Scale Formation on HSLA Steel during Continuous Casting Part I: The Effect of Temperature–Time on Oxidation Kinetics
Abstract
1. Introduction
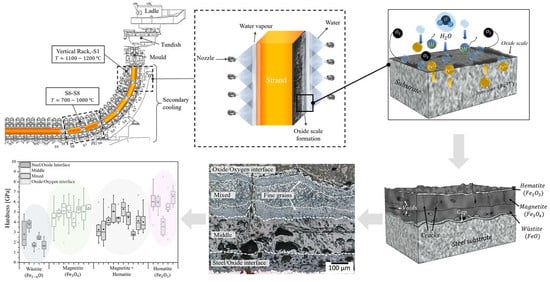
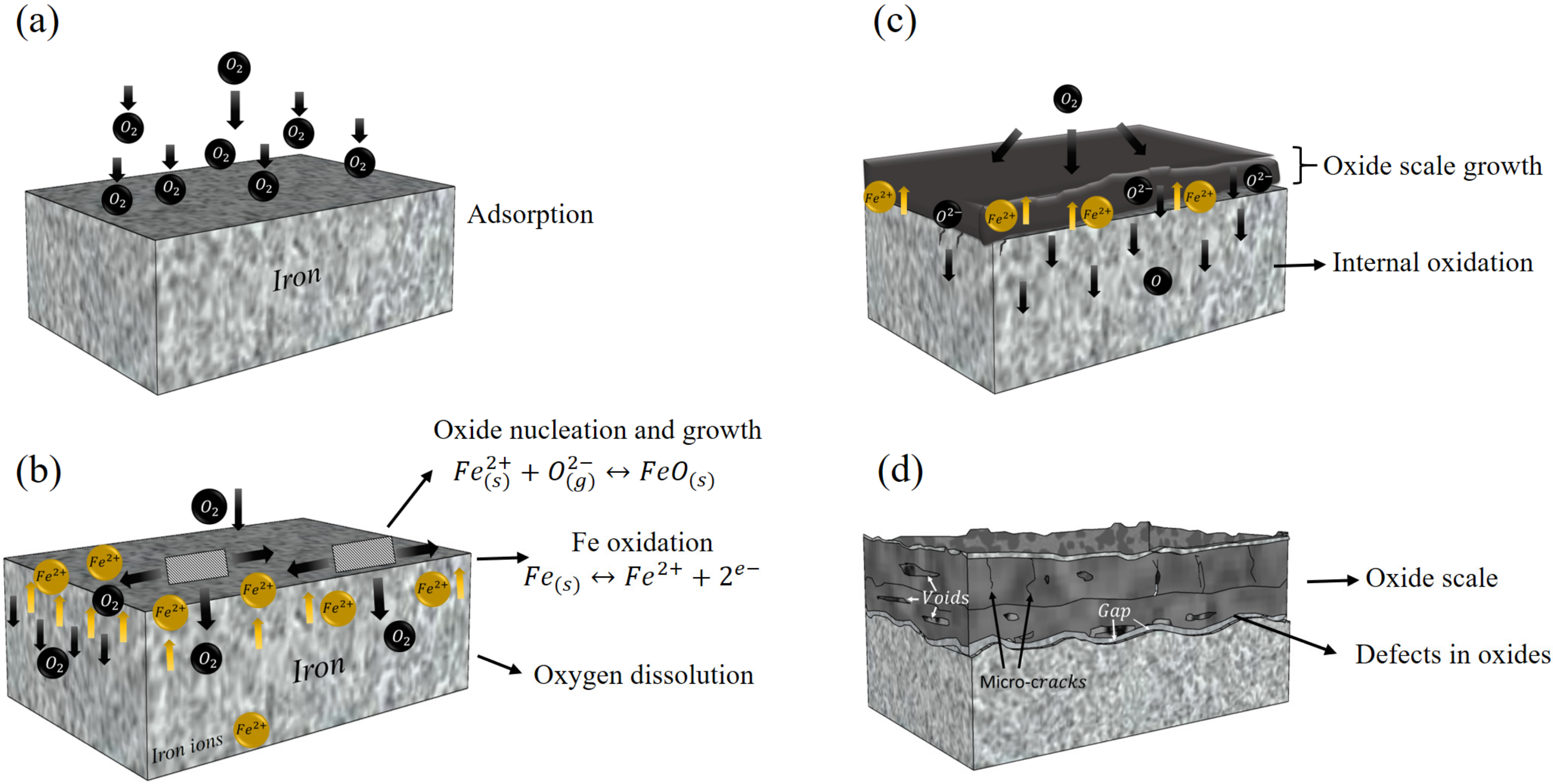
1.1. Oxidation During Casting
1.2. Phases in Oxides
1.3. Temperature–Time Effect on the Kinetics
2. Experiment Setup
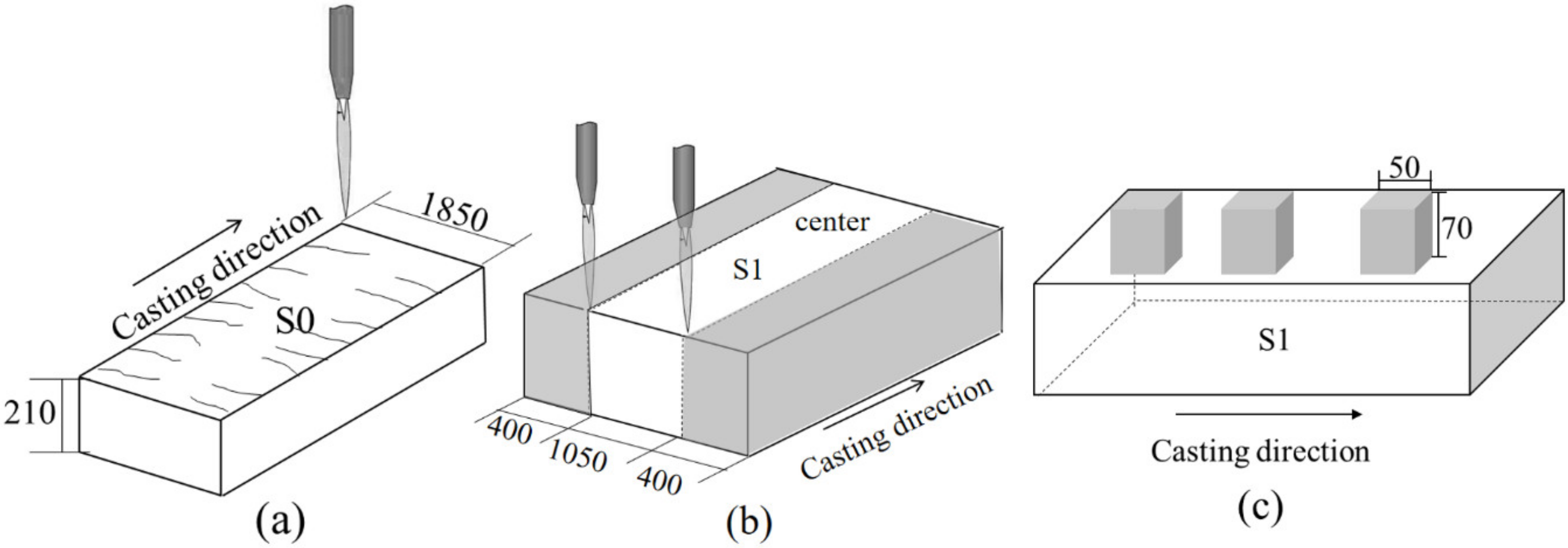
2.1. Sample Identification
2.2. Oxidation Tests
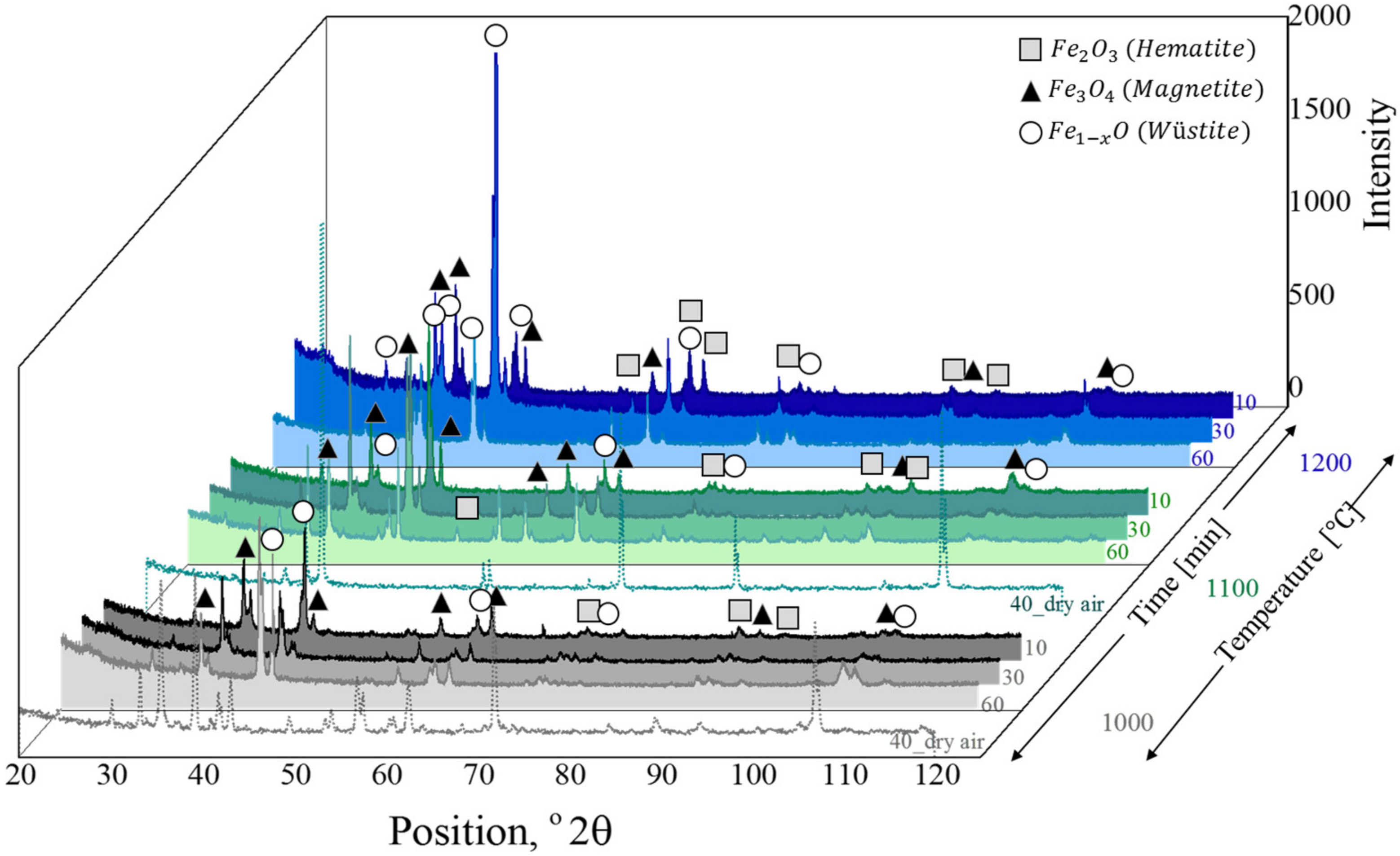
2.3. Characterization of Oxides
2.4. Nanoindentation
3. Results
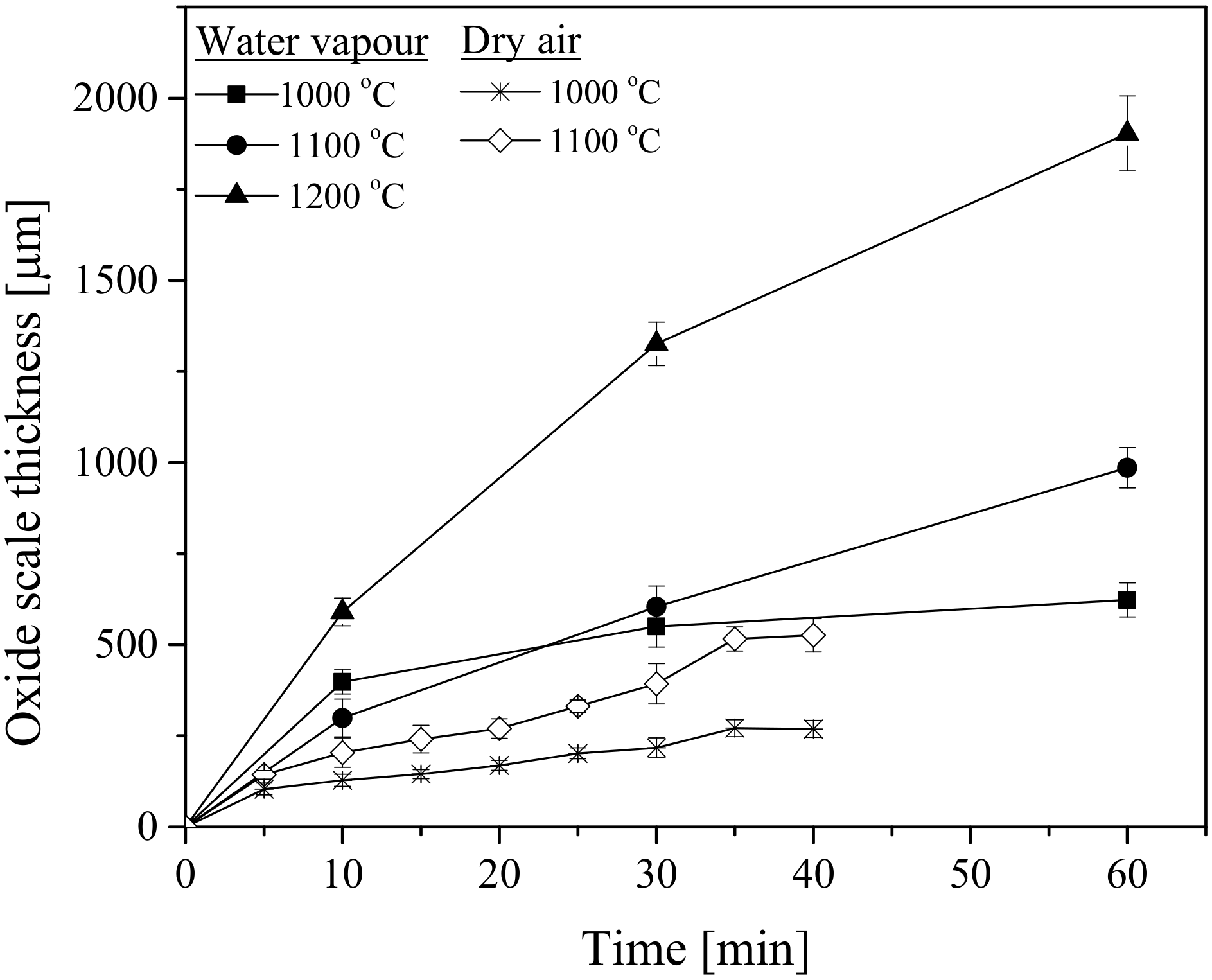
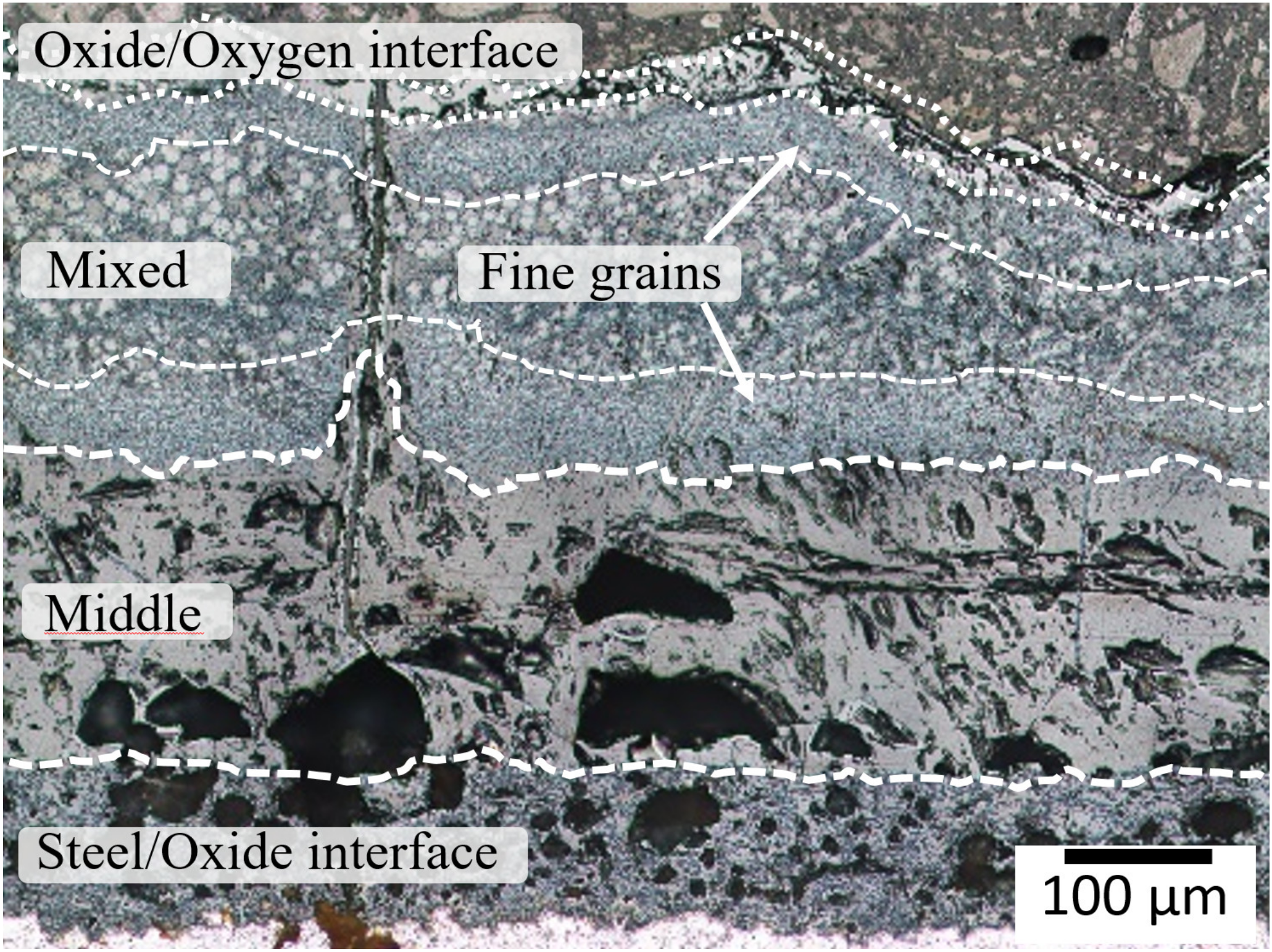
3.1. Oxide Scale Growth
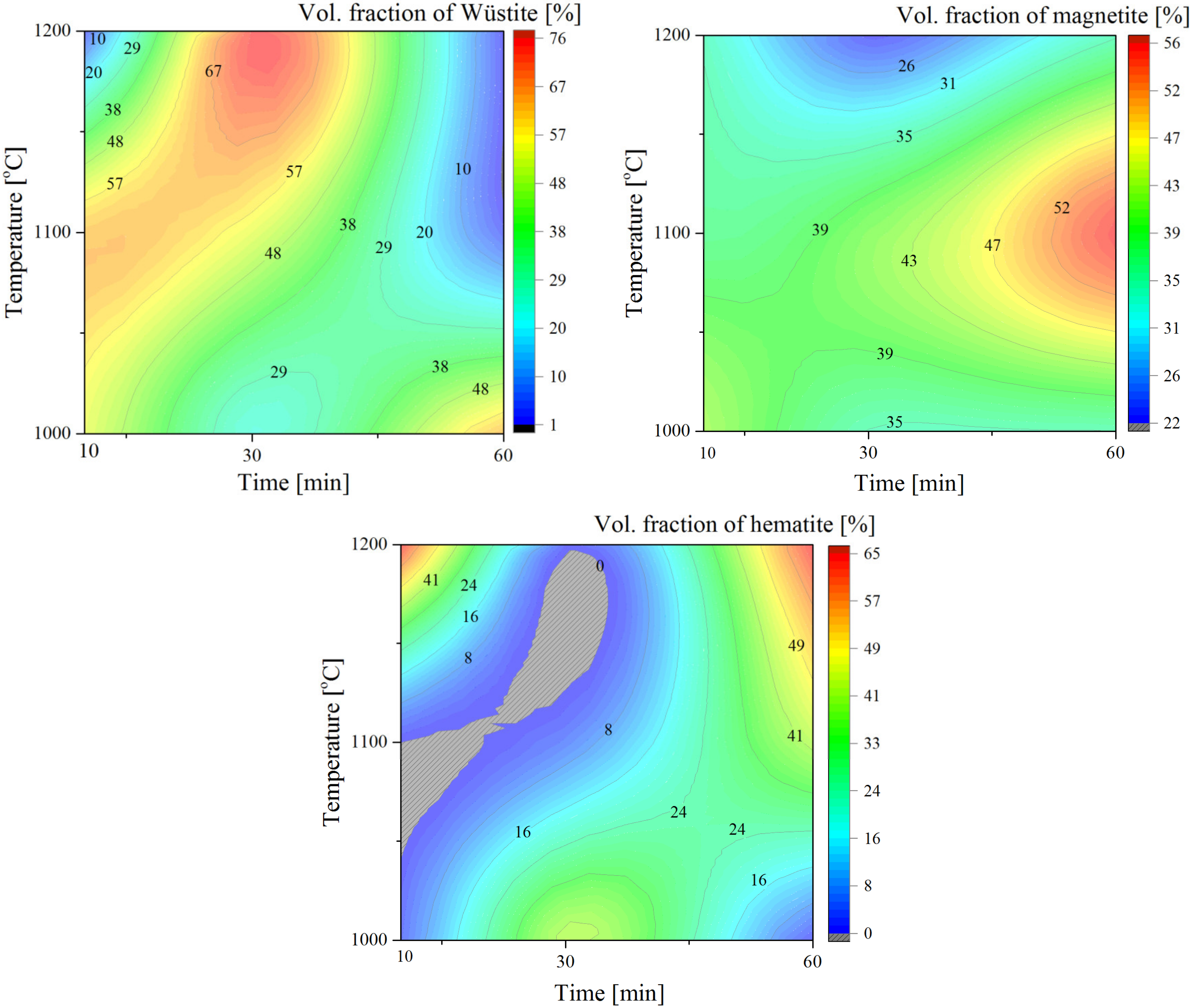
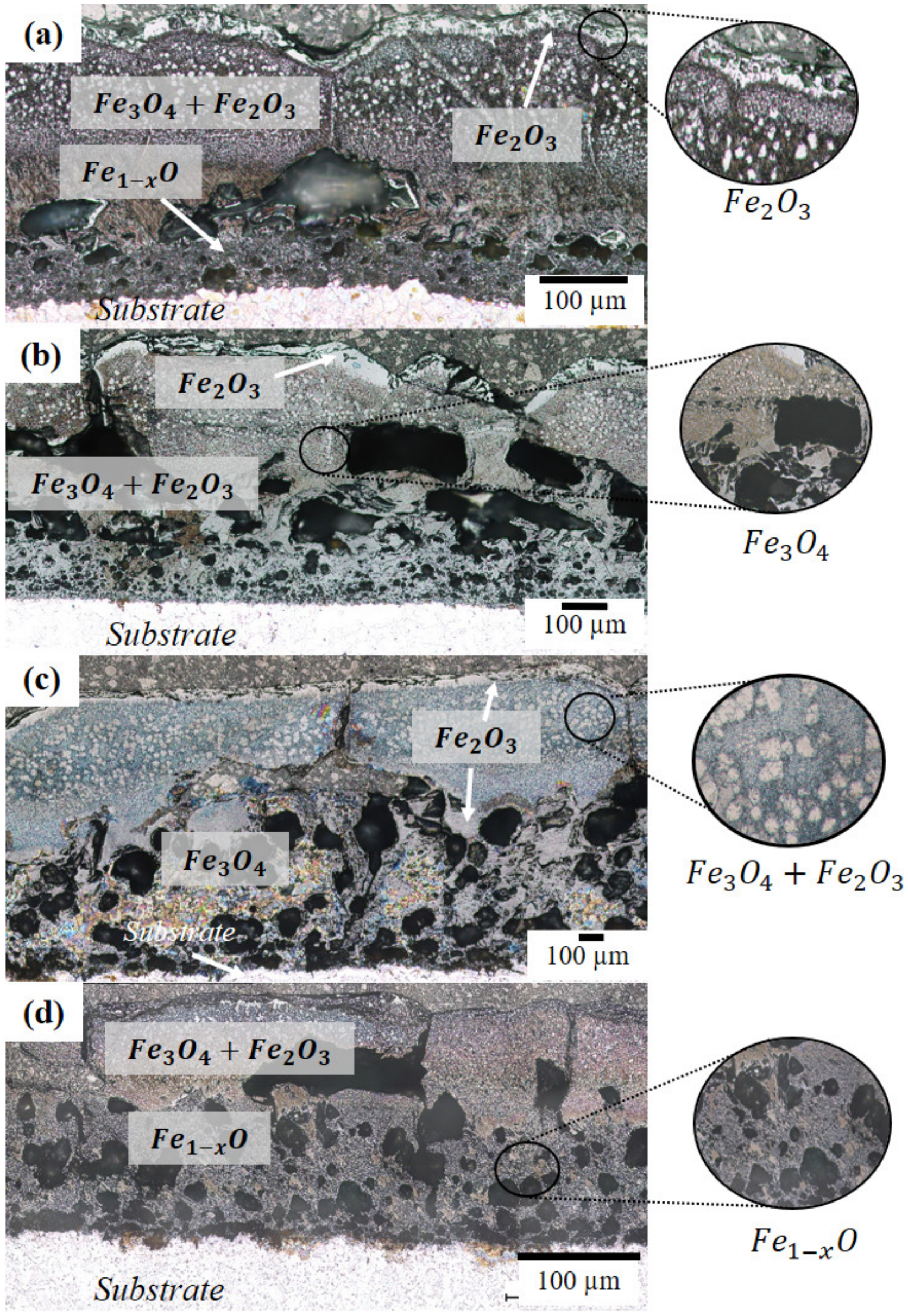
3.2. Microstructure and Phase Analysis
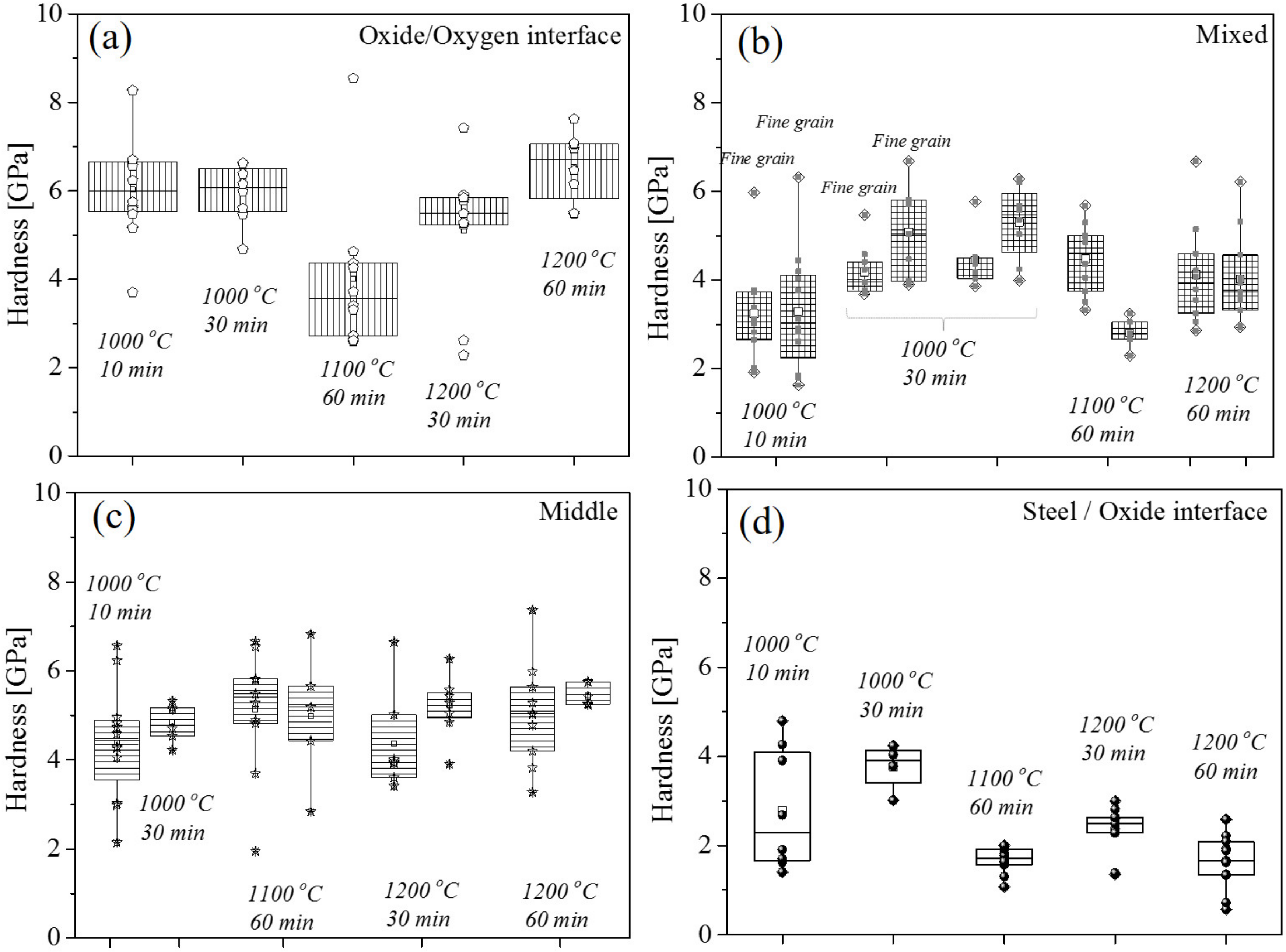
3.3. Micromechanics of Oxides
3.4. Numerical Modelling
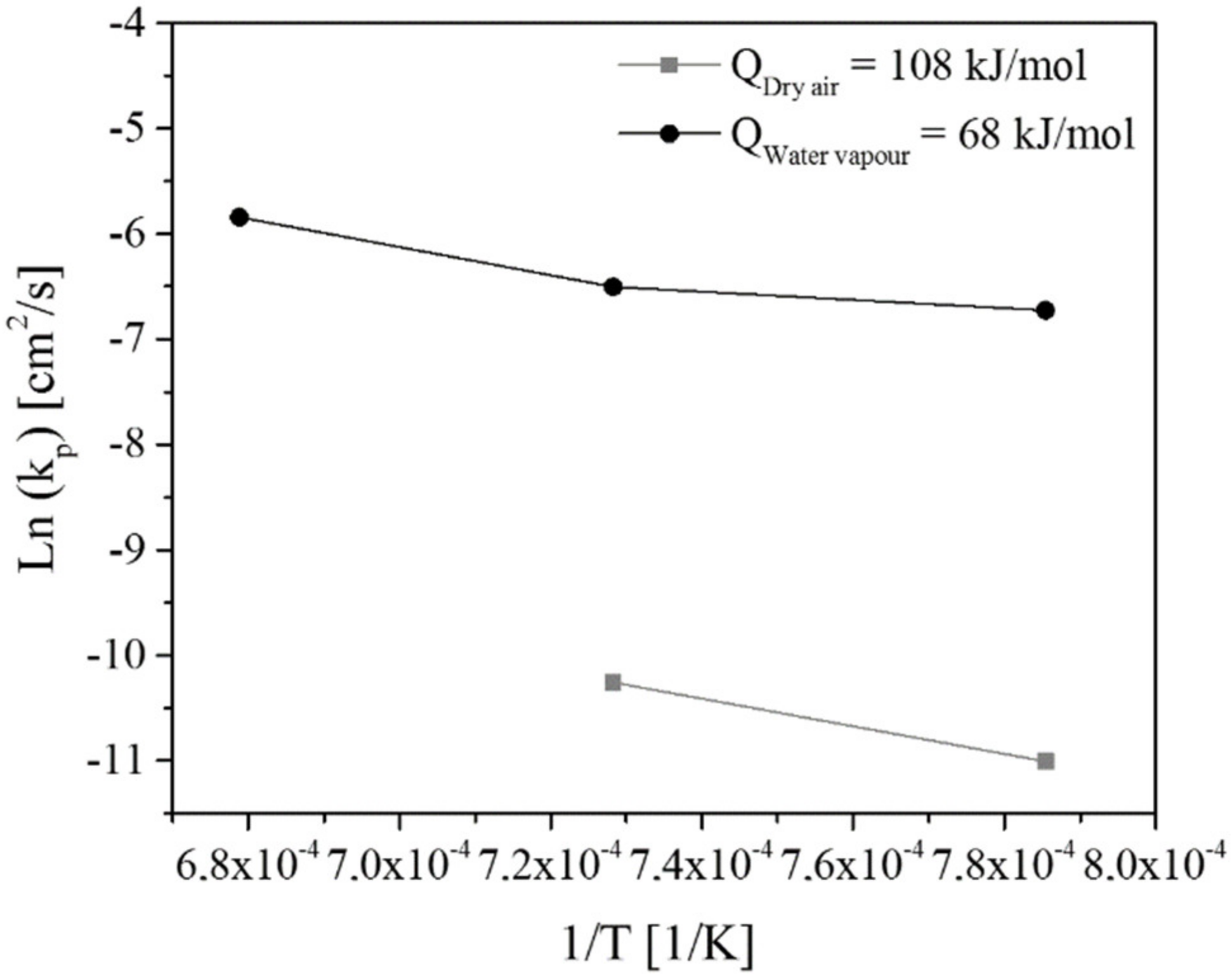
3.4.1. Oxidation-Rate Determination
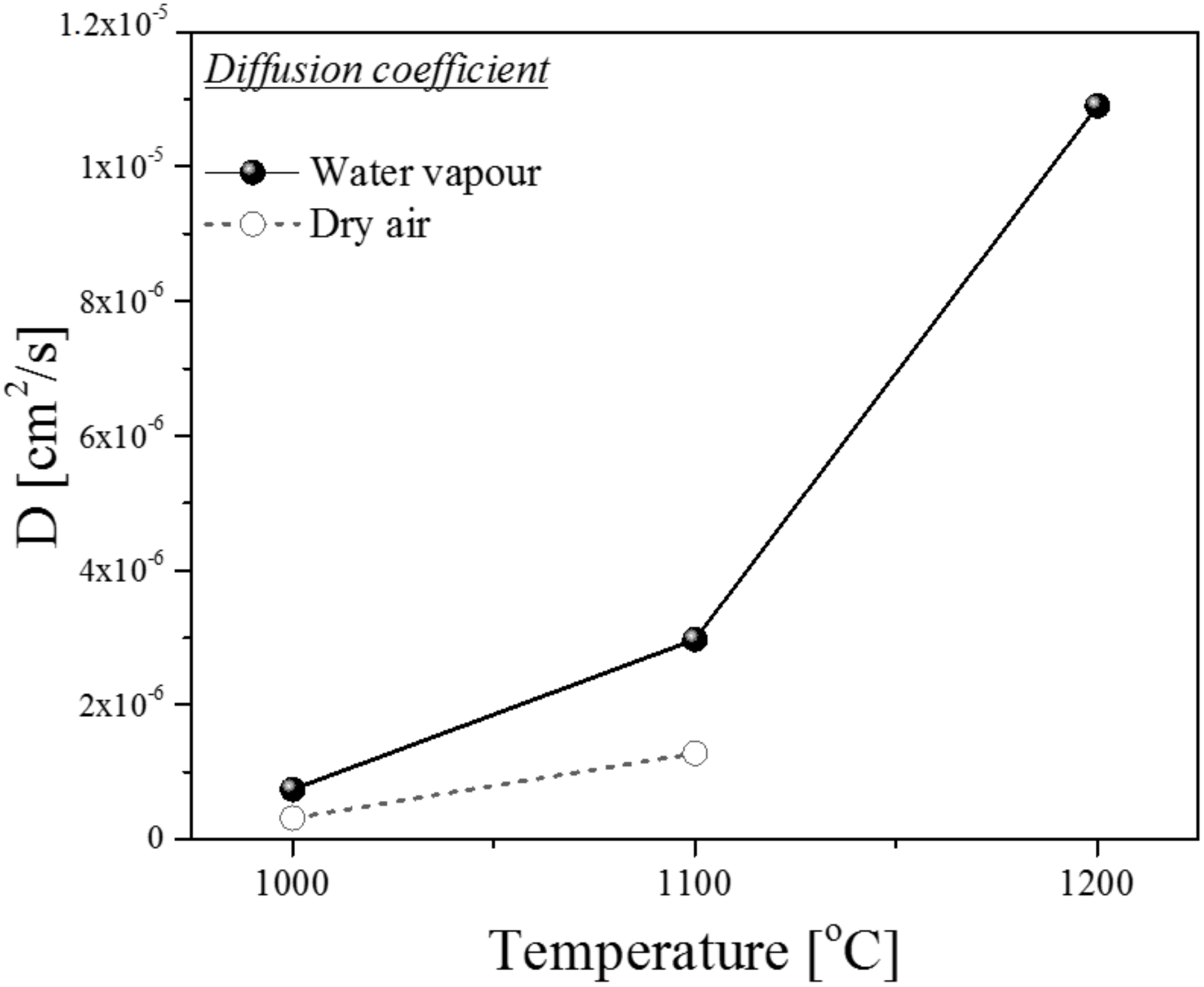
3.4.2. Diffusion Coefficient
4. Discussion
4.1. Microstructure and Phase Analysis
4.2. Micromechanical Analysis
4.3. Numerical Analysis
5. Conclusions
- Higher temperatures and longer holding times promoted thicker oxide scale formation.
- Defects such as pores, voids, and micro-cracks formed during oxide scale growth. The number and size of defects increased with temperature, and these were related to the transport of water molecules and gas formation.
- The micromechanics of the oxide scales showed the highest plasticity with lowest hardness and elastic modulus for the wüstite phase in comparison with magnetite and hematite.
- A study of kinetics revealed that the diffusion of oxygen through the scale was faster during oxidation under water vapor in comparison with the dry air atmosphere.
- Finally, this work demonstrated that a lower amount of wüstite can be achieved under the dry air condition in comparison with the water vapor condition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abuluwefa, H.; Guthrie, R.; Ajersch, F. The effect of oxygen concentration on the oxidation of low-carbon steel in the temperature range 1000 to 1250 °C. Oxid. Metals 1996, 46, 5–6. [Google Scholar] [CrossRef]
- Li, Y.; Sellars, C. Comparative investigations of interfacial heat transfer behaviour during hot forging and rolling of steel with oxide scale formation. J. Mater. Process. Technol. 1998, 282–286. [Google Scholar] [CrossRef]
- Marston, H.; Bolt, P.; Leprince, G.; Röder, M.; Klima, R.; Niska, J.; Jarl, M. Challenges in the modelling of scale formation and decarburisation of high carbon, special and general steels. Ironmak. Steelmak. 2004, 31, 57–65. [Google Scholar] [CrossRef]
- Sun, W.; Tieu, A.; Jiang, Z.; Lu, C. High temperature oxide scale characteristics of low carbon steel in hot rolling. J. Mater. Process. Technol. 2004, 155, 1307–1312. [Google Scholar] [CrossRef]
- Cao, G.-M.; Liu, X.-J.; Sun, B.; Liu, Z. Morphology of Oxide Scale and Oxidation Kinetics of Low Carbon Steel. J. Iron Steel Res. Int. 2014, 21, 335–341. [Google Scholar] [CrossRef]
- Chen, R.Y.; Yuen, W.Y.D. Oxide-Scale Structures Formed on Commercial Hot-Rolled Steel Strip and Their Formation Mechanisms. Oxid. Met. 2001, 56, 89–118. [Google Scholar] [CrossRef]
- Chen, R.; Yeun, W. Review of the High-Temperature Oxidation of Iron and Carbon Steels in Air or Oxygen. Oxid. Met. 2003, 59, 433–468. [Google Scholar] [CrossRef]
- Hu, X.-J.; Zhang, B.-M.; Chen, S.-H.; Fang, F.; Jiang, J. Oxide Scale Growth on High Carbon Steel at High Temperatures. J. Iron Steel Res. Int. 2013, 20, 47–52. [Google Scholar] [CrossRef]
- Liu, S.; Tang, D.; Wu, H.; Wang, L. Oxide scales characterization of micro-alloyed steel at high temperature. J. Mater. Process. Technol. 2013, 213, 1068–1075. [Google Scholar] [CrossRef]
- Suarez, L.; Houbaert, Y.; Eynde, X.V.; Colás, R. High temperature deformation of oxide scale. Corros. Sci. 2009, 51, 309–315. [Google Scholar] [CrossRef]
- Takada, J.; Adachi, M. Determination of diffusion coefficient of oxygen in α-iron from internal oxidation measurements in Fe-Si alloys. J. Mater. Sci. 1986, 21, 2133–2137. [Google Scholar] [CrossRef]
- Chabičovský, M.; Hnízdil, M.; Tseng, A.; Raudenský, M. Effects of oxide layer on Leidenfrost temperature during spray cooling of steel at high temperatures. Int. J. Heat Mass Transf. 2015, 88, 236–246. [Google Scholar] [CrossRef]
- Hu, P.; Ying, L.; Li, Y.; Liao, Z. Effect of oxide scale on temperature-dependent interfacial heat transfer in hot stamping process. J. Mater. Process. Technol. 2013, 213, 1475–1483. [Google Scholar] [CrossRef]
- Wendelstorf, R.; Spitzer, K.-H.; Wendelstorf, J. Effect of oxide layers on spray water cooling heat transfer at high surface temperatures. Int. J. Heat Mass Transf. 2008, 51, 4892–4901. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, S.-H.; Thomas, B.G.; Lee, G.-G.; Park, J.-M.; Sengupta, J. Measurement and Prediction of Lubrication, Powder Consumption, and Oscillation Mark Profiles in Ultra-low Carbon Steel Slabs. ISIJ Int. 2006, 46, 1635–1644. [Google Scholar] [CrossRef]
- Slowik, J.; Borchardt, G.; Köhler, C.; Jeschar, R.; Scholz, R. Influence of oxide scales on heat transfer in secondary cooling zones in the continuous casting process, part 2: Determination of material properties of oxide scales on steel under spray-water cooling conditions. Steel Res. 1990, 61, 302–311. [Google Scholar] [CrossRef]
- Wang, N.; Dong, J.-H.; Huang, W.-J.; Li, B.; Chen, M. Growth Rate, Microstructure and Phase Composition of Oxide Scales for Three Typical Steels in Simulated Continuous Casting Process. J. Iron Steel Res. Int. 2014, 21, 1065–1072. [Google Scholar] [CrossRef]
- Köhler, C.; Jeschar, R.; Scholz, R.; Slowik, J.; Borchardt, G. Influence of oxide scales on heat transfer in secondary cooling zones in the continuous casting process, part 1: Heat transfer through hot-oxidized steel surfaces cooled by spray-water. Steel Res. 1990, 61, 295–301. [Google Scholar] [CrossRef]
- Kofstad, P. High temperature corrosion. In Elsevier Applied Science; Crown House: Barking, UK, 1988. [Google Scholar]
- Landolt, D. Corrosion and Surface Chemistry of Metals, 1st ed.; Taylor and Francis Group, LLC: New York, NY, USA, 2007; ISBN 978-2-940222-11-7. [Google Scholar]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Baud, J.; Ferrier, A.; Manenc, J. The oxidation and decarburizing of Fe-C alloys in air and the influence of relative humidity. Oxid. Met. 1975, 9, 69–97. [Google Scholar] [CrossRef]
- Gesmundo, F.; Gleeson, B. Oxidation of multicomponent two-phase alloys. Oxid. Met. 1995, 44, 211–237. [Google Scholar] [CrossRef]
- Anusha, A.; Parameswaran, C.; Revathi, P.; Velmurugan, V. Numerical simulation of dry and wet oxidation of Silicon by TCAD Sprocess. In Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies, Chennai, India, 24–26 July 2013; pp. 513–516. [Google Scholar]
- Wang, N.; Wen, H.; Huang, W.; Dou, N.; Chen, M. Effect of Steel Composition on the Scale Layer Composition in Continuous Casting. In Proceedings of the 8th Pacific Rim International Congress on Advanced Materials and Processing, Waikoloa, HI, USA, 4–9 August 2013. [Google Scholar]
- Darken, L.S.; Gurry, R.W. The System Iron-Oxygen. I. The Wüstite Field and Related Equilibria. J. Am. Chem. Soc. 1945, 67, 1398–1412. [Google Scholar] [CrossRef]
- Janković, B.Ž.; Adnađević, B.; Mentus, S. The kinetic analysis of non-isothermal nickel oxide reduction in hydrogen atmosphere using the invariant kinetic parameters method. Thermochim. Acta 2007, 456, 48–55. [Google Scholar] [CrossRef]
- Ríos, L.M.M.; Muñoz, P.V.C.; Giraldo, J.M.C.; Gaviria, G.H.; González, A.F.R.; Mantilla, J.I.C. Modelos cinéticos de degradación térmica de polímeros: Una revisión. Rev. Ing. Univ. Medellín 2013, 12, 113–129. [Google Scholar] [CrossRef]
- Aboulkas, A.; El Harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Petzow, G.; Carle, V.; Harnisch, U. Techniques for Metallography, Ceramography, Plastography. In Metallographic Etching; ASM International: Geauga County, OH, USA, 1999. [Google Scholar]
- Walker, P.; Tarn, W.H. CRC Handbook of Metal Etchants; CRC Press LLC: Boca Raton, FL, USA, 1991. [Google Scholar]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Ye, S.; Endoh, T. Edge effect in the oxidation of three-dimensional nano-structured silicon. Mater. Sci. Semicond. Process. 2019, 93, 266–273. [Google Scholar] [CrossRef]
- Zambrano, O.; Coronado, J.; Rodriguez, S. Mechanical properties and phases determination of low carbon steel oxide scales formed at 1200 °C in air. Surf. Coat. Technol. 2015, 282, 155–162. [Google Scholar] [CrossRef]
- Mehrer, H. Diffusion in Solid Metals and Alloys. In Landolt-Börnstein Numerical Data and Functional Relationships in Science and Technology; Group III; Springer: Berlin Heidelberg; New York, NY, USA, 1990; p. 26. [Google Scholar]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R.V. Kinetics of Hematite to Wüstite by Hydrogen for Chemical Looping Combustion. Energy Fuels 2014, 28, 5406–5414. [Google Scholar] [CrossRef]
- Hidaka, Y.; Anraku, T.; Otsuka, N. Deformation of iron oxides upon tensile tests at 600–1250 °C. Oxid. Metals 2003, 59, 97–113. [Google Scholar] [CrossRef]
- Javurek, M.; Ladner, P.; Watzinger, J.; Wimmer, P.; Shan, G. Secondary cooling: Roll heat transfer during dry casting. In Proceedings of the METEC ESTAD, Düsseldorf, Germany, 19 June 2015. [Google Scholar]
- Raudensky, M.; Hnizdil, M.; Hwang, J.Y.; Lee, S.H.; Kim, S.Y. Influence of the water temperature on the cooling intensity of mist nozzles in continuous casting. Mater. Tehnol. 2012, 46, 3. [Google Scholar]
- Yin, H.; Yuen, W.Y.D.; Young, D.J. Effects of water vapour and oxygen partial pressures on low carbon steel oxidation in N2-H2-H2O mixtures. Mater. Corros. 2012, 63, 869–877. [Google Scholar] [CrossRef]
- Issartel, C.; Buscail, H.; Wang, Y.; Rolland, R.; Vilasi, M.; Aranda, L. Water Vapour Effect on Ferritic 4509 Steel Oxidation Between 800 and 1000 °C. Oxid. Met. 2011, 76, 127–147. [Google Scholar] [CrossRef]
- Buscail, H.; Rolland, R.; Perrier, S. Influence of water vapour on 316L oxidation at high temperature—In situ X-Ray diffraction. Ann. Chim. Sci. Matér. 2015, 39, 107–114. [Google Scholar] [CrossRef]









| T (°C) | Holding Time (min) | Average Thickness (µm) | |
|---|---|---|---|
| Dry Air | Water Vapor | ||
| 1000 | 10 | 128 | 400 |
| 30 | 217 | 566 | |
| 40 | 269 | - | |
| 60 | - | 627 | |
| 1100 | 10 | 204 | 236 |
| 30 | 393 | 618 | |
| 40 | 526 | - | |
| 60 | - | 992 | |
| 1200 | 10 | - | 587 |
| 30 | - | 1337 | |
| 40 | - | - | |
| 60 | - | 1945 | |
| T (°C) | Time (min) | ||||||
|---|---|---|---|---|---|---|---|
| Dry Air | Water Vapor | Dry Air | Water Vapor | Dry Air | Water Vapor | ||
| 1000 | 10 | - | 54.5 | - | 43.0 | - | 2.5 |
| 30 | - | 23.1 | - | 34.6 | - | 42.3 | |
| 40 | 13 | - | 38 | - | 48 | - | |
| 60 | - | 64.2 | - | 34.7 | - | 1 | |
| 1100 | 10 | - | 63.3 | - | 36.5 | - | 0.2 |
| 30 | - | 54.2 | - | 41.1 | - | 4.7 | |
| 40 | 0 | - | 45 | - | 55 | - | |
| 60 | - | 2.3 | - | 55.7 | - | 42.0 | |
| 1200 | 10 | - | 0.7 | - | 34.4 | - | 64.9 |
| 30 | - | 76.2 | - | 22.4 | - | 1.4 | |
| 60 | - | 76.3 | - | 22.8 | - | 0.9 | |
| Phase | Micromechanics of Oxides Formed Under Water Vapour | ||||
|---|---|---|---|---|---|
| HN (GPa) | E (GPa) | Plasticity Index | Plastic Work (GPa) | Elastic Work (GPa) | |
| Wüstite | 2.7 | 138 | 0.86 | 12.2 | 2.0 |
| Magnetite | 5.0 | 144 | 0.78 | 10.0 | 2.8 |
| Hematite | 5.5 | 151 | 0.75 | 9.6 | 3.1 |
| T (°C) | Oxidation Rate, | Q (Oxidation) | Diffusion Coefficient, D | Q (Diffusion) | ||||
|---|---|---|---|---|---|---|---|---|
| Dry Air | Water Vapour | Dry Air | Water Vapour | Dry Air | Water Vapour | Dry Air | Water Vapour | |
| 1000 | 1.66 × 10−5 | 1.20 × 10−3 | 108 | 68 | 3.20 × 10−7 | 7.43 × 10−7 | 202 | 209 |
| 1100 | 3.51 × 10−5 | 1.50 × 10−3 | 1.28 × 10−6 | 3.09 × 10−6 | ||||
| 1200 | - | 2.90 × 10−3 | - | - | 1.09 × 10−5 | - | ||
| Comparison of Parabolic Oxidation Rate Constants, kp (cm2/s) | ||||||
|---|---|---|---|---|---|---|
| Oxidation Under Dry Air | ||||||
| T (°C) | This Work | Liu et al. (I) | Chen et al. (II) | Abuluwefa et al. (III) | Jacek et al. (IV) | Reference |
| 900 | - | 2.44 × 10−8 | 3.51 × 10−8 | - | 4.71 × 10−8 | (I) [9] |
| 1000 | 1.66 × 10−5 | 3.78 × 10−7 | 1.11 × 10−7 | 4.90 × 10−7 | - | (II) [7] |
| 1100 | 3.51 × 10−5 | 7.21 × 10−7 | 2.98 × 10−7 | 1.25 × 10−6 | - | (III) [1] |
| 1200 | - | 1.39 × 10−6 | 6.98 × 10−7 | 2.60 × 10−6 | 1.09 × 10−8 | (IV) [16] |
| Oxidation Under Water Vapour | ||||||
| T (°C) | This Work | Issartel C. et al. (V) | Buscail et al. (VI) | Yin H. et al. (VII) | Jacek et al. (VIII) | Reference |
| 900 | - | 1.70 × 106 | 5.80 × 10−7 | 1.70 × 10−4 | 1.03 × 10−8 | (V) [41] |
| 1000 | 1.20 × 10−3 | 5.50 × 10−6 | 1.90 × 10−6 | 2.80 × 10−4 | 4.56 × 10−8 | (VI) [42] |
| 1100 | 1.50 × 10−3 | - | - | - | - | (VII) [40] |
| 1200 | 2.90 × 10−3 | - | - | - | - | (VIII) [16] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda Huitron, R.M.; Ramírez López, P.E.; Vuorinen, E.; Nazen Jalali, P.; Pelcastre, L.; Kärkkäinen, M. Scale Formation on HSLA Steel during Continuous Casting Part I: The Effect of Temperature–Time on Oxidation Kinetics. Metals 2020, 10, 1243. https://doi.org/10.3390/met10091243
Pineda Huitron RM, Ramírez López PE, Vuorinen E, Nazen Jalali P, Pelcastre L, Kärkkäinen M. Scale Formation on HSLA Steel during Continuous Casting Part I: The Effect of Temperature–Time on Oxidation Kinetics. Metals. 2020; 10(9):1243. https://doi.org/10.3390/met10091243
Chicago/Turabian StylePineda Huitron, Rosa Maria, Pavel Ernesto Ramírez López, Esa Vuorinen, Pooria Nazen Jalali, Leonardo Pelcastre, and Maija Kärkkäinen. 2020. "Scale Formation on HSLA Steel during Continuous Casting Part I: The Effect of Temperature–Time on Oxidation Kinetics" Metals 10, no. 9: 1243. https://doi.org/10.3390/met10091243
APA StylePineda Huitron, R. M., Ramírez López, P. E., Vuorinen, E., Nazen Jalali, P., Pelcastre, L., & Kärkkäinen, M. (2020). Scale Formation on HSLA Steel during Continuous Casting Part I: The Effect of Temperature–Time on Oxidation Kinetics. Metals, 10(9), 1243. https://doi.org/10.3390/met10091243