Effect of Sample Preparation Pressure on Transformation Law of Low-Valent Titanium Oxide in a Multi-Stage Reduction Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedures
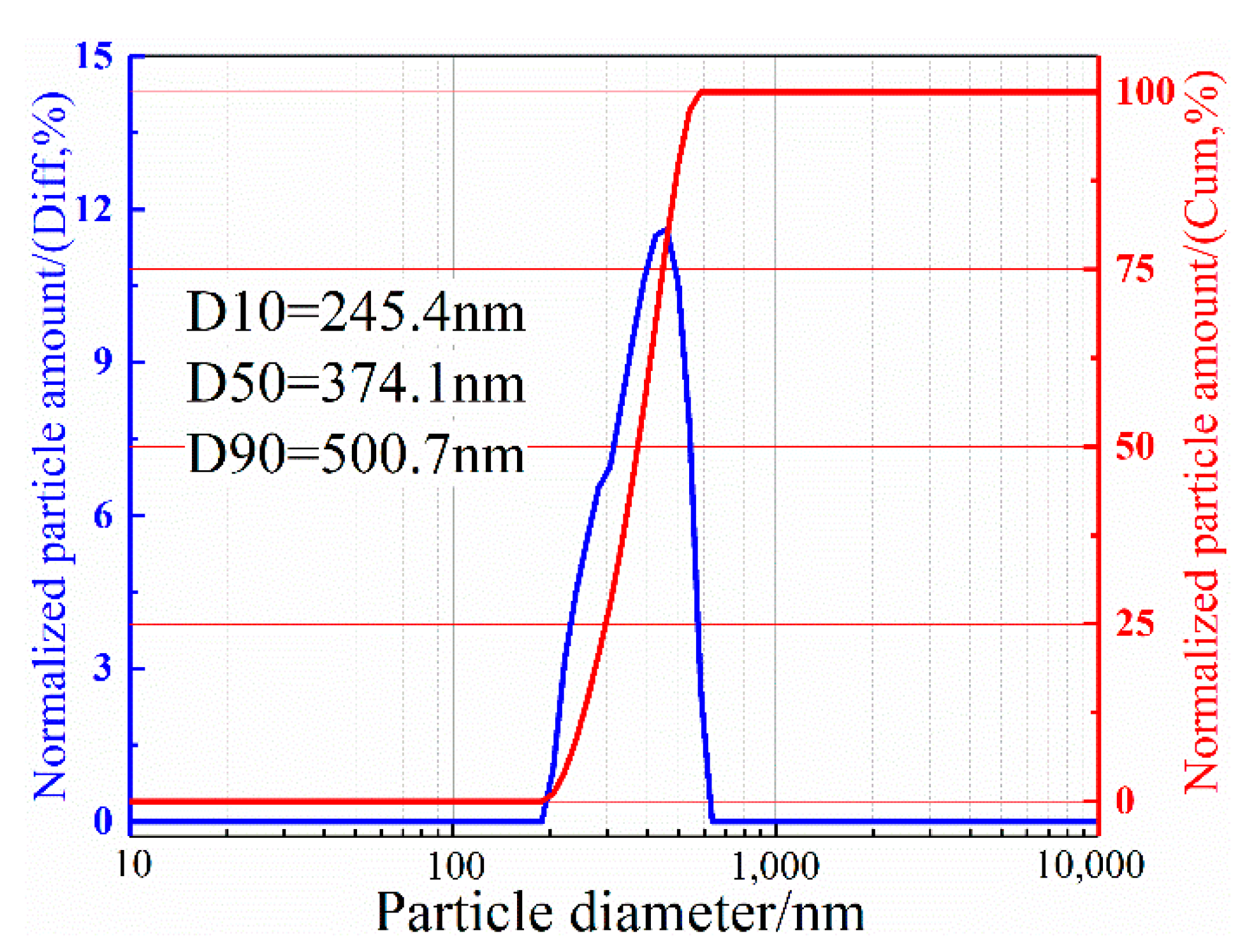
2.2. Analysis and Characterization
3. Results and Discussion
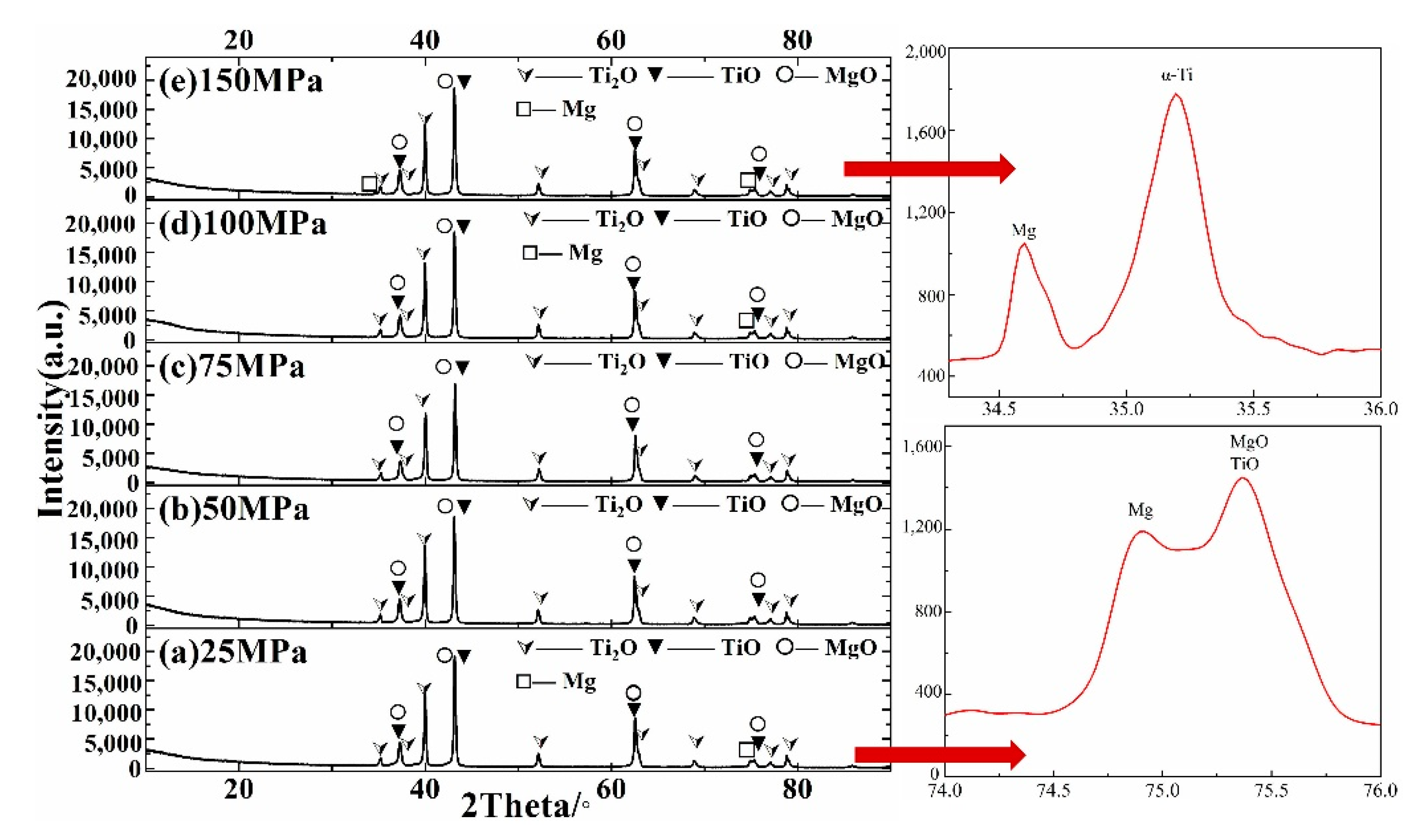
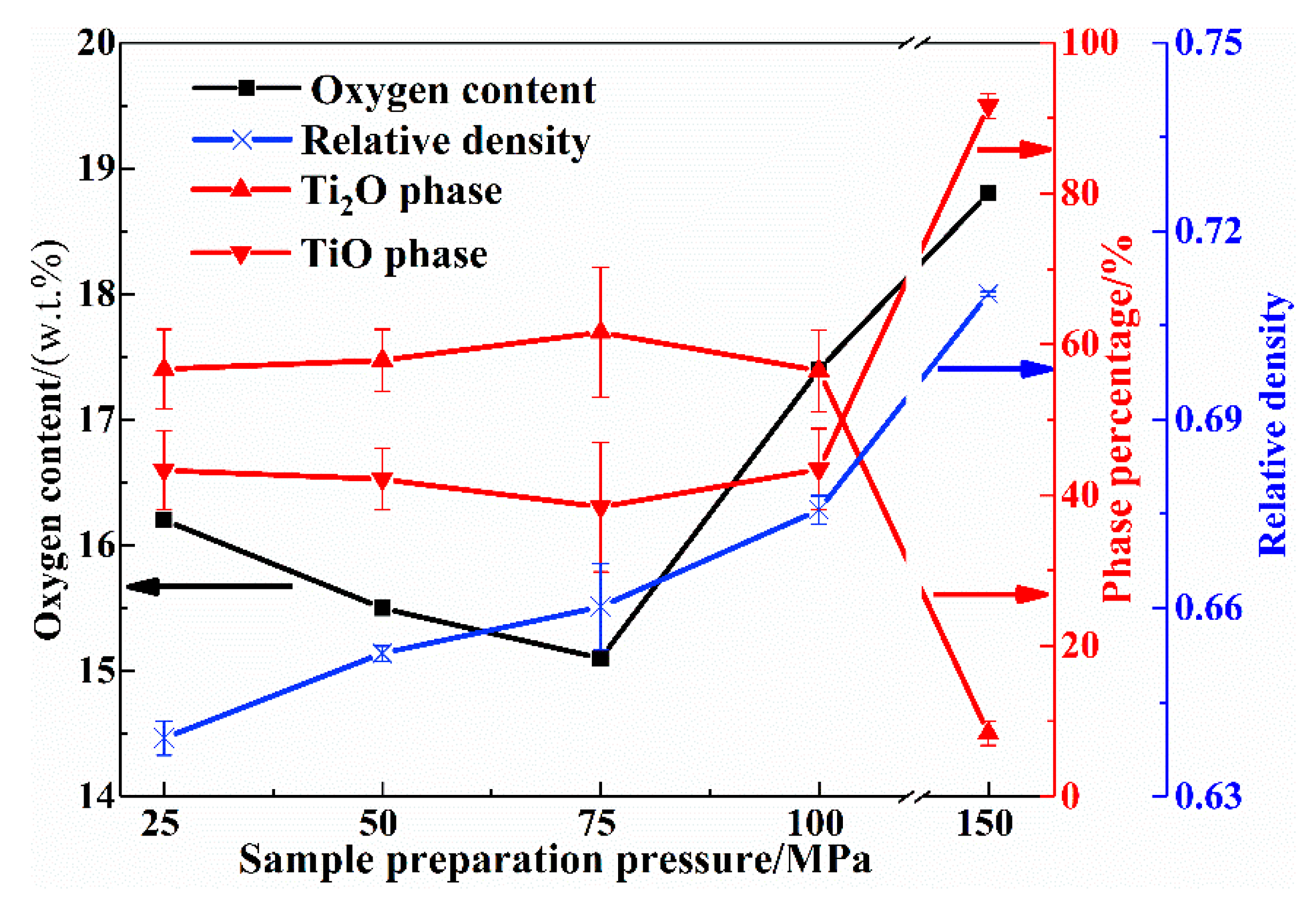
3.1. Phase Analyses
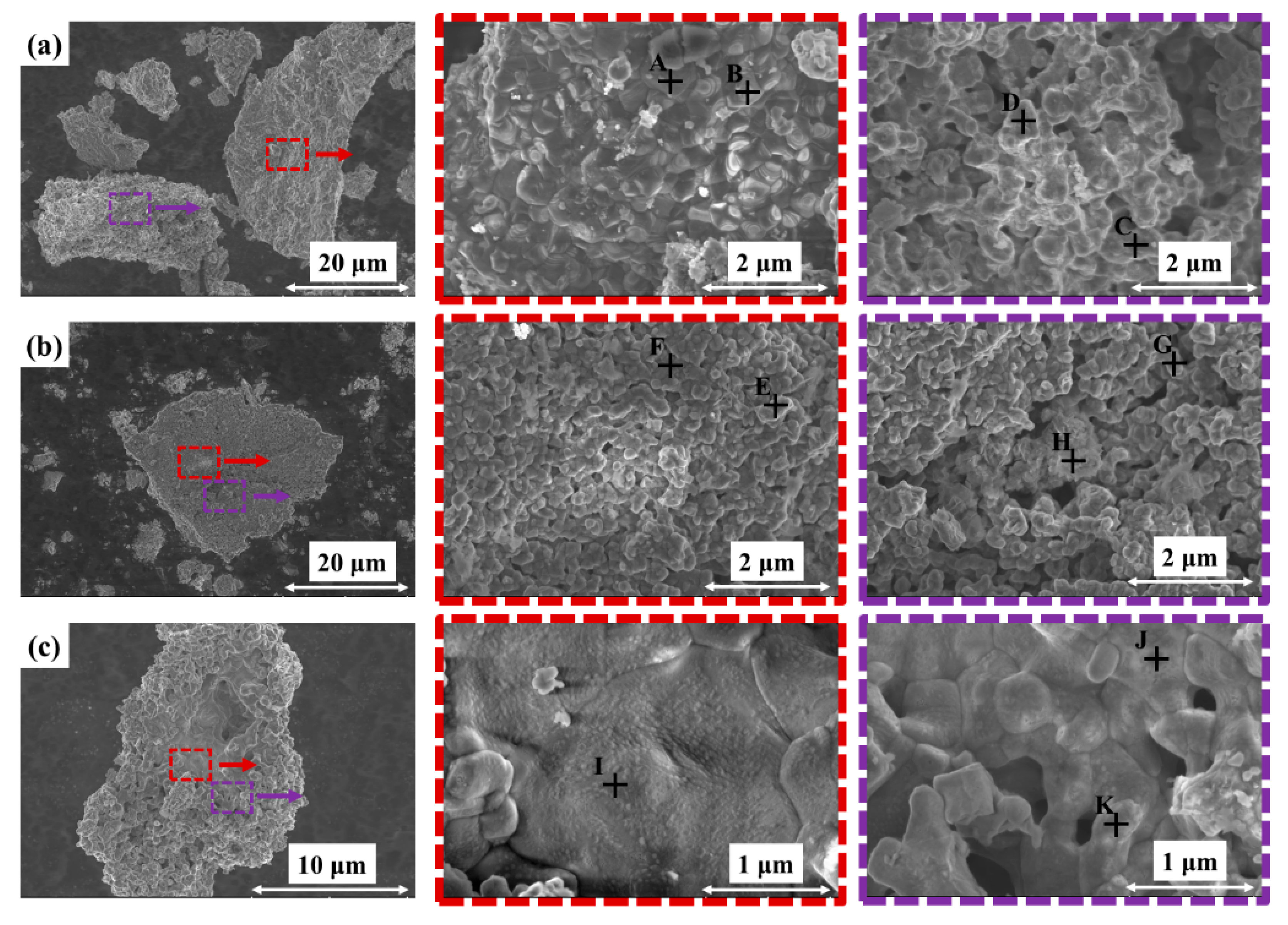
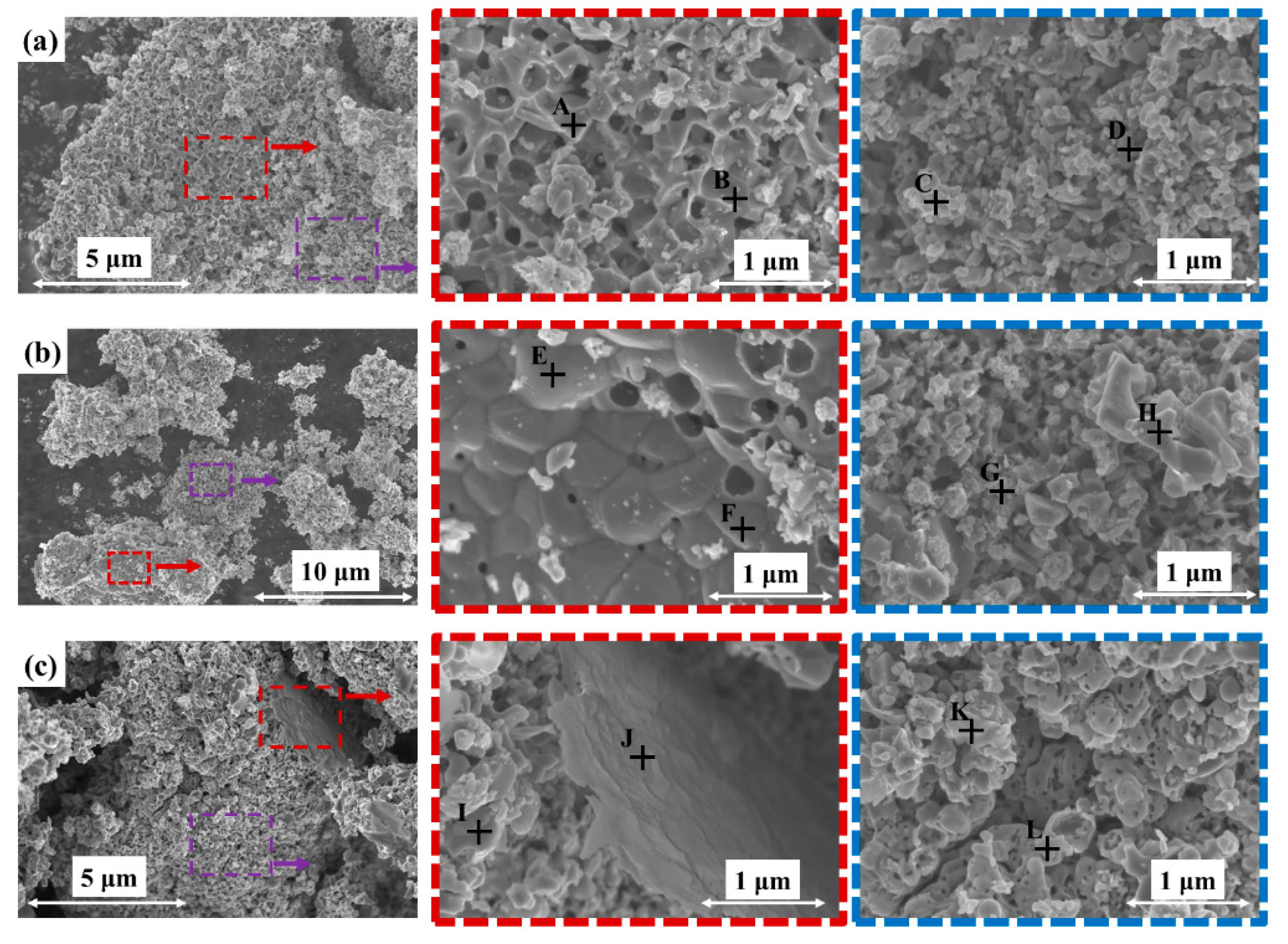
3.2. Micromorphology Analysis
3.3. Action Mechanism of Sample Preparation Pressure on Combustion Reaction
- (1)
- Initial stage: the material in the heating wire heating zone reaches the reaction initiation temperature, and the combustion reaction starts. Because the reaction mode is a solid–solid reaction, the reaction can only occur at the surface of TiO2 which is in direct contact with Mg;
- (2)
- Flow phases generation stage: The elevated temperature generated in the previous stage causes local excess Mg liquefaction and gasification (calculation result in Figure 7), and the generated fluid Mg diffuses through the pores of the particles under the action of surface tension and gravity (compact was ignited from the upper end);
- (3)
- Spreading stage: The thermal fluid generated in the previous stage diffuses to the surface of TiO2 which is not in direct contact with the solid Mg and undergoes forced heat exchange, and the combustion continues when the temperature reaches the combustion initiation temperature.
4. Conclusions
- (1)
- The generation of Mg thermal fluid is the key link of self-sustaining chemical reaction. When combustion is terminated, titanium exists in TiO and α-Ti phase, and constitutes a non-stoichiometric low-valent titanium oxide;
- (2)
- The titanium-containing phase exists in a solid state and simultaneous sintering and reduction reactions occur during the combustion reaction, and a skeleton form composed of low-valent titanium oxide particles is finally exhibited. Excessive sample preparation pressure causes the titanium-containing compound to change from an open cell structure to a closed cell structure;
- (3)
- The sample preparation pressure determines the proportion of pores reserved for Mg diffusion in the compacts and the contact area of the reactants, thereby affecting the partitioning behavior of Mg thermal fluid and the heat transfer effect during the combustion process. When the sample preparation pressure is 75 MPa (relative density 0.66 ± 0.01), the combustion effect is optimal, and a low-valent titanium oxide precursor with an oxygen content of 15.1% can be obtained. After the deep reduction of low-valent titanium oxide precursor, a titanium powder product with oxygen content of 0.27% can be obtained.
Author Contributions
Funding
Conflicts of Interest
References
- Kroll, W.J. The Production of Ductile Titanium. J. Electrochem. Soc. 1940, 78, 34–47. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Panton, B.; Zeng, Z.; Andrei, C.M.; Zhou, Y.; Miranda, R.M.; Fernandes, F.M.B. Laser joining of NiTi to Ti6Al4V using a Niobium interlayer. Acta Mater. 2016, 105, 9–15. [Google Scholar] [CrossRef]
- Callegari, B.; Oliveira, J.P.; Aristizabal, K.; Coelho, R.S.; Brito, P.P.; Wu, L.; Schell, N.; Soldera, F.A.; Mücklich, F.; Pinto, H.C. In-situ synchrotron radiation study of the aging response of Ti-6Al-4V alloy with different starting microstructures. Mater. Charact. 2020, 165, 110400. [Google Scholar] [CrossRef]
- Callegari, B.; Oliveira, J.P.; Coelho, R.S. New insights into the microstructural evolution of Ti-5Al-5Mo-5V-3Cr alloy during hot working. Mater. Charact. 2020, 162, 110180. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J.; Farthing, T.W. Direct Electrochemical Reduction of Titanium Dioxide to Titanium in Molten Calcium Chloride. Nature 2000, 407, 361–364. [Google Scholar] [CrossRef]
- Ono, K.; Suzuki, R.O. A New Concept for Producing Ti Sponge: Calciothermie Reduction. JOM 2002, 54, 59–61. [Google Scholar] [CrossRef]
- Abiko, T.; Toru, H. Okabe. Production of titanium powder directly from TiO2 in CaCl2 through an electronically mediated reaction (EMR). J. Phys. Chem. Solids 2005, 66, 410–413. [Google Scholar]
- Zhao, Z.; Lu, X.; Ding, W. A new technology using som to produce titanium sponge. Shanghai Met. 2005, 27, 40–43. [Google Scholar]
- Pal, U.B.; Powell, A.C. The use of solid-oxide-membrane technology for electrometallurgy. JOM 2007, 59, 44–49. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J.; Farthing, T.W. Cathodic deoxygenation of the alpha case on titanium and alloys in molten calcium chloride. Metall. Mater. Trans. B 2001, 32, 1041–1052. [Google Scholar] [CrossRef]
- Chen, G.Z.; Fray, D.J. Electro-Deoxidation of Metal Oxides. In Proceedings of the Light Metals (the 130th TMS Annual Meeting), New Orleans, LA, USA, 11–15 February 2001; pp. 1147–1151. [Google Scholar]
- Fray, D.J. Emerging molten salt technologies for metals production. JOM 2001, 53, 27–31. [Google Scholar] [CrossRef]
- Zhu, H.; Jiao, S.; Gu, X. A Production Method of Pure Titanium by Electrolysis from Titanium Oxide/Titanium Carbide Soluble Anode. CN 200510011684.6, 28 December 2005. [Google Scholar]
- Jiao, S.; Zhu, H. Electrolysis of Ti-C-O solid solution prepared by TiC and TiO2. J. Alloy. Compd. 2007, 438, 243–246. [Google Scholar] [CrossRef]
- Choi, K.; Choi, H.; Sohn, I. Understanding the Magnesiothermic Reduction Mechanism of TiO2 to Produce Ti. Metall. Mater. Trans. B 2017, 48, 922–932. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Z.Z.; Xia, Y.; Sun, P.; Van Devener, B.; Free, M.; Lefler, H.; Zheng, S. Hydrogen assisted magnesiothermic reduction of TiO2. Chem. Eng. J. 2017, 308, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Fang, Z.Z.; Zhang, Y.; Lefler, H.; Zhang, T.; Sun, P.; Huang, Z. Hydrogen assisted magnesiothermic reduction (HAMR) of commercial TiO2 to produce titanium powder with controlled morphology and particle size. Mater. Trans. 2017, 58, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Okabe, T.H.; Oda, T.; Mitsuda, Y. Titanium powder production by preform reduction process (PRP). J. Alloy. Compd. 2004, 364, 156–163. [Google Scholar] [CrossRef]
- Wan, H.L.; Xu, B.Q.; Dai, Y.N.; Yang, B.; Liu, D.C.; Sen, W. Preparation of titanium powders by calciothermic reduction of titanium dioxide. J. Cent. South Univ. 2012, 19, 2434–2439. [Google Scholar] [CrossRef]
- Won, C.W.; Nersisyan, H.H.; Won, H.I. Titanium powder prepared by a rapid exothermic reaction. Chem. Eng. J. 2010, 157, 270–275. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Won, H.I.; Won, C.W.; Jo, A.; Kim, J.H. Direct magnesiothermic reduction of titanium dioxide to titanium powder through combustion synthesis. Chem. Eng. J. 2014, 235, 67–74. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Won, H.I.; Won, C.W.; Kim, J.B.; Park, S.M.; Kim, J.H. Combustion synthesis of porous titanium microspheres. Mater. Chem. Phys. 2013, 141, 283–288. [Google Scholar] [CrossRef]
- Fan, S.G.; Dou, Z.H.; Zhang, T.A.; Liu, Y.; Niu, L.P. Deoxidation Mechanism in Reduced Titanium Powder Prepared by Multistage Deep Reduction of TiO2. Metall. Mater. Trans. B 2019, 50, 282–290. [Google Scholar] [CrossRef]
- Munir, Z.A.; Holt, J.B. Combustion and Plasma Synthesis of High-Temperature Materials, 3rd ed.; Material Neurk VCH Publ.: New York, NY, USA, 1990; pp. 51–53. [Google Scholar]
- Mossino, P. Some aspects in self-propagating high-temperature synthesis. Ceram. Int. 2004, 30, 311–332. [Google Scholar] [CrossRef]
- Yeh, C.L.; Yeh, C.C. Preparation of CoAl intermetallic compound by combustion synthesis in self-propagating mode. J. Alloy. Compd. 2005, 388, 249. [Google Scholar] [CrossRef]
- Yeh, C.L.; Yeh, C.C. Preparation of CoTi intermetallics by self-propagating combustion synthesis. J. Alloy. Compd. 2005, 396, 232. [Google Scholar] [CrossRef]
- Gennari, S.; Tamburini, U.A.; Maglia, F.; Spinolo, G.; Munir, Z.A. A new approach to the modeling of SHS reactions: Combustion synthesis of transition metal aluminides. Acta Mater. 2006, 54, 2343–2351. [Google Scholar] [CrossRef]
- Cui, H.; Liu, W.; Cao, L.; Song, Q.; Tian, J.; Teng, F.; Wang, J. Effects of B4C particle size on pore structures of porous TiB2–TiC by reaction synthesis. J. Eur. Ceram. Soc. 2015, 35, 3381–3388. [Google Scholar] [CrossRef]
- Makino, A. Fundamental aspects of the heterogeneous flame in the self-propagating high-temperature synthesis (SHS) process. Prog. Energy Combust. 2001, 27, 71–74. [Google Scholar] [CrossRef]
- Yeh, C.L.; Su, S.H.; Chang, H.Y. Effects of TiC addition on combustion synthesis of NiAl in SHS mode. J. Alloy. Compd. 2005, 398, 90–93. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Liu, Y.; Guo, Z.; Tosto, S. Effect of process parameter on the combustion temperature of laser-induced self-propagating high-temperature synthesized Al/TiC composites. J. Mater. Process. Technol. 2009, 209, 2564–2569. [Google Scholar] [CrossRef]
- Morsi, K.; Shinde, S.; Olevsky, E.A. Effect of nickel particle size on the compaction behavior of rotator mixed and mechanically alloyed nickel and aluminum powders. Mater. Sci. Eng. A-Struct. 2006, 426, 283–288. [Google Scholar] [CrossRef]
- Yeo, S.C.; Jeon, S.W.; Lee, J.E.; Singh, V.; Kim, S.H. Combustion characteristics of molybdenum–silicon mixtures. Ceram. Int. 2015, 41, 1711–1723. [Google Scholar] [CrossRef]
- Adeli, M.; Seyedein, S.H.; Aboutalebi, M.R.; Kobashi, M.; Kanetake, N. A study on the combustion synthesis of titanium aluminide in the self-propagating mode. J. Alloy. Compd. 2010, 497, 100–104. [Google Scholar] [CrossRef]
- Biswas, A. Porous NiTi by thermal explosion mode of SHS: Processing, mechanism and generation of single phase microstructure. Acta Mater. 2005, 53, 1415–1425. [Google Scholar] [CrossRef]
- Borovinskaya, I.P.; Merzhanov, A.G.; Novikov, N.P.; Filonenko, A.K. Gasless combustion of mixtures of powdered transition metals with boron. Combust. Explos. Shock 1974, 10, 2–10. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Zhao, R.Y.; Liang, Y.H.; Zhan, L.; Jiang, Q.C. Effects of C particle size on the ignition and combustion characteristics of the SHS reaction in the 20 wt.% Ni-Ti-C system. J. Alloy. Compd. 2008, 460, 276–282. [Google Scholar] [CrossRef]
- Zou, B.; Shen, P.; Jiang, Q. Dependence of the SHS reaction behavior and product on B4C particle size in Al–Ti–B4C and Al–TiO2–B4C systems. Mater. Res. Bull. 2009, 44, 499–504. [Google Scholar] [CrossRef]
- Yang, Y.F.; Wang, H.Y.; Wang, J.G. Effect of C particle size on the mechanism of self-propagation high-temperature synthesis in the Ni–Ti–C system. J. Alloy. Compd. 2011, 509, 7060–7065. [Google Scholar] [CrossRef]
- Sharifitabar, M.; Sabzevar, M.H. Effects of Fe additions on self-propagating high temperature synthesis characteristics of TiO2–Al–C system. Int. J. Refract. Met. Hard Mater. 2014, 47, 93–101. [Google Scholar] [CrossRef]
- Xiao, G.; Fan, Q.; Gu, M.; Jin, Z. Microstructural evolution during the combustion synthesis of TiC–Al cermet with larger metallic particles. Mater. Sci. Eng. A-Struct. 2006, 425, 318–325. [Google Scholar] [CrossRef]
- Liang, Y.; Han, Z.; Li, X. Study on the reaction mechanism of self-propagating high-temperature synthesis of TiC in the Cu–Ti–C system. Mater. Chem. Phys. 2012, 137, 200–206. [Google Scholar] [CrossRef]
- Zhang, T.A.; Dou, Z.H. Research growth mechanism of TiB2 powder Prepared by SHS-metallurgy. J. Inorg. Mater. 2006, 21, 583–590. [Google Scholar]
- Zarrinpour, H.; Firoozi, S.; Milani, V. Ignition and chemical mechanisms of volume combustion synthesis of titanium diboride. Ceram. Int. 2016, 42, 11217–11223. [Google Scholar] [CrossRef]
- Weimin, W.; Zhengyi, F.; Hao, W.; Runzhang, Y. Chemistry reaction processes during combustion synthesis of B2O3–TiO2–Mg system. J. Mater. Process. Technol. 2002, 128, 162–168. [Google Scholar] [CrossRef]



| No. | Sample Preparation Pressure/MPa | Mole Ratio of TiO2/Mg |
|---|---|---|
| 1 | 25 | 1:2 |
| 2 | 50 | 1:2 |
| 3 | 75 | 1:2 |
| 4 | 100 | 1:2 |
| 5 | 150 | 1:2 |
| No. | Ti/(wt.%)O/(wt.%) | Mg/(wt.%) | Atomic Ratio of Ti/O/Mg | |
|---|---|---|---|---|
| A | 38.31 | 26.63 | 35.06 | 1:2.1:1.8 |
| B | 34.40 | 30.07 | 35.53 | 1:2.6:2.0 |
| C | 49.16 | 18.84 | 32.00 | 1:1.2:1.3 |
| D | 49.71 | 20.87 | 29.42 | 1:1.3:1.2 |
| E | 34.80 | 29.81 | 35.39 | 1:2.6:2.0 |
| F | 39.86 | 22.66 | 37.48 | 1:1.7:1.9 |
| G | 42.74 | 29.07 | 28.19 | 1:2.0:1.3 |
| H | 53.13 | 29.71 | 17.16 | 1:1.7:0.6 |
| I | 47.88 | 20.05 | 32.08 | 1:1.3:1.3 |
| J | 50.85 | 21.47 | 27.69 | 1:1.3:1.1 |
| K | 38.27 | 31.33 | 30.40 | 1:2.5:1.6 |
| No. | Ti/(wt.%) | O/(wt.%) | Mg/(wt.%) |
|---|---|---|---|
| A | 88.31 | 11.22 | 0.47 |
| B | 86.20 | 13.80 | 0.00 |
| C | 87.39 | 12.07 | 0.54 |
| D | 85.90 | 13.75 | 0.35 |
| E | 77.32 | 21.99 | 0.69 |
| F | 74.14 | 25.22 | 0.64 |
| G | 89.53 | 9.58 | 0.89 |
| H | 73.70 | 25.55 | 0.75 |
| I | 71.28 | 27.61 | 0.71 |
| J | 81.28 | 15.43 | 3.29 |
| K | 84.97 | 14.42 | 0.71 |
| L | 88.13 | 11.61 | 0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Dou, Z.; Zhang, T.; Yan, J.-s.; Niu, L.-p. Effect of Sample Preparation Pressure on Transformation Law of Low-Valent Titanium Oxide in a Multi-Stage Reduction Process. Metals 2020, 10, 1259. https://doi.org/10.3390/met10091259
Fan S, Dou Z, Zhang T, Yan J-s, Niu L-p. Effect of Sample Preparation Pressure on Transformation Law of Low-Valent Titanium Oxide in a Multi-Stage Reduction Process. Metals. 2020; 10(9):1259. https://doi.org/10.3390/met10091259
Chicago/Turabian StyleFan, Shigang, Zhihe Dou, Ting’an Zhang, Ji-sen Yan, and Li-ping Niu. 2020. "Effect of Sample Preparation Pressure on Transformation Law of Low-Valent Titanium Oxide in a Multi-Stage Reduction Process" Metals 10, no. 9: 1259. https://doi.org/10.3390/met10091259
APA StyleFan, S., Dou, Z., Zhang, T., Yan, J.-s., & Niu, L.-p. (2020). Effect of Sample Preparation Pressure on Transformation Law of Low-Valent Titanium Oxide in a Multi-Stage Reduction Process. Metals, 10(9), 1259. https://doi.org/10.3390/met10091259




