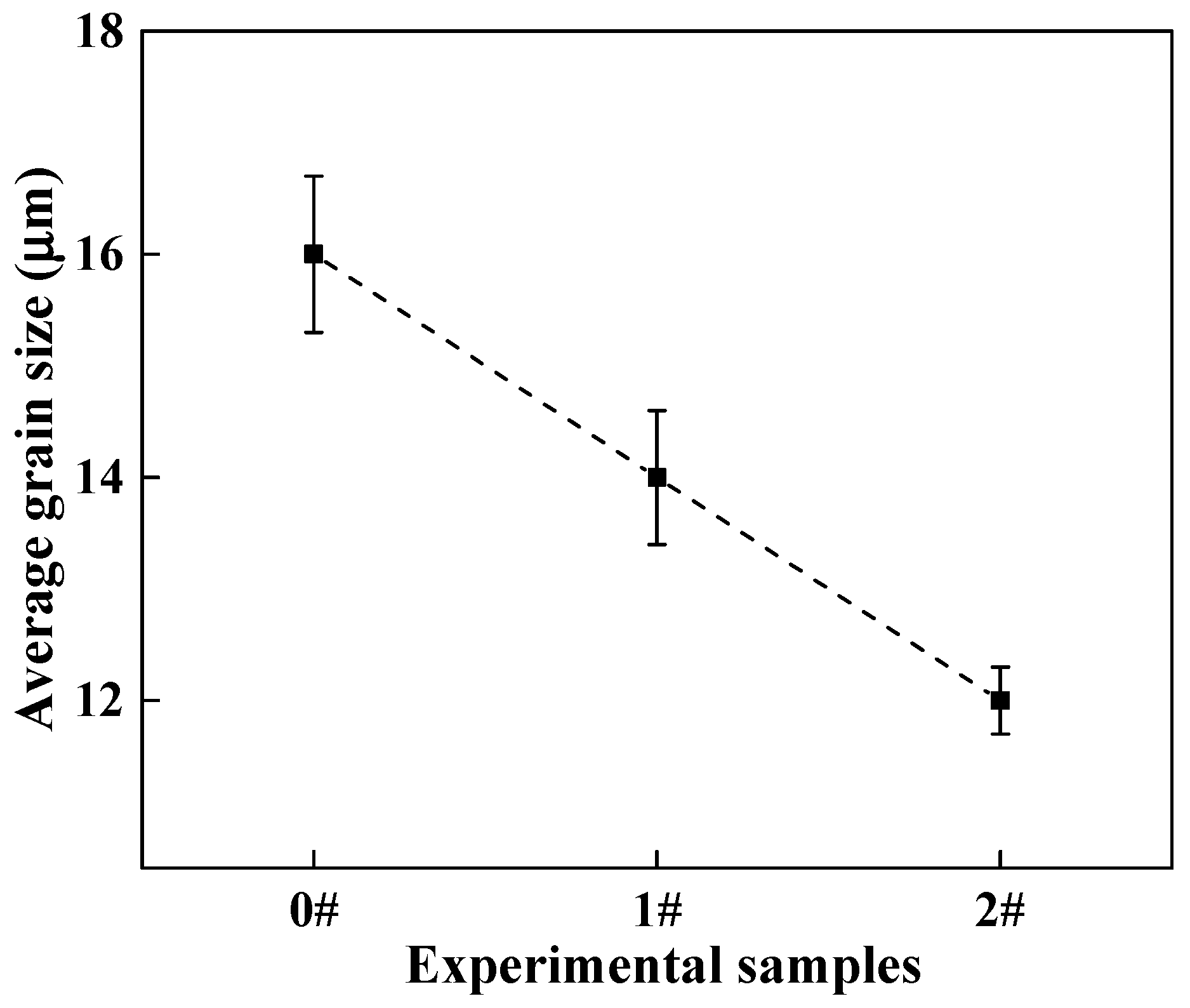
3.3. Effect of Ce on Microstructure and Phase Composition
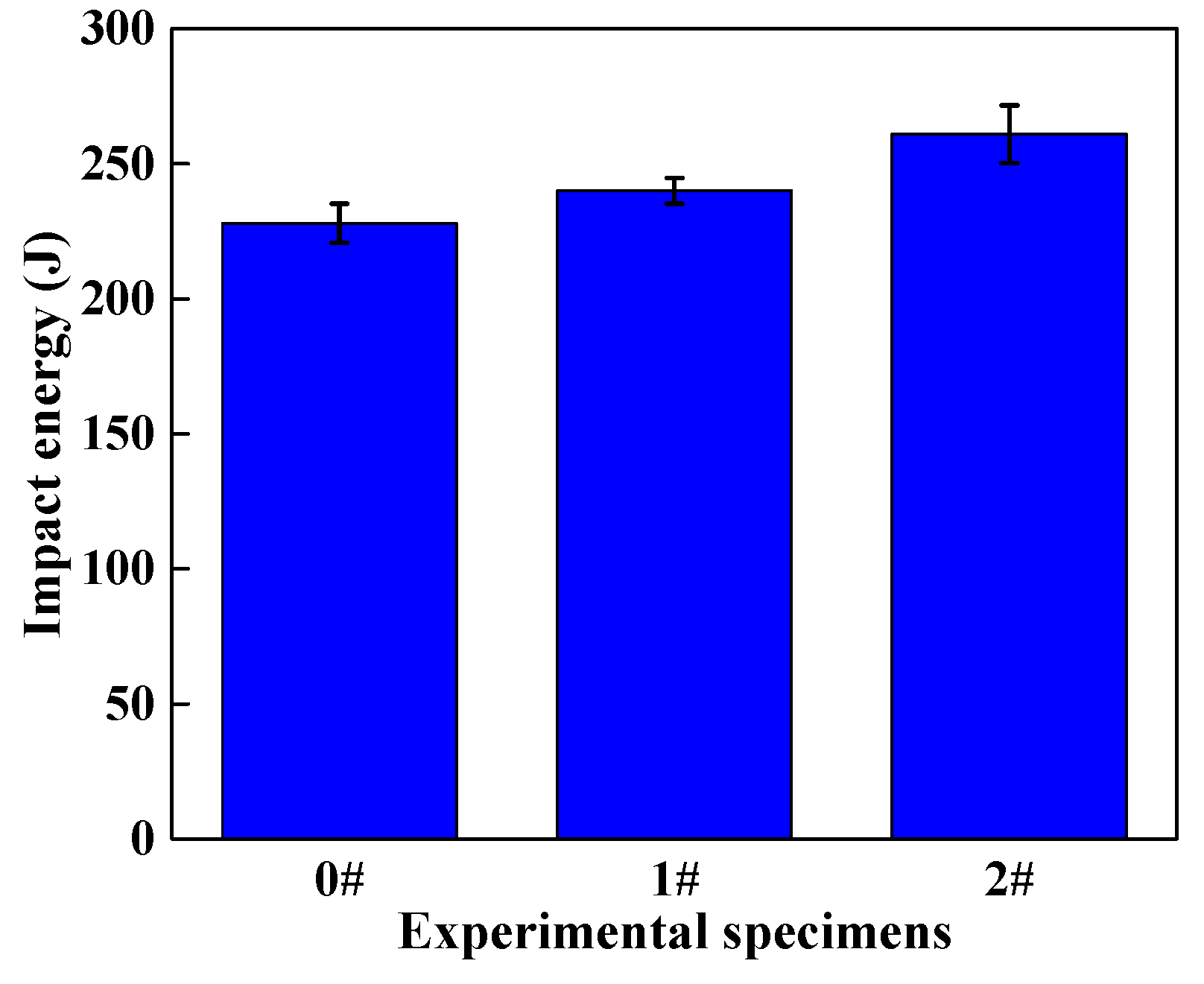
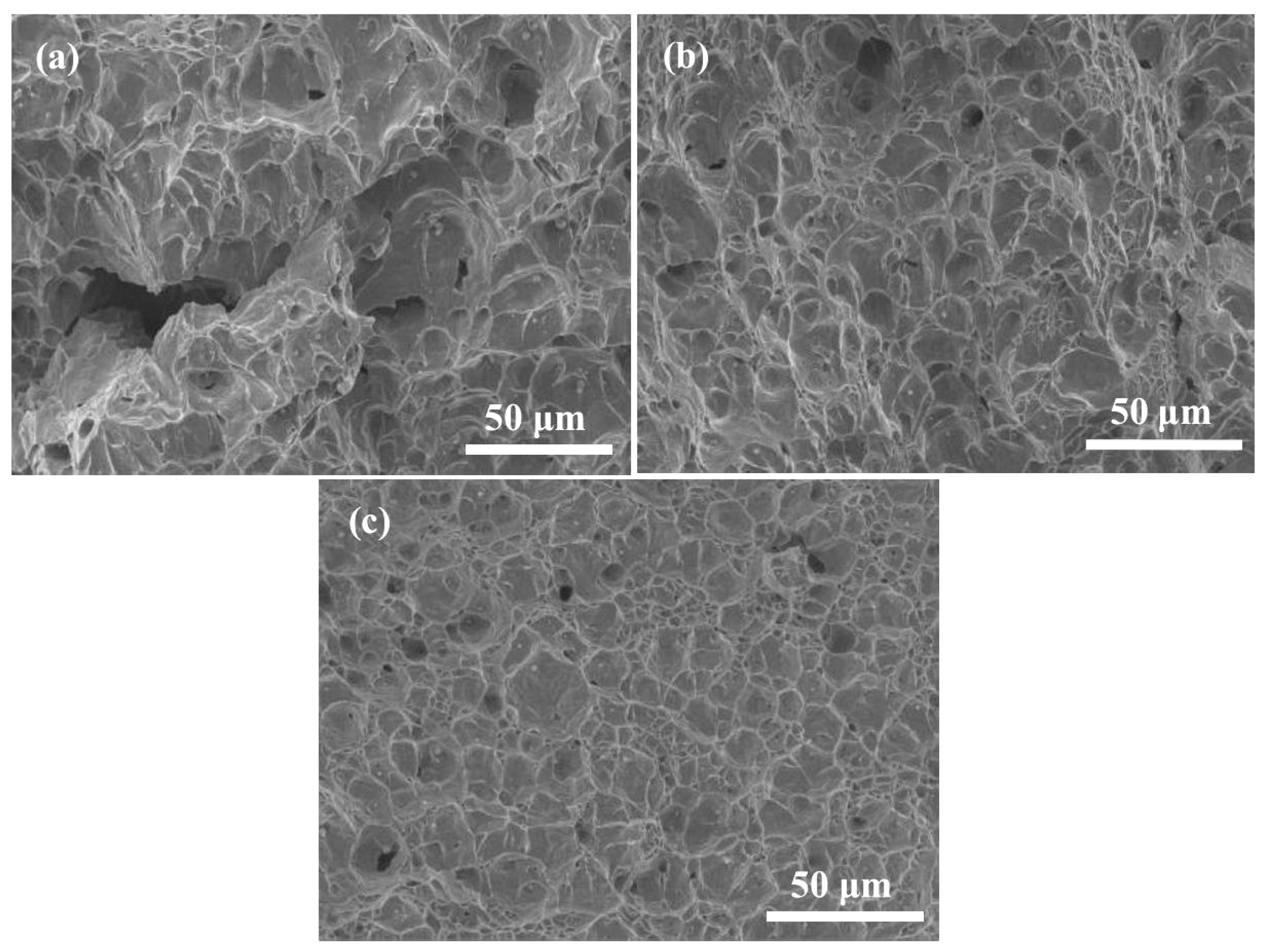
3.4. Effect of Ce on Mechanical Properties
4. Conclusions
- (1)
- When the content of Ce in steel is 0%, the main inclusions in steel are irregular and large size Al2O3 and Al2O3-MnS. When the content of Ce in steel is 0.005% or 0.02%, the inclusions in steel are mainly the spherical and small size inclusions CeAlO3, Ce2O2S and Ce2O3. Simultaneously, Ce can purify the molten steel, which reduces the number of inclusions per unit area. In addition, Ce can promote grain refinement and reduce the average grain size of LDX2101 duplex stainless steel.
- (2)
- Ce can improve the mechanical properties of LDX2101 duplex stainless steel. The tensile strength and yield strength of the steels are increased by 4.69% and 2.83% via grain refinement strengthening and solid solution strengthening. At the same time, the plasticity of the steels is increased by 4.9% by grain refinement strengthening and the impact toughness of the steels are increased by 14.5% via reducing the size and quantity of inclusions, modifying inclusions and refining grains.
Author Contributions
Funding
Conflicts of Interest
References
- Song, Z.G.; Feng, H.; Hu, S.M. Development of Chinese duplex stainless steel in recent years. J. Iron Steel Res. Int. 2017, 24, 121–130. [Google Scholar] [CrossRef]
- Strubbia, R.; Herenu, S.; Marinelli, M.C.; Alvarez, A.I. Short crack nucleation and growth in lean duplex stainless steels fatigued at room temperature. Int. J. Fatigue 2012, 41, 90–94. [Google Scholar] [CrossRef]
- Gedge, G. Structural uses of stainless steel–buildings and civil engineering. J. Constr. Steel Res. 2008, 64, 1194–1198. [Google Scholar] [CrossRef]
- Tang, R.R.; Gong, L.H. Effect of solution temperature on Microstructure and properties of 2101 nickel duplex stainless steel. Iron Steel Vanadium Titan. 2013, 34, 73–77. [Google Scholar]
- Jiang, W.Y.; Feng, Y.C.; Wang, L.P.; Wang, J.Q.; Zhou, P.C.; Guo, E.J. Effect of RE content on microstructure and properties of Cr25Ni5Mo2Cu3REx duplex stainless steel. Chin. Rare Earths 2013, 34, 56–60. [Google Scholar]
- Zhang, W.; Jiang, L.J.; Hu, J.C.; Song, H.M. Effect of ageing on precipitation and impact energy of 2101 economical duplex stainless steel. Mater. Charact. 2009, 60, 50–55. [Google Scholar] [CrossRef]
- Liljas, M.; Johansson, P.; Liu, H.P.; Olsson, C. Development of a lean duplex stainless steel. Steel Res. Int. 2008, 79, 466–473. [Google Scholar] [CrossRef]
- Sieurin, H.; Sandstrom, R.; Westin, E.M. Fracture toughness of the lean duplex stainless steel LDX 2101. Metall. Mater. Trans. A 2006, 37A, 2975–2981. [Google Scholar] [CrossRef]
- Lv, J.L.; Liang, T.X.; Wang, C.; Dong, L.M. Effect of ultrafine grain on tensile behaviour and corrosion resistance of the duplex stainless steel. Mater. Sci. Eng. C 2016, 62, 558–563. [Google Scholar]
- Wan, J.Q.; Ruan, H.H.; Shi, S.Q. Excellent combination of strength and ductility in 15Cr-2Ni duplex stainless steel based on ultrafine-grained austenite phase. Mater. Sci. Eng. A 2017, 690, 96–103. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Du, X.J.; Wang, L.M. Influence of rare earth element on microstructure and mechanical properties of 2205 duplex stainless steel. Mater. Mech. Eng. 2010, 34, 46–49. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.M. Effects of rare earth addition on the inclusions and mechanical properties of 2205 duplex stainless steel. Adv. Mater. Res. 2012, 503–504, 463–468. [Google Scholar] [CrossRef]
- Ma, X.C.; An, Z.J.; Chen, L.; Mao, T.Q.; Wang, J.F.; Long, H.J.; Xue, H.Y. The effect of rare earth alloying on the hot workability of duplex stainless steel. Mater. Design 2015, 86, 848–854. [Google Scholar] [CrossRef]
- Zhao, L.P.; Zhang, H.M.; Cui, C.Y.; Sun, X.S. Mechanical property and corrosion resistant of cerium-bearing 2Cr13 stainless steel. Adv. Mater. Res. 2011, 308–310, 701–705. [Google Scholar] [CrossRef]
- Chen, L.; Ma, X.C.; Wang, L.M.; Ye, X.N. Effect of rare earth element yttrium addition on microstructures and properties of a 21Cr-11Ni austenitic heat-resistant stainless steel. Mater. Design 2011, 32, 2206–2212. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, S.T.; Lee, I.S.; Song, C.B. Effects of rare earth metals addition and aging treatment on the corrosion resistance and mechanical properties of super duplex stainless steels. Met. Mater. Int. 2002, 8, 309–318. [Google Scholar] [CrossRef]
- Wang, X.F.; Cheng, W.Q. Influence of cerium on hot workability of 00Cr25Ni7Mo4N super duplex stainless steel. J. Rare Earths 2010, 28, 295–300. [Google Scholar] [CrossRef]
- Yu, S.C.; Wu, S.Q.; Yan, J.Q.; Gong, Y.J.; Gong, Q.S. Influence of rare earth on microstructure and mechanical properties of 5Cr21Mn9Ni4N steel. J. Rare Earths 2004, 22, 122–125. [Google Scholar]
- Kim, S.M.; Kim, J.S.; Kim, K.T.; Park, K.T.; Lee, C.S. Effect of Ce addition on secondary phase transformation and mechanical properties of 27Cr-7Ni hyper duplex stainless steels. Mater. Sci. Eng. A 2013, 573, 27–36. [Google Scholar] [CrossRef]
- Cai, G.G.; Li, C.S. Effects of Ce on inclusions, microstructure, mechanical properties, and corrosion behavior of AISI 202 stainless steel. J. Mater. Eng. Perform. 2015, 24, 3989–4009. [Google Scholar] [CrossRef]
- Zhang, S.H.; Yu, Y.C.; Wang, S.B.; Li, H. Effects of cerium addition on solidification structure and mechanical properties of 434 ferritic stainless steel. J. Rare Earths 2017, 35, 518–524. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.C.; Yang, L.; Gao, X.Z. Effect of Ce on inclusions and impact property of 2Cr13 stainless steel. J. Iron Steel Res. Int. 2010, 17, 59–64. [Google Scholar] [CrossRef]
- Yang, Q.X.; Zhao, Y.H.; Li, Y.L.; Yao, M. Thermodynamics of modifying effect of rare earth oxide on inclusions in hardfacing metal of medium-high carbon steel. J. Rare Earth 2002, 20, 291–294. [Google Scholar]
- Li, N.; Liu, Q.Y.; Wang, Y.Q.; Zhu, Z.H.; Li, X.; Qiu, S.T. Effect of Ce on inclusions modification in 2.9%Si-0.8%Al non-oriented electrical steel. J. Iron Steel Res. 2017, 29, 570–576. [Google Scholar]
- Yang, J.C.; Li, H.W.; Zhang, J.; Zhou, L.; Zhao, G.S. Thermodynamic analysis and observation of cerium inclusions in cerium-containing IF steel. Chin. Rare Earths 2018, 39, 1–8. [Google Scholar]
- Bai, Q.Q.; Zhang, Z.H. Effect of solution treatment temperature on phase ratio and mechanical properties of 2507 super duplex stainless steel. Heat Treat. Met. 2019, 44, 123–127. [Google Scholar]
- Park, Y.H.; Lee, Z.H. The effect of nitrogen and heat treatment on the microstructure and tensile properties of 25Cr-7Ni-1.5Mo-3W-xN duplex stainless steel castings. Mater. Sci. Eng. A 1999, 297, 78–84. [Google Scholar] [CrossRef]
- Li, J. Effect of solid solution treatment on structure and properties of super duplex stainless steel S32750. Spec. Steel 2012, 33, 64–66. [Google Scholar]
- Ahn, J.H.; Jung, H.D.; Im, J.H.; Jung, K.H.; Moon, B.M. Influence of the addition of gadolinium on the microstructure and mechanical properties of duplex stainless steel. Mater. Sci. Eng. A 2016, 658, 255–262. [Google Scholar] [CrossRef]
- Li, Y.; Yang, G.; Jiang, Z.H.; Chen, C.Y.; Sun, S.; Du, P.F. Effects of Ce on the microstructure and properties of 27Cr-3.8Mo-2Ni super-ferritic stainless steels. Ironmak. Steelmak. 2020, 47, 67–76. [Google Scholar] [CrossRef]
- Gao, J.Z.; Fu, P.X.; Liu, H.W.; Li, D.Z. Effects of rare earth on the microstructure and impact toughness of H13 steel. Metals 2015, 5, 383–394. [Google Scholar] [CrossRef] [Green Version]


| Steels | C | Si | Mn | P | S | Cr | Mo | Ni | Al | Cu | O | N | Ce | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0# | 0.0347 | 0.398 | 4.83 | 0.0067 | 0.0045 | 21.97 | 0.418 | 1.48 | 0.0092 | 0.384 | 0.0033 | 0.25 | 0 | Bal |
| 1# | 0.0309 | 0.427 | 4.94 | 0.0046 | 0.0043 | 21.96 | 0.405 | 1.50 | 0.0122 | 0.383 | 0.0031 | 0.25 | 0.005 | Bal |
| 2# | 0.0331 | 0.426 | 4.97 | 0.0053 | 0.0036 | 21.87 | 0.425 | 1.49 | 0.0149 | 0.388 | 0.0029 | 0.25 | 0.020 | Bal |
| Reactions | ΔGθ (J·mol−1) |
|---|---|
| [Ce] + 2[O] = CeO2(s) | −852,720 + 249.96T |
| [Ce] + 3/2[O] = 1/2Ce2O3(s) | −714,380 + 179.70T |
| [Ce] + [O] + 1/2[S] = 1/2Ce2O2S(s) | −675,700 + 165.50T |
| [Ce] + 4/3[S] = 1/3Ce3S4(s) | −497,670 + 146.30T |
| [Ce] + 3/2[S] = 1/2Ce2S3(s) | −536,420 + 163.86T |
| [Ce] + [S] = CeS(s) | −422,100 + 120.38T |
| [Ce] + [Al] + 3[O] = CeAlO3(s) | −1,366,460 + 364.30T |
| [Ce] + [N] = CeN(s) | −72,890 + 161.09T |
| [Ce] + 2[C] = CeC2(s) | −131,000 + 121.40T |
| O | C | S | P | Si | Mn | Ni | Cr | Mo | Cu | N | Al | Ce | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | −0.20 | −0.45 | −0.133 | −0.070 | −0.131 | −0.021 | 0.006 | −0.040 | 0.0035 | −0.0130 | 0.057 | −3.900 | −0.570 |
| S | −0.27 | 0.11 | −0.028 | 0.029 | 0.063 | −0.026 | 0 | −0.011 | 0.0027 | −0.0084 | 0.010 | 0.035 | −1.910 |
| Ce | −5.03 | 0.35 | −8.360 | 1.770 | ̶ | ̶ | ̶ | ̶ | ̶ | ̶ | −6.560 | −2.250 | 0.003 |
| Al | −6.60 | 0.09 | 0.030 | ̶ | 0.0056 | ̶ | ̶ | ̶ | ̶ | 0.0060 | −0.053 | 0.045 | −0.430 |
| C | −0.34 | 0.14 | 0.046 | 0.051 | 0.0800 | −0.012 | 0.012 | −0.024 | −0.0083 | 0.0160 | 0.110 | 0.043 | −0.024 |
| N | 0.05 | 0.13 | 0.007 | 0.045 | 0.0470 | −0.021 | 0.010 | −0.047 | −0.0110 | 0.0090 | ̶ | −0.028 | ̶ |
| Reactions | ΔG (J·mol−1) | |
|---|---|---|
| 1# (0.005% Ce) | 2# (0.02% Ce) | |
| [Ce] + 2[O] = CeO2(s) | 16,490.47 | −1863.26 |
| [Ce] + 3/2[O] = 1/2Ce2O3(s) | −41,084.91 | −60,272.51 |
| [Ce] + [O] + 1/2[S] = 1/2Ce2O2S(s) | −44,780.79 | −62,915.73 |
| [Ce] + 4/3[S] = 1/3Ce3S4(s) | 49,649.23 | 32,990.78 |
| [Ce] + 3/2[S] = 1/2Ce2S3(s) | 60,018.70 | 43,989.16 |
| [Ce] + [S] = CeS(s) | 44,211.77 | 26,295.62 |
| [Ce] + [Al] + 3[O] = CeAlO3(s) | −84,694.90 | −104,321.50 |
| [Ce] + [N] = CeN(s) | 433,241.58 | 411,426.72 |
| [Ce] + 2[C] = CeC2(s) | 383,974.43 | 360,024.53 |
| Transformation Reactions | ΔGθ (J·mol−1) |
|---|---|
| CeO2(s) + [Ce] + [S] = Ce2O2S(s) | −462,280 + 81.04T |
| Ce2O3(s) + [S] = Ce2O2S(s) + [O] | 77,360 − 28.48T |
| Al2O3(s) + [Ce] = CeAlO3(s) + [Al] | −102,119 − 25.70T |
| Transformation Reactions | ΔG (J·mol−1) | |
|---|---|---|
| 1# (0.005% Ce) | 2# (0.02% Ce) | |
| CeO2(s) + [Ce] + [S] = Ce2O2S(s) | - | −87,568.20 |
| Ce2O3(s) + [S] = Ce2O2S(s) + [O] | −7391.76 | −5286.44 |
| Al2O3(s) + [Ce] = CeAlO3(s) + [Al] | −76,402.16 | −95,150.77 |
| Steels | Austenite (%) | Ferrite (%) |
|---|---|---|
| 0# | 47.6 ± 0.4 | 52.4 ± 0.8 |
| 1# | 50.5 ± 1.1 | 49.5 ± 0.4 |
| 2# | 51.4 ± 0.7 | 48.6 ± 1.2 |
| Steels | Tensile Strength (MPa) | Yield Strength (MPa) | Elongation (%) |
|---|---|---|---|
| 0# | 725 ± 5.3 | 460 ± 4.7 | 42.9 ± 2.7 |
| 1# | 736 ± 7.2 | 466 ± 2.5 | 44.3 ± 1.4 |
| 2# | 759 ± 11.2 | 473 ± 4.1 | 45.0 ± 2.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, W.; Wang, P.; Zhang, L.; Jiang, Z. Effects of Ce on Microstructure and Mechanical Properties of LDX2101 Duplex Stainless Steel. Metals 2020, 10, 1233. https://doi.org/10.3390/met10091233
Gong W, Wang P, Zhang L, Jiang Z. Effects of Ce on Microstructure and Mechanical Properties of LDX2101 Duplex Stainless Steel. Metals. 2020; 10(9):1233. https://doi.org/10.3390/met10091233
Chicago/Turabian StyleGong, Wei, Pengfei Wang, Lei Zhang, and Zhouhua Jiang. 2020. "Effects of Ce on Microstructure and Mechanical Properties of LDX2101 Duplex Stainless Steel" Metals 10, no. 9: 1233. https://doi.org/10.3390/met10091233




