Effect of Basicity on the Structure, Viscosity and Crystallization of CaO-SiO2-B2O3 Based Mold Fluxes
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Simulation
2.2. Viscosity Measurement
2.3. Thermodynamic Calculation
2.4. Fourier Transform Infrared (FTIR) Spectroscopy
3. Results and Discussion
3.1. Effect of Basicity on the Structure of CaO-SiO2-B2O3 System
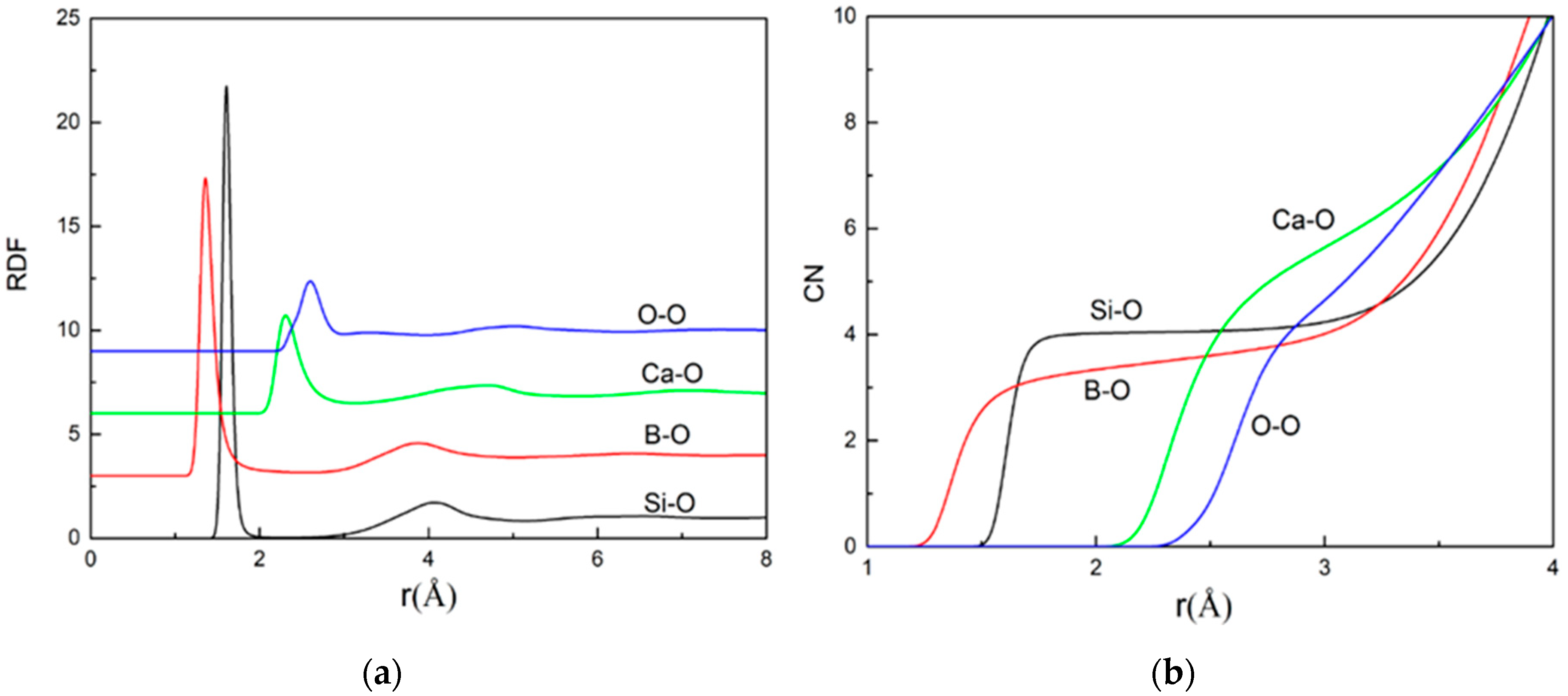
3.1.1. Average Bond Length and Average Coordination Number
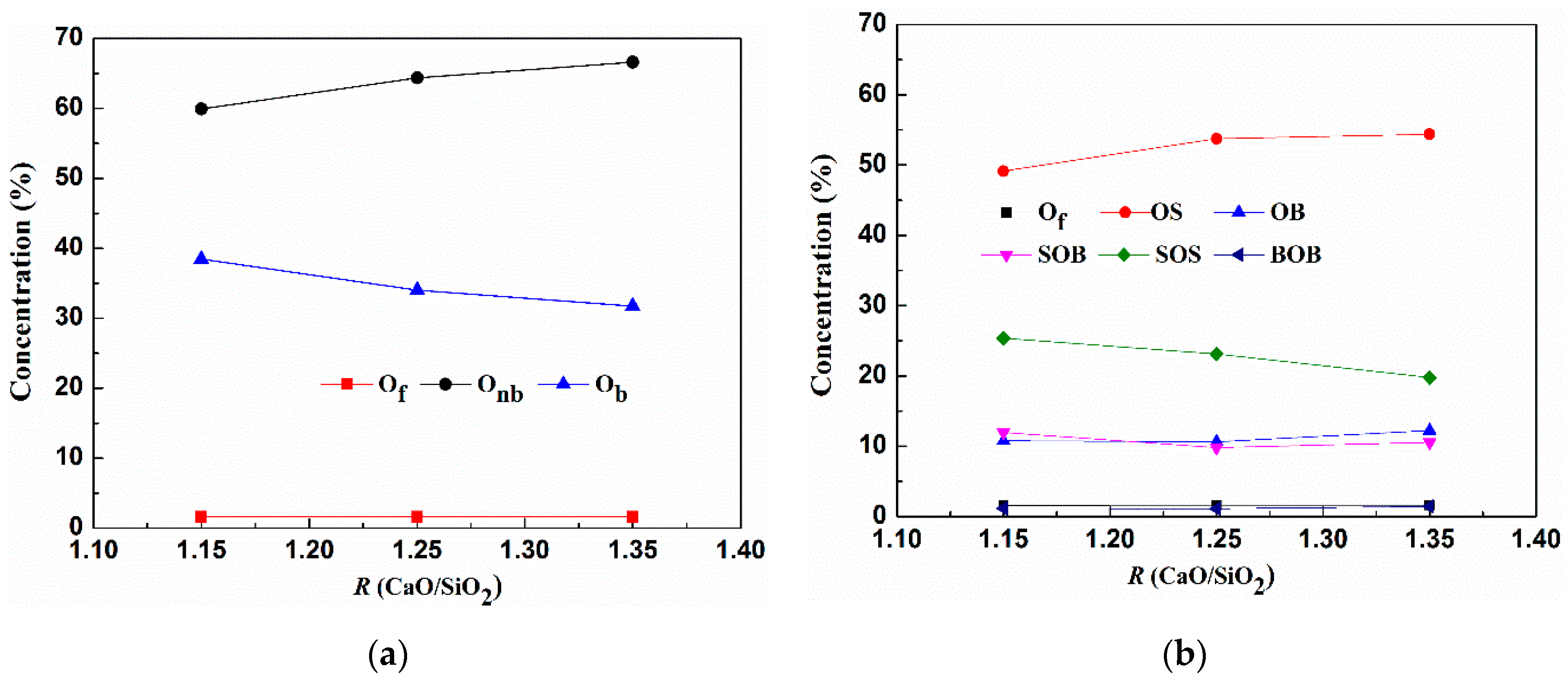
3.1.2. Distribution of Oxygen Types
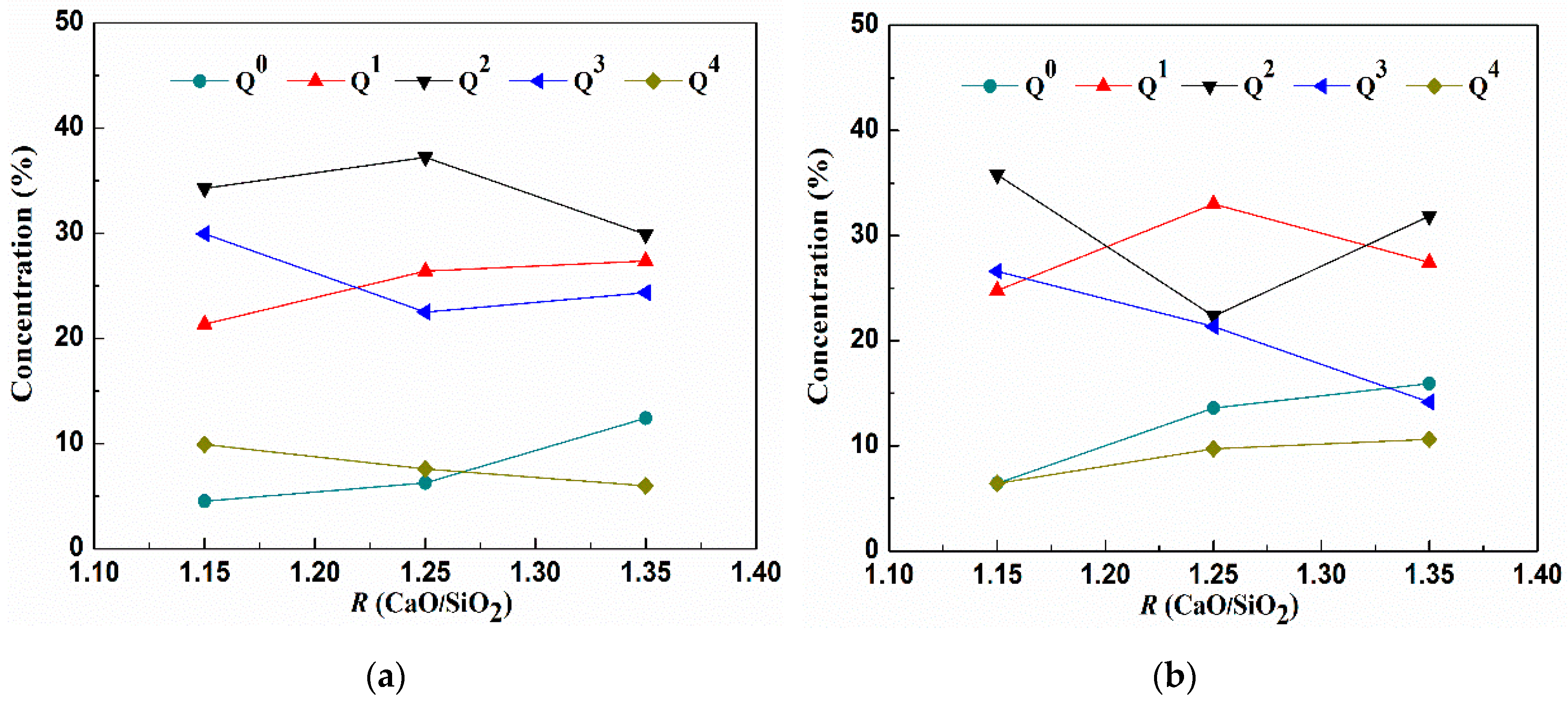
3.1.3. Distribution of Structure Units Qn
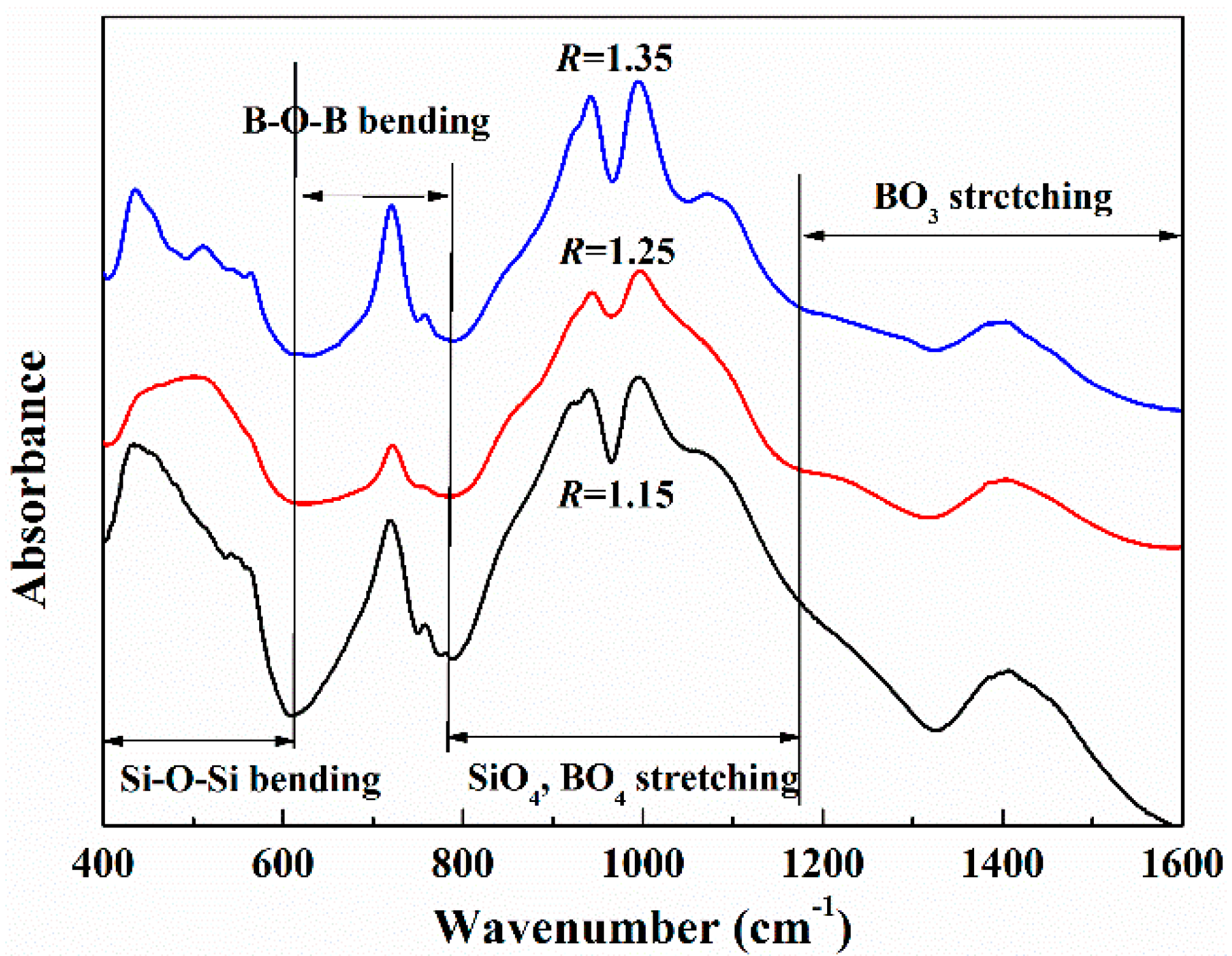
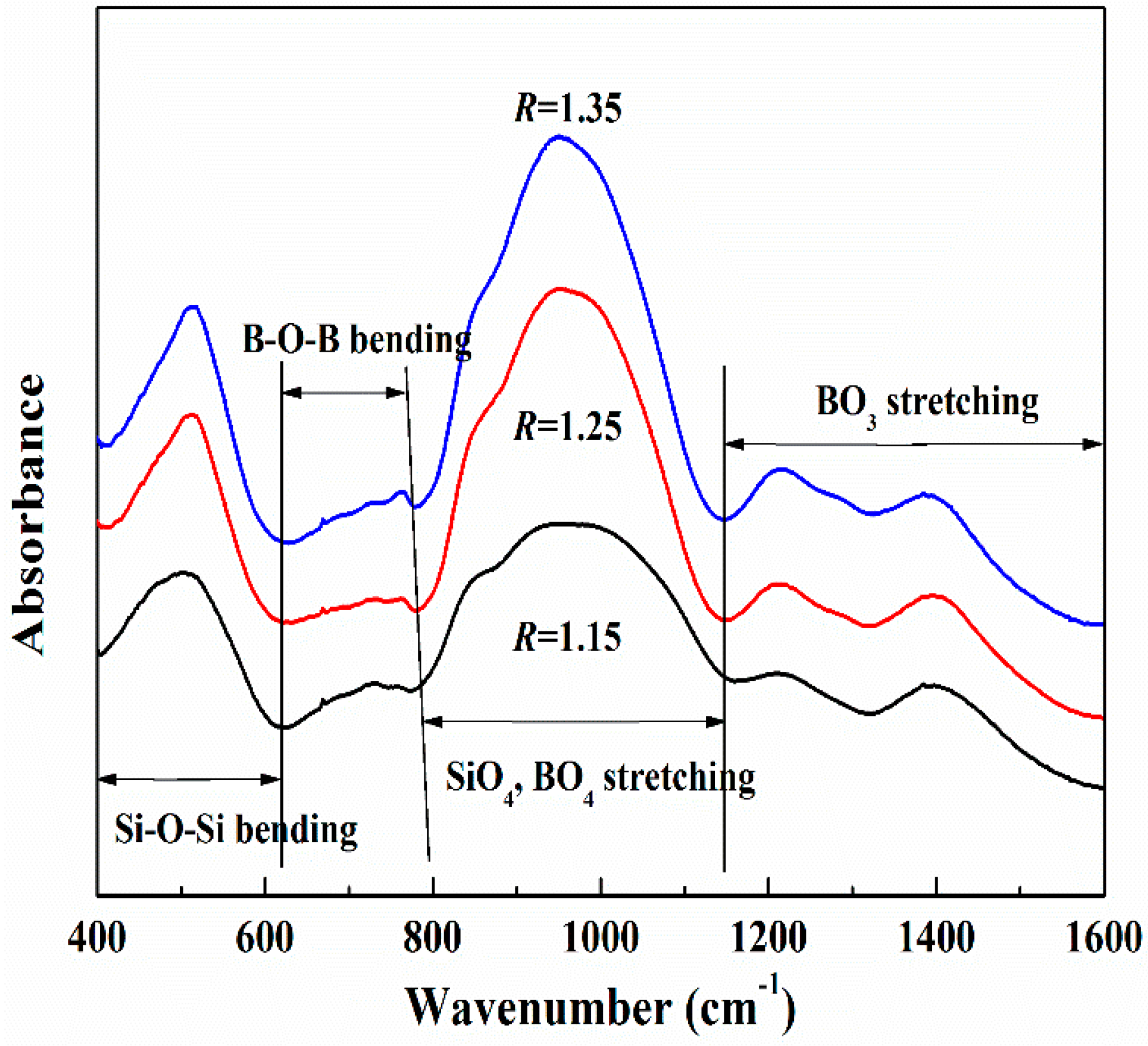
3.1.4. FTIR Experimental Results
3.2. Effect of Basicity on the Viscosity of F-Free Mold Fluxes
3.2.1. Effect of Basicity on the Viscosity and Its Correlation to the Structure
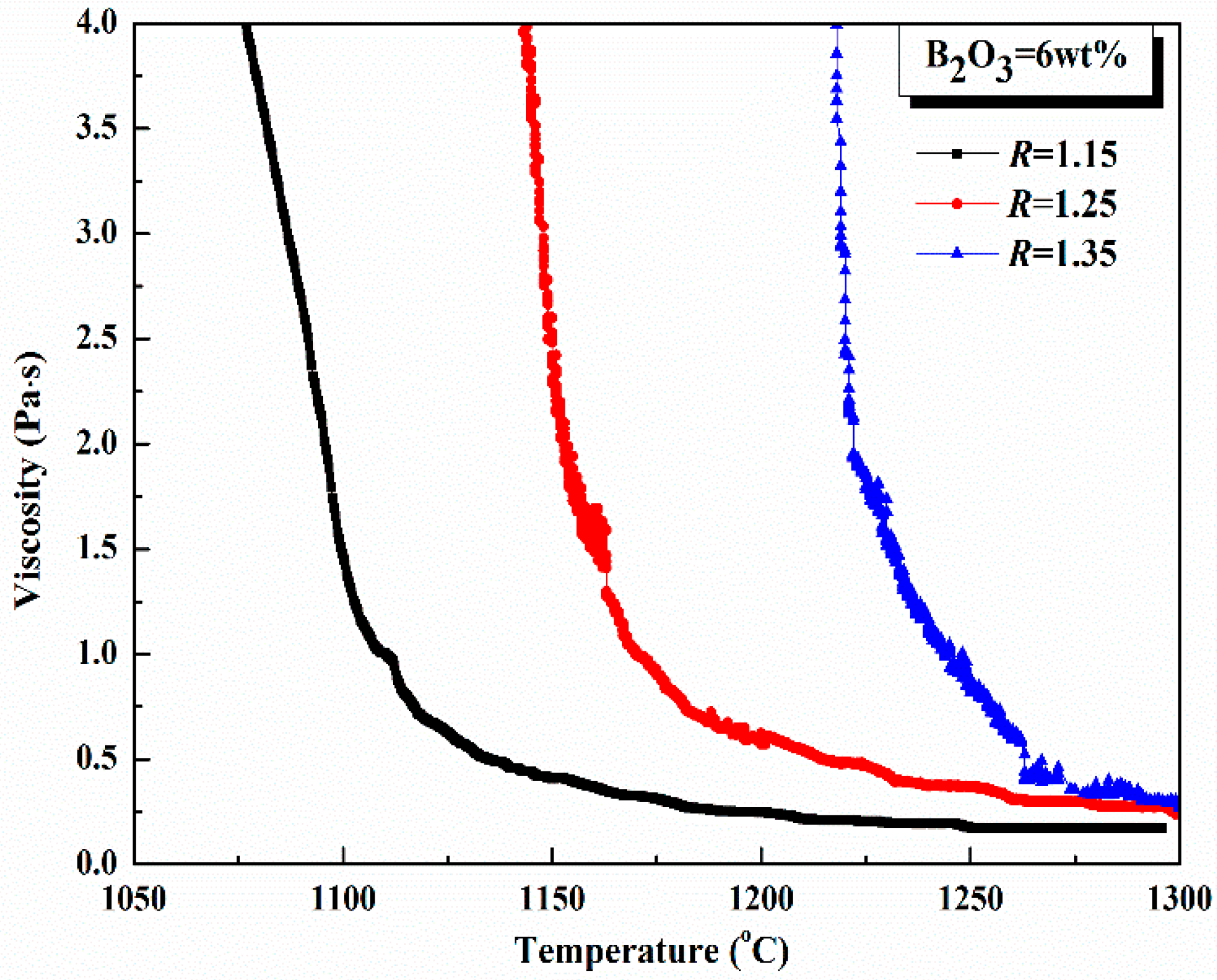
3.2.2. Effect of Basicity on the Properties of Viscosity-Temperature Curve
4. Conclusions
- Combing the MD and FTIR results of CaO-SiO2-B2O3 system, stable structural units of [SiO4]4− tetrahedral, [BO3]3− trihedral, and [BO4]5− tetrahedral are formed, and the Si-O and B-O structure depolymerizes with the basicity increase from 1.15 to 1.25, then the B-O structure becomes complex with the basicity further increase from 1.25 to 1.35. It means that with the increase of basicity from 1.15 to 1.35, the slag structure becomes simpler first and then changes to complex structure, which leads to decrease and then increase of slag viscosity theoretically.
- In fluorine-free mold fluxes shown in this paper, with the basicity increase, the viscosity at 1300 °C increases, the liquidus temperature decreases and then increases, the network structure polymerizes. It is therefore concluded that the structural complexity plays a predominant role in increasing the viscosity at 1300 °C since the increase of alkalinity will provide more cations to balance the negative excess of [BO4]5− tetrahedral.
- In fluorine-free mold fluxes shown in this paper, due to the changes in crystallization phase and solid solution ratio, the figure curve of fluorine-free slag shows the characteristics of alkaline slag and the breaking temperature increases with the basicity increase. Thus, it is of critical importance to set suitable basicity to achieve the coordinate control of lubrication and heat transfer of fluoride-free mold fluxes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mills, K.C.; Fox, A.B.; Li, Z.; Thackeray, R.P. The performance and properties of mould fluxes. Ironmak. Steelmak. 2005, 32, 26–34. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. The effect of CaF2 on the viscosities and structure of CaO-SiO2(-MgO)-CaF2 slags. Metall. Mater. Trans. B 2002, 33, 723. [Google Scholar] [CrossRef]
- Watanabe, T.; Fukuyama, H.; Susa, M.; Nagata, K. Phase diagram Cuspidine (3CaO·2SiO2·CaF2)-CaF2. Metall. Mater. Trans. B 2000, 31, 1273. [Google Scholar] [CrossRef]
- Cho, J.; Shibata, H. Effect of solidification of mold fluxes on the heat transfer in casting mold. J. Non-Cryst. Solids 2001, 282, 110. [Google Scholar] [CrossRef]
- Persson, M.; Seetharaman, S.; Seetharaman, S. Kinetic studies of fluoride evaporation from slags. ISIJ Int. 2007, 47, 1711–1717. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Lee, D.-H.; Shin, D.-W.; Choi, S.-Y.; Cho, J.-W.; Park, J.-M. Properties of F-free glass system as a mold flux: Viscosity, thermal conductivity and crystallization behavior. J. Non-Cryst. Solids 2004, 345–346, 157–160. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Zhou, K. Viscosity and crystallization behavior of F-free mold flux for casting medium carbon steels. ISIJ Int. 2015, 55, 1916–1924. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Wei, J.; Zhou, K. Melting and heat transfer behavior of fluorine-free mold fluxes for casting medium carbon steels. ISIJ Int. 2015, 55, 821–829. [Google Scholar] [CrossRef]
- Lu, B.; Wang, W.; Li, J.; Zhao, H.; Huang, D. Effects of basicity and B2O3 on the crystallization and heat transfer behaviors of low fluorine mold flux for casting medium carbon steels. Metall. Mater. Trans. B 2013, 44, 365–377. [Google Scholar] [CrossRef]
- Kim, H.; Sohn, I. Effect of CaF2 and Li2O additives on the viscosity of CaO-SiO2-Na2O slags. ISIJ Int. 2011, 51, 1–8. [Google Scholar] [CrossRef]
- Nakada, H.; Nagata, K. Crystallization of CaO-SiO2-TiO2 slag as a candidate for fluorine free mold flux. ISIJ Int. 2006, 46, 441–449. [Google Scholar] [CrossRef]
- Wang, W.; Cai, D.; Zhang, L. A review of fluorine-free mold flux development. ISIJ Int. 2018, 58, 1957–1964. [Google Scholar] [CrossRef]
- He, S.P.; You, J.L.; Wang, Q.; Xu, C.S. Effect of fluorine in mould fluxes on microstructural units of silicates slag. Spectros. Spect. Anal. 2008, 28, 2579–2582. [Google Scholar]
- Yang, J.; Zhang, J.; Sasaki, Y.; Ostrovski, O.; Zhang, C.; Cai, D.; Kashiwaya, Y. Crystallization behavior and heat transfer of fluorine-free mold fluxes with different Na2O concentration. Metall. Mater. Trans. B 2016, 47, 2447–2458. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Y.J.; He, S.P.; Mills, K.C.; Li, Z.S. Formation of TiN and Ti (C, N) in TiO2 containing, fluoride free mould fluxes at high temperature. Ironmak. Steelmak. 2011, 38, 297–301. [Google Scholar] [CrossRef]
- Wu, T. Study on Structure and Macroscopic Properties of Low Reactive Mold Fluxes. Ph.D. Thesis, Chongqing University, Chongqing, China, 2017. [Google Scholar]
- Woodcock, L.V.; Angell, C.A.; Cheeseman, P. Molecular dynamics studies of the vitreous state: Simple ionic system and silica. J. Chem. Phys. 1976, 65, 1565–1577. [Google Scholar] [CrossRef]
- Feuston, B.P.; Garofalinim, S.H. Empirical three-body potential for vitreous silica. J. Chem. Phys. 1988, 89, 5818–5824. [Google Scholar] [CrossRef]
- Shimoda, K.; Saito, K. Detailed structure elucidation of the blast furnace slag by molecular dynamics simulation. ISIJ Int. 2007, 44, 1275–1279. [Google Scholar] [CrossRef]
- Mills, K.C.; Keene, B.J. Physical properties of BOS slags. Int. Mater. Rev. 1987, 32, 1–120. [Google Scholar] [CrossRef]
- Cramb, A.W. The solidification behavior of slags: Phenomena related to mold slags. ISIJ Int. 2014, 54, 2665–2671. [Google Scholar] [CrossRef][Green Version]
- Park, J.H.; Min, D.J.; Song, H.S. FT-IR spectroscopic study on structure of CaO-SiO2 and CaO-SiO2-CaF2 slags. ISIJ Int. 2002, 42, 344–351. [Google Scholar] [CrossRef]
- Kim, H.; Matsuura, H.; Tsukihashi, F.; Wang, W.; Min, D.J.; Sohn, I. Effect of Al2O3 and CaO/SiO2 on the viscosity of calcium-silicate-based slags containing 10 mass Pct MgO. Metall. Mater. Trans. B 2013, 44, 5. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Sohn, I.; Min, D.J. The effect of MgO on the viscosity of the CaO-SiO2–20 wt%Al2O3-MgO slag system. Steel Res. Int. 2010, 81, 261–264. [Google Scholar] [CrossRef]
- Shen, Y.; Chong, J.; Huang, Z.; Tian, J.; Zhang, W.; Tang, X.; Ding, W.; Du, X. Viscosity and Structure of a CaO-SiO2-FeO-MgO System during a Modified Process from Nickel Slag by CaO. Materials 2019, 12, 2562. [Google Scholar] [CrossRef] [PubMed]
- Kamitsos, E.I.; Karakassides, M.A.; Chryssikos, G.D. Vibrational spectra of magnesium-sodium-borate glasses. 2. Raman and mid-infrared investigation of the network structure. J. Phys. Chem. 1987, 91, 1073–1079. [Google Scholar] [CrossRef]
- Padmaja, G.; Kistaiah, P. Infrared and Raman spectroscopic studies on alkali borate glasses: Evidence of mixed alkali effect. J. Phys. Chem. A 2009, 113, 2397–2404. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Liao, J.L.; Zheng, K.; Wang, X.D.; Zhang, Z.T. Effect of B2O3 on the structure and viscous behavior of Ti-bearing blast furnace slags. JOM 2014, 66, 2168–2175. [Google Scholar] [CrossRef]


| Atom1 | Atom2 | Aij (eV) | Bij (1/Å) | Cij (eV·Å6) |
|---|---|---|---|---|
| O | O | 1,497,049.00 | 5.88 | 17.34 |
| O | B | 10,699.19 | 6.06 | 0 |
| O | Si | 62,794.37 | 6.06 | 0 |
| O | Ca | 717,827.00 | 6.06 | 8.67 |
| Ca | Ca | 329,051.60 | 6.25 | 4.335 |
| Ca | Si | 26,674.68 | 6.25 | 0 |
| Ca | B | 4300.43 | 6.25 | 0 |
| Si | Si | 2162.39 | 6.25 | 0 |
| B | B | 56.20 | 6.25 | 0 |
| Groups | Basicity | Mass Fraction (%) | Atomic Number | Density(g/cm3) | Box Length (Å) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | B2O3 | Ca | Si | B | O | Total | ||||
| R1 | 1.15 | 50 | 44 | 6 | 809 | 665 | 154 | 2370 | 3998 | 2.669 | 38.35426 |
| R2 | 1.25 | 52 | 42 | 6 | 847 | 638 | 156 | 2357 | 3998 | 2.678 | 38.39251 |
| R3 | 1.35 | 54 | 40 | 6 | 885 | 612 | 158 | 2346 | 4001 | 2.687 | 38.43881 |
| NO. | Basicity | CaO | SiO2 | B2O3 | Al2O3 | Na2O | MgO | Li2O |
|---|---|---|---|---|---|---|---|---|
| F1 | 1.15 | 42.26 | 36.74 | 6 | 4 | 8 | 2 | 1 |
| F2 | 1.25 | 43.89 | 35.11 | 6 | 4 | 8 | 2 | 1 |
| F3 | 1.35 | 45.38 | 33.62 | 6 | 4 | 8 | 2 | 1 |
| Property | Sample | Ca-Ca | Ca-Si | Ca-B | Si-Si | Si-B | B-B | Ca-O | Si-O | B-O | O-O |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | R1 | 3.38 | 3.53 | 3.25 | 3.19 | 2.96 | 2.75 | 2.31 | 1.61 | 1.36 | 2.60 |
| Bond | R2 | 3.41 | 3.53 | 3.15 | 3.19 | 2.96 | 2.75 | 2.31 | 1.61 | 1.36 | 2.60 |
| Length | R3 | 3.39 | 3.54 | 3.21 | 3.20 | 2.97 | 2.75 | 2.31 | 1.61 | 1.36 | 2.60 |
| Average | R1 | 6.79 | 4.73 | 1.05 | 1.92 | 0.41 | 0.59 | 5.89 | 4.05 | 3.63 | 4.83 |
| Coordination | R2 | 7.11 | 4.54 | 1.07 | 1.76 | 0.41 | 0.55 | 5.96 | 4.05 | 3.66 | 4.65 |
| Number | R3 | 7.36 | 4.41 | 1.08 | 1.61 | 0.40 | 0.41 | 5.98 | 4.05 | 3.58 | 4.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadaf, S.; Wu, T.; Zhong, L.; Liao, Z.Y.; Wang, H.C. Effect of Basicity on the Structure, Viscosity and Crystallization of CaO-SiO2-B2O3 Based Mold Fluxes. Metals 2020, 10, 1240. https://doi.org/10.3390/met10091240
Sadaf S, Wu T, Zhong L, Liao ZY, Wang HC. Effect of Basicity on the Structure, Viscosity and Crystallization of CaO-SiO2-B2O3 Based Mold Fluxes. Metals. 2020; 10(9):1240. https://doi.org/10.3390/met10091240
Chicago/Turabian StyleSadaf, Shama, Ting Wu, Lei Zhong, Zhi You Liao, and Hai Chuan Wang. 2020. "Effect of Basicity on the Structure, Viscosity and Crystallization of CaO-SiO2-B2O3 Based Mold Fluxes" Metals 10, no. 9: 1240. https://doi.org/10.3390/met10091240
APA StyleSadaf, S., Wu, T., Zhong, L., Liao, Z. Y., & Wang, H. C. (2020). Effect of Basicity on the Structure, Viscosity and Crystallization of CaO-SiO2-B2O3 Based Mold Fluxes. Metals, 10(9), 1240. https://doi.org/10.3390/met10091240




