Corrosion Behavior of As-Cast Ti–10Mo–6Zr–4Sn–3Nb and Ti–6Al–4V in Hank’s Solution: A Comparison Investigation
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
2.2. Microstructural Characterizations
2.3. Electrochemical Measurements
3. Results
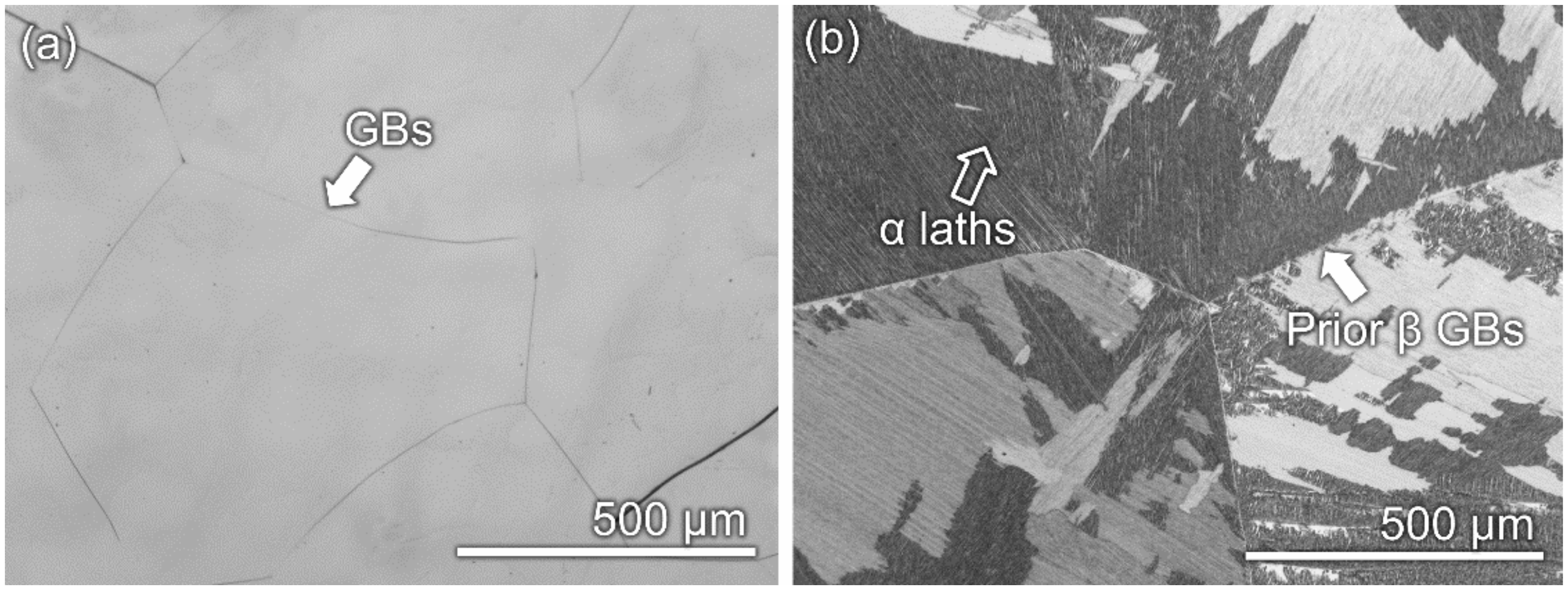
3.1. Microstructural Features
3.2. Open Circuit Potential
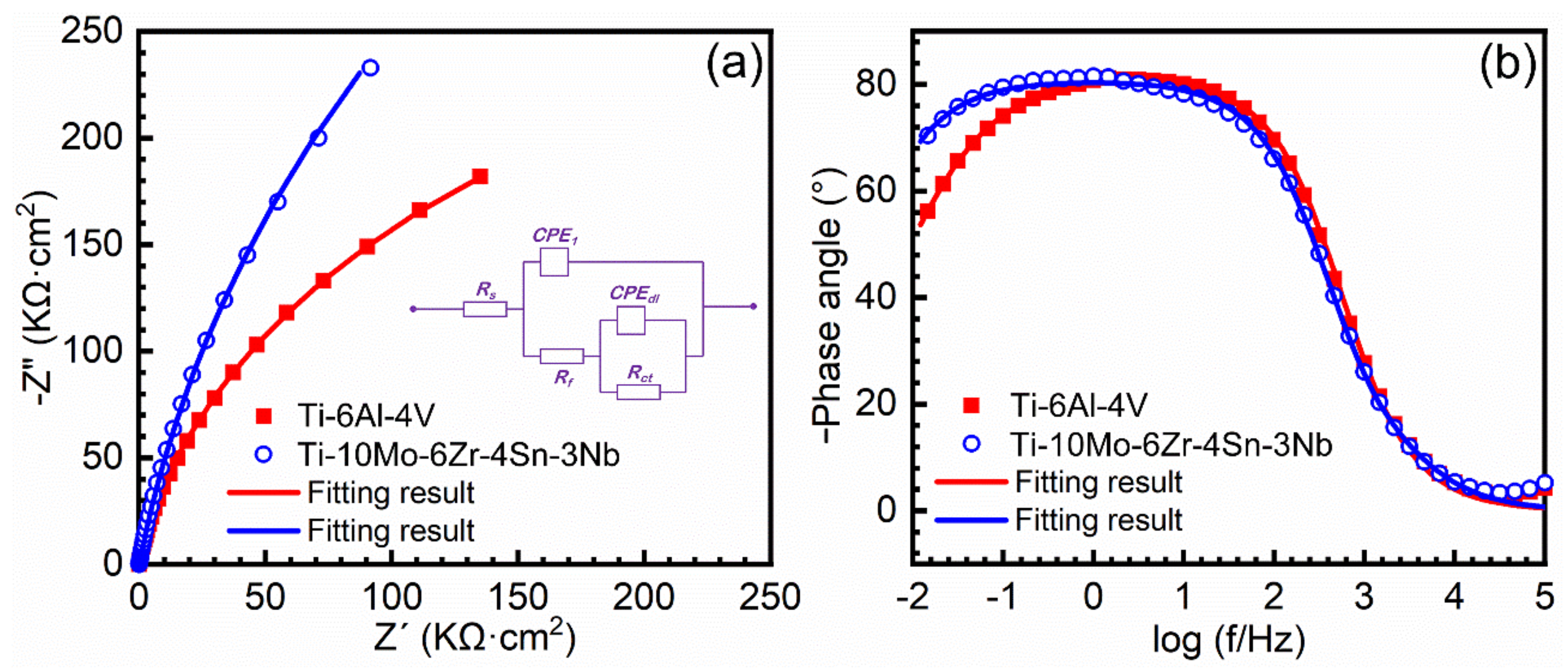
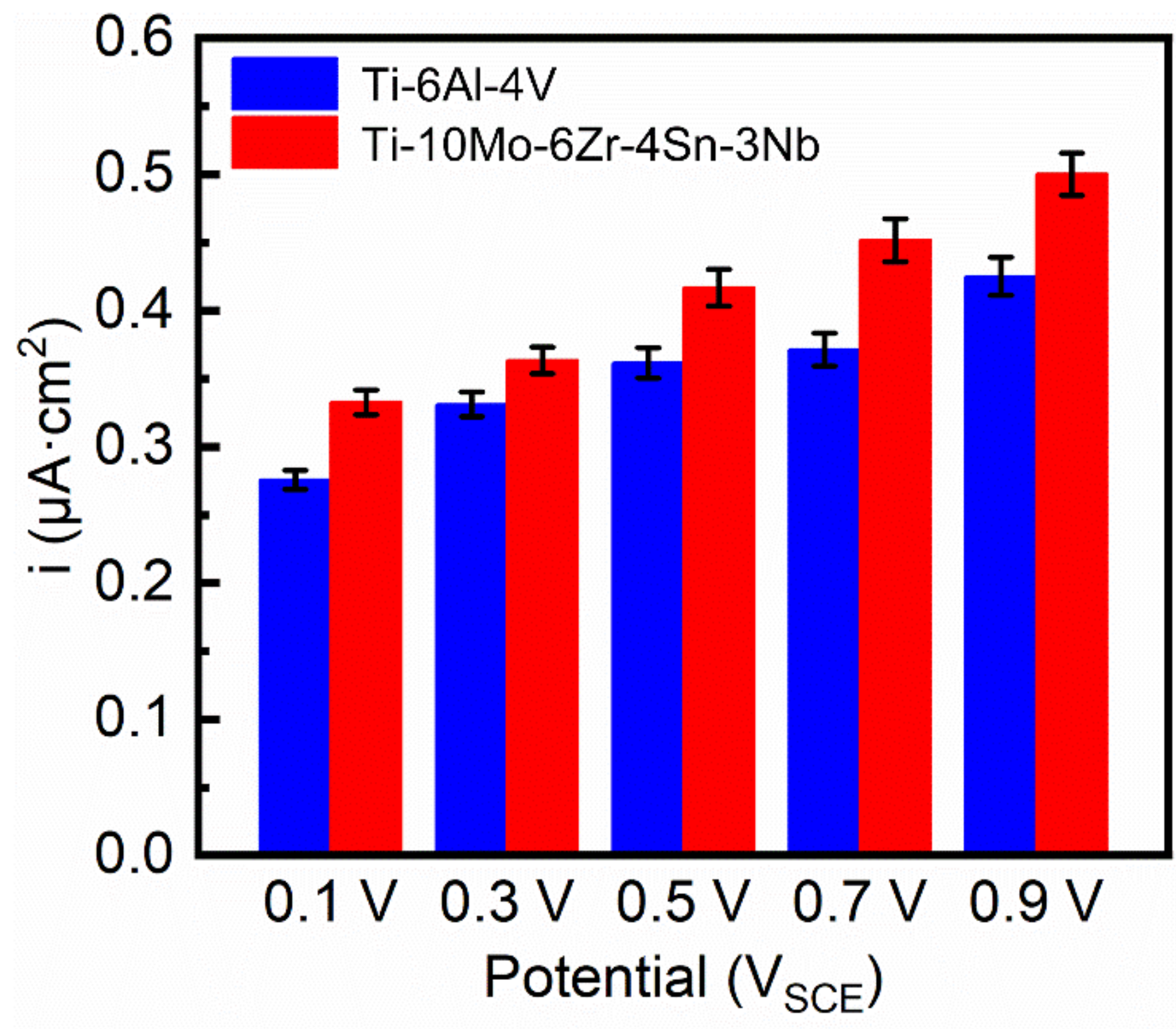
3.3. Potentiodynamic Polarization Test and Electrochemical Impedance Spectroscopy
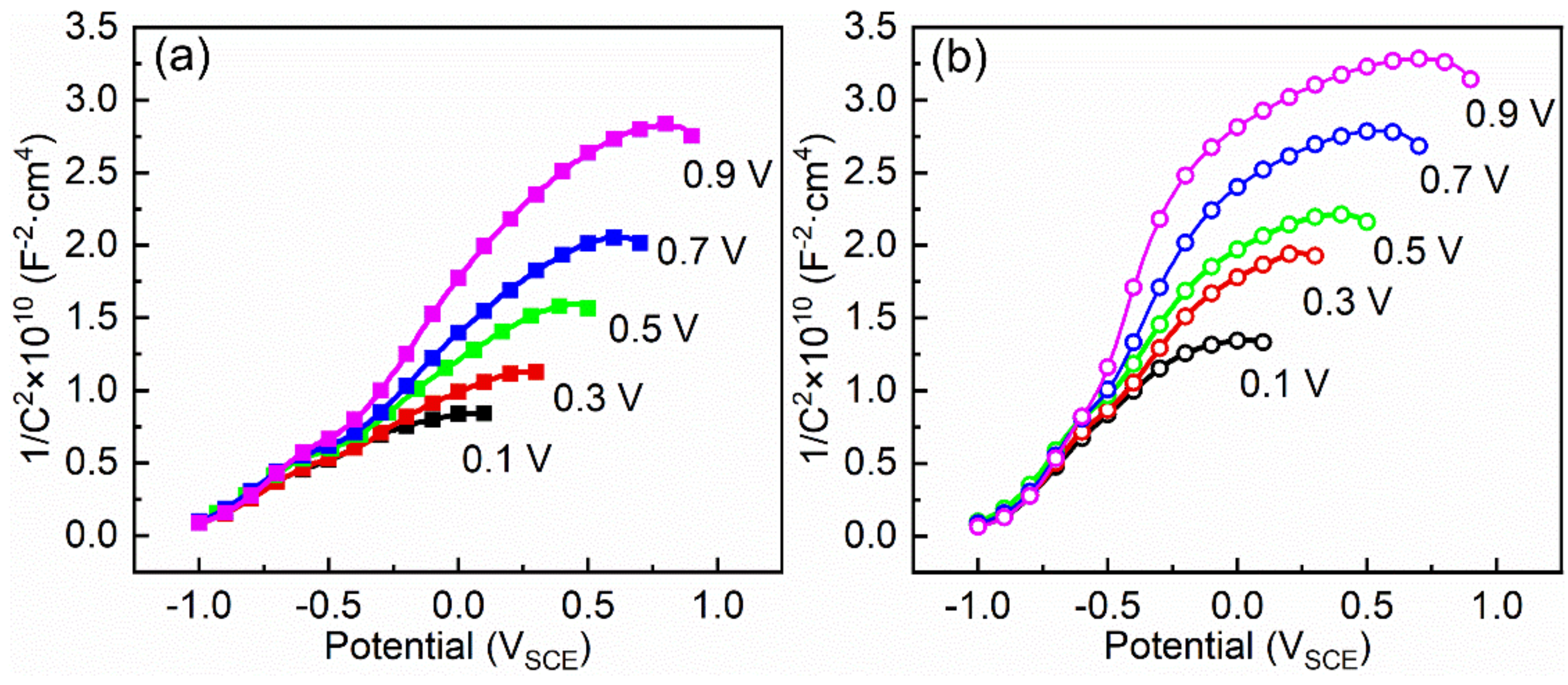
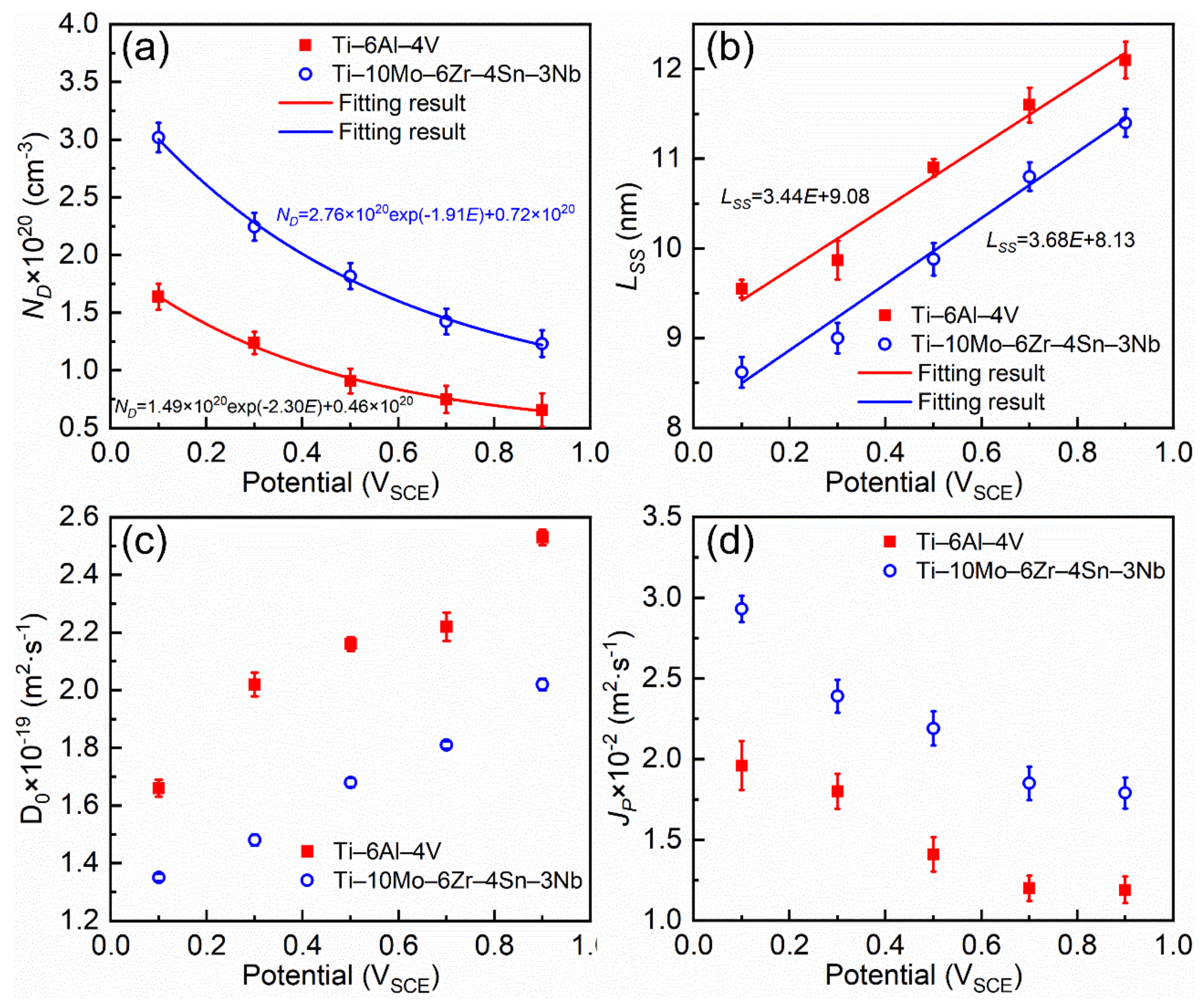
3.4. Mott–Schottky Analysis
4. Discussion
5. Conclusions
- As-cast Ti–10Mo–6Zr–4Sn–3Nb has a monolithic microstructure composed of β-phase, whilst as-cast Ti–6Al–4V has dual α- and β-phases. The volume fractions of α- and β-phases in Ti–6Al–4V are about 5% and 95%, respectively.
- Ti–10Mo–6Zr–4Sn–3Nb has a higher open circuit potential (−0.3869 VSCE) than Ti–6Al–4V (−0.4923 VSCE) after 1800 s immersion. Meanwhile, Ti–10Mo–6Zr–4Sn–3Nb also has a higher corrosion potential (−0.430 ± 0.012 VSCE) and a lower corrosion current density (0.007 ± 0.002 μA·cm−2) than Ti–6Al–4V (−0.504 ± 0.044 VSCE and 0.020 ± 0.008 μA·cm−2). Electrochemical impedance spectroscopy results indicate that the charge transfer resistance of Ti–10Mo–6Zr–4Sn–3Nb (1.3297 ± 0.0858 MΩ cm2) is higher than that of Ti–6Al–4V (0.7367 ± 0.0082 MΩ cm2). Such results indicate the better corrosion resistance of Ti–10Mo–6Zr–4Sn–3Nb in Hank’s solution at 37 °C as compared to Ti–6Al–4V, which is beneficial for Ti–10Mo–6Zr–4Sn–3Nb to be used as biomaterials.
- Ti–10Mo–6Zr–4Sn–3Nb shows higher passivation current density in both potentiodynamic polarization and potentiostatic polarization tests compared with Ti–6Al–4V. By Mott–Schottky examination, the result shows that more donors (mainly referring to oxygen vacancies) are presented in the passive film formed on Ti–10Mo–6Zr–4Sn–3Nb under the effect of the electric field. This is because more alloying elements are contained in Ti–10Mo–6Zr–4Sn–3Nb and such alloying elements and oxygen have a lower difference in the electronegativities compared with that between Ti and O. Therefore, more oxygen vacancies are produced in the passive film on Ti–10Mo–6Zr–4Sn–3Nb, resulting in a higher flux of oxygen vacancy, which finally leads to the higher passivation current density of Ti–10Mo–6Zr–4Sn–3Nb. This finding illustrates that the passive film on Ti–10Mo–6Zr–4Sn–3Nb is less protective compared with that on Ti–6Al–4V when applying an anodic potential in their passivation range.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef] [Green Version]
- Rabadia, C.D.; Liu, Y.J.; Zhao, C.H.; Wang, J.C.; Jawed, S.F.; Wang, L.Q.; Chen, L.Y.; Sun, H.; Zhang, L.C. Improved trade-off between strength and plasticity in titanium based metastable beta type Ti-Zr-Fe-Sn alloys. Mater. Sci. Eng. A 2019, 766, 138340. [Google Scholar] [CrossRef]
- Rabadia, C.D.; Liu, Y.J.; Chen, L.Y.; Jawed, S.F.; Wang, L.Q.; Sun, H.; Zhang, L.C. Deformation and strength characteristics of Laves phases in titanium alloys. Mater. Des. 2019, 179, 107891. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous Titanium Implants: A Review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Kang, L.; Yang, C. A Review on High-Strength Titanium Alloys: Microstructure, Strengthening, and Properties. Adv. Eng. Mater. 2019, 21, 1801359. [Google Scholar] [CrossRef]
- Marc, L.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar]
- Zhang, L.-C.; Attar, H.; Calin, M.; Eckert, J. Review on manufacture by selective laser melting and properties of titanium based materials for biomedical applications. Mater. Technol. 2016, 31, 66–76. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, J.; Chen, Y.; Zhang, L.-C. Heat treatment degrading the corrosion resistance of selective laser melted Ti-6Al-4V alloy. J. Electrochem. Soc. 2017, 164, C428–C434. [Google Scholar] [CrossRef]
- Polozov, I.; Sufiiarov, V.; Popovich, A.; Masaylo, D.; Grigoriev, A. Synthesis of Ti-5Al, Ti-6Al-7Nb, and Ti-22Al-25Nb alloys from elemental powders using powder-bed fusion additive manufacturing. J. Alloys Compd. 2018, 763, 436–445. [Google Scholar] [CrossRef]
- Ninomi, M.; Gong, B.; Kobayashi, T.; Ohyabu, T.; Toriyama, O. Fracture characteristics of Ti–6Al–4V and Ti–5Al–2.5 Fe with refined microstructure using hydrogen. Metal Mater. Trans. A 1995, 26, 1141–1151. [Google Scholar] [CrossRef]
- Cecchel, S.; Ferrario, D.; Cornacchia, G.; Gelfi, M. Development of Heat Treatments for Selective Laser Melting Ti6Al4V Alloy: Effect on Microstructure, Mechanical Properties, and Corrosion Resistance. Adv. Eng. Mater. 2020, 22, 2000359. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The Relationship Between Stress Shielding and Bone Resorption Around Total Hip Stems and the Effects of Flexible Materials. Clin. Orthop. Relat. Res. 1992, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Liu, J.; Liu, L.; Wang, R.L.W.; Ma, Y.; Zhong, W.L.; Zhang, L.C. Superelastic behavior of in-situ eutectic-reaction manufactured high strength 3D porous NiTi-Nb scaffold. Scripta Mater. 2020, 181, 121–126. [Google Scholar] [CrossRef]
- Azadbakht, R.; Almasi, T.; Keypour, H.; Rezaeivala, M. A new asymmetric Schiff base system as fluorescent chemosensor for Al3+ ion. Inorg. Chem. Commun. 2013, 33, 63–67. [Google Scholar] [CrossRef]
- Bottino, M.C.; Coelho, P.G.; Yoshimoto, M.; König, B.; Henriques, V.A.R.; Bressiani, A.H.A.; Bressiani, J.C. Histomorphologic evaluation of Ti–13Nb–13Zr alloys processed via powder metallurgy. A study in rabbits. Mater. Sci. Eng. C 2008, 28, 223–227. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Hou, W.; Wang, S.; Hao, Y.; Yang, R.; Sercombe, T.B.; Zhang, L.-C. Electron beam melted beta-type Ti–24Nb–4Zr–8Sn porous structures with high strength-to-modulus ratio. J. Mater. Sci. Technol. 2016, 32, 505–508. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; Li, X.P.; Zhang, L.C.; Sercombe, T.B. Processing and properties of topologically optimised biomedical Ti–24Nb–4Zr–8Sn scaffolds manufactured by selective laser melting. Mater. Sci. Eng. A 2015, 642, 268–278. [Google Scholar] [CrossRef]
- Qin, P.; Chen, Y.; Liu, Y.-J.; Zhang, J.; Chen, L.-Y.; Li, Y.; Zhang, X.; Cao, C.; Sun, H.; Zhang, L.-C. Resemblance in corrosion behavior of selective laser melted and traditional monolithic β Ti-24Nb-4Zr-8Sn alloy. ACS Biomater. Sci. Eng. 2019, 5, 1141–1149. [Google Scholar] [CrossRef]
- Zhang, L.C.; Klemm, D.; Eckert, J.; Hao, Y.L.; Sercombe, T.B. Manufacture by selective laser melting and mechanical behavior of a biomedical Ti-24Nb-4Zr-8Sn alloy. Scripta Mater. 2011, 65, 21–24. [Google Scholar] [CrossRef]
- Wang, L.; Qu, J.; Chen, L.; Meng, Q.; Zhang, L.C.; Qin, J.; Zhang, D.; Lu, W. Investigation of Deformation Mechanisms in β-Type Ti-35Nb-2Ta-3Zr Alloy via FSP Leading to Surface Strengthening. Metall. Mater. Trans. A 2015, 46, 4813–4818. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Lv, Y.; Zhang, L.-C.; Chen, L.; Meng, Q.; Qu, J.; Zhang, D.; Lu, W. Microstructure evolution and superelastic behavior in Ti-35Nb-2Ta-3Zr alloy processed by friction stir processing. Acta Mater. 2017, 131, 499–510. [Google Scholar] [CrossRef] [Green Version]
- Saji, V.S.; Choe, H.C.; Brantley, W.A. An electrochemical study on self-ordered nanoporous and nanotubular oxide on Ti–35Nb–5Ta–7Zr alloy for biomedical applications. Acta Biomater. 2009, 5, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, H.; Li, J.; Gai, J.; Ru, J.; Du, Z.; Fan, J.; Niu, J.; Song, H.; Yu, Z. The effect of cold swaging deformation on the microstructures and mechanical properties of a novel metastable β type Ti-10Mo-6Zr-4Sn-3Nb alloy for biomedical devices. Front. Mater. 2020, 7, 228. [Google Scholar] [CrossRef]
- Guo, H.; Du, Z.; Wang, X.; Cheng, J.; Liu, F.; Cui, X.; Liu, H. Flowing and dynamic recrystallization behavior of new biomedical metastable β titanium alloy. Mater. Res. Exp. 2019, 6, 0865d0862. [Google Scholar] [CrossRef]
- Du, Z.; Guo, H.; Liu, J.; Cheng, J.; Zhao, X.; Wang, X.; Liu, F.; Cui, X. Microstructure evolution during aging heat treatment and its effects on tensile properties and dynamic Young’s modulus of a biomedical β titanium alloy. Mater. Sci. Eng. A 2020, 139677. [Google Scholar] [CrossRef]
- Chen, L.Y.; Shen, P.; Zhang, L.; Lu, S.; Chai, L.; Yang, Z.; Zhang, L.C. Corrosion behavior of non-equilibrium Zr-Sn-Nb-Fe-Cu-O alloys in high-temperature 0.01 M LiOH aqueous solution and degradation of the surface oxide films. Corros. Sci. 2018, 136, 221–230. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Zhang, L.C.; Lu, W.; Zhang, L.; Wang, L.; Zhang, D. Effects of alloyed Si on the autoclave corrosion performance and periodic corrosion kinetics in Zr-Sn-Nb-Fe-O alloys. Corros. Sci. 2015, 100, 651–662. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, Q.; Li, J.; Lu, J.; Zhang, Y.; Zhang, L.-C.; Qin, X.; Lu, W.; Zhang, L.; Wang, L.; et al. Effect of microstructure on corrosion behavior of a Zr-Sn-Nb-Fe-Cu-O alloy. Mater. Des. 2016, 92, 888–896. [Google Scholar] [CrossRef]
- Bai, Y.; Gai, X.; Li, S.; Zhang, L.-C.; Liu, Y.; Hao, Y.; Zhang, X.; Yang, R.; Gao, Y. Improved corrosion behaviour of electron beam melted Ti-6Al–4V alloy in phosphate buffered saline. Corros. Sci. 2017, 123, 289–296. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, R.L.; Majumdar, T.; Mantri, S.A.; Ravi, V.A.; Banerjee, R.; Birbilis, N. A closer look at the in vitro electrochemical characterisation of titanium alloys for biomedical applications using in-situ methods. Acta Biomater. 2017, 54, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Gai, X.; Bai, Y.; Li, J.; Li, S.; Hou, W.; Hao, Y.; Zhang, X.; Yang, R.; Misra, R.D.K. Electrochemical behaviour of passive film formed on the surface of Ti-6Al-4V alloys fabricated by electron beam melting. Corros. Sci. 2018, 145, 80–89. [Google Scholar] [CrossRef]
- Atapour, M.; Pilchak, A.L.; Frankel, G.S.; Williams, J.C. Corrosion behavior of β titanium alloys for biomedical applications. Mater. Sci. Eng. C 2011, 31, 885–891. [Google Scholar] [CrossRef]
- Guan, L.; Li, Y.; Wang, G.; Zhang, Y.; Zhang, L.-C. pH dependent passivation behavior of niobium in acid fluoride-containing solutions. Electrochim. Acta 2018, 285, 172–184. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Chen, Q.; Wu, M. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Xu, T.; Lu, S.; Wang, Z.-X.; Chen, S.; Zhang, L.-C. Improved hardness and wear resistance of plasma sprayed nanostructured NiCrBSi coating via short-time heat treatment. Surf. Coat. Technol. 2018, 350, 436–444. [Google Scholar] [CrossRef]
- Zhang, L.C.; Xu, J.; Ma, E. Mechanically Alloyed Amorphous Ti50(Cu0.45Ni0.55)44–xAlxSi4B2 Alloys with Supercooled Liquid Region. J. Mater. Res. 2002, 17, 1743–1749. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, J.; Zhang, Y.; Lu, W.; Zhang, L.C.; Wang, L.; Zhang, D. Effect of low-temperature pre-deformation on precipitation behavior and microstructure of a Zr-Sn-Nb-Fe-Cu-O alloy during fabrication. J. Nucl. Sci. Technol. 2016, 53, 496–507. [Google Scholar] [CrossRef]
- Chai, L.J.; Wang, S.Y.; Wu, H.; Guo, N.; Pan, H.C.; Chen, L.Y.; Murty, K.L.; Song, B. α→β Transformation characteristics revealed by pulsed laser-induced non-equilibrium microstructures in duplex-phase Zr alloy. Sci. China Technol. Sci. 2017, 60, 1255–1262. [Google Scholar] [CrossRef]
- Chen, L.Y.; Sang, P.; Zhang, L.; Song, D.; Chu, Y.Q.; Chai, L.; Zhang, L.C. Homogenization and growth behavior of second-phase particles in a deformed Zr-Sn-Nb-Fe-Cu-Si-O alloy. Metals 2018, 8, 759. [Google Scholar] [CrossRef] [Green Version]
- Xiang, K.; Chen, L.-Y.; Chai, L.; Guo, N.; Wang, H. Microstructural characteristics and properties of CoCrFeNiNbx high-entropy alloy coatings on pure titanium substrate by pulsed laser cladding. Appl. Surf. Sci. 2020, 517, 146214. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behaviour of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Qin, P.; Liu, Y.; Sercombe, T.B.; Li, Y.; Zhang, C.; Cao, C.; Sun, H.; Zhang, L.-C. Improved Corrosion Resistance on Selective Laser Melting Produced Ti-5Cu Alloy after Heat Treatment. ACS Biomater. Sci. Eng. 2018, 4, 2633–2642. [Google Scholar] [CrossRef]
- Qin, X.; Guo, X.; Lu, J.; Chen, L.; Qin, J.; Lu, W. Erosion-wear and intergranular corrosion resistance properties of AISI 304L austenitic stainless steel after low-temperature plasma nitriding. J. Alloys Compd. 2017, 698, 1094–1101. [Google Scholar] [CrossRef]
- Sang, P.; Chen, L.-Y.; Zhao, C.; Wang, Z.-X.; Wang, H.; Lu, S.; Song, D.; Xu, J.-H.; Zhang, L.-C. Particle Size-Dependent Microstructure, Hardness and Electrochemical Corrosion Behavior of Atmospheric Plasma Sprayed NiCrBSi Coatings. Metals 2019, 9, 1342. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Chen, L.-Y.; Zhao, C.; Liu, Y.; Zhang, L.-C. Calculation of oxygen diffusion coefficients in oxide films formed on low-temperature annealed Zr alloys and their related corrosion behavior. Metals 2019, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Huang, J.; Lin, C.; Pan, C.; Chen, S.; Yang, T.; Lin, D.; Lin, H.; Jang, J. Anisotropic response of Ti-6Al-4V alloy fabricated by 3D printing selective laser melting. Mater. Sci. Eng. A 2017, 682, 389–395. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N.; Davies, C.H.J. Revealing the relationship between grain size and corrosion rate of metals. Scripta Mater. 2010, 63, 1201–1204. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Dai, N.; Qin, P.; Attar, H.; Zhang, L.-C. Corrosion behaviour of selective laser melted Ti-TiB biocomposite in simulated body fluid. Electrochim. Acta 2017, 232, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Lu, Y.; Wu, S.; Liu, L.; He, M.; Zhao, C.; Gan, Y.; Lin, J.; Luo, J.; Xu, X.; et al. Preliminary study on the corrosion resistance, antibacterial activity and cytotoxicity of selective-laser-melted Ti6Al4V-xCu alloys. Mater. Sci. Eng. C 2017, 72, 631–640. [Google Scholar] [CrossRef]
- Zhang, H.; Man, C.; Wang, L.; Dong, C.; Wang, L.; Kong, D.; Wang, X. Different corrosion behaviors between α and β phases of Ti6Al4V in fluoride-containing solutions: Influence of alloying element Al. Corros. Sci. 2020, 169, 108605. [Google Scholar] [CrossRef]
- Kumar, S.; Sankara Narayanan, T.S.N.; Saravana Kumar, S. Influence of fluoride ion on the electrochemical behaviour of β-Ti alloy for dental implant application. Corros. Sci. 2010, 52, 1721–1727. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Zhang, X.; Ni, Q.; Chen, Y.; Wu, M.; Yang, C. Distinction in corrosion resistance of selective laser melted Ti-6Al-4V alloy on different planes. Corros. Sci. 2016, 111, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Domene, R.M.; Blasco-Tamarit, E.; García-García, D.M.; García-Antón, J. Passive and transpassive behaviour of Alloy 31 in a heavy brine LiBr solution. Electrochim. Acta 2013, 95, 1–11. [Google Scholar] [CrossRef]
- Armelin, E.; Moradi, S.; Hatzikiriakos, S.G.; Alemán, C. Designing Stainless Steel Surfaces with Anti-Pitting Properties Applying Laser Ablation and Organofluorine Coatings. Adv. Eng. Mater. 2018, 20, 1700814. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yu, H.; Wang, S.; Chen, B.; Wang, Y.; Fan, W.; Sun, D. Quantitative analysis of local fine structure on diffusion of point defects in passive film on Ti. Electrochim. Acta 2019, 314, 161–172. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cui, Y.-W.; Zhang, L.-C. Recent Development in Beta Titanium Alloys for Biomedical Applications. Metals 2020, 10, 1139. [Google Scholar] [CrossRef]
- Alves, A.P.R.; Santana, F.A.; Rosa, L.A.A.; Cursino, S.A.; Codaro, E.N. A study on corrosion resistance of the Ti-10Mo experimental alloy after different processing methods. Mater. Sci. Eng. C 2004, 24, 693–696. [Google Scholar] [CrossRef]
- Bai, Y.; Li, S.J.; Prima, F.; Hao, Y.L.; Yang, R. Electrochemical corrosion behavior of Ti-24Nb-4Zr-8Sn alloy in a simulated physiological environment. Appl. Surf. Sci. 2012, 258, 4035–4040. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Chen, G.-Q.; Chen, L.-Y.; Xu, L.; Lu, S. Degradation Behavior of Micro-Arc Oxidized ZK60 Magnesium Alloy in a Simulated Body Fluid. Metals 2018, 8, 724. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Lin, X.; Wang, J.; Zheng, M.; Guo, P.; Zhang, Y.; Ren, Y.; Liu, J.; Huang, W. Effect of stress-relief annealing on anodic dissolution behaviour of additive manufactured Ti-6Al-4V via laser solid forming. Corros. Sci. 2019, 153, 314–326. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Zheng, M.; Wang, J.; Guo, P.; Qin, T.; Zhu, M.; Huang, W.; Yang, H. Distinction in anodic dissolution behavior on different planes of laser solid formed Ti-6Al-4V alloy. Electrochim. Acta 2018, 283, 1482–1489. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Y.; Liu, J.; Yao, J. Influence of the Ti alloy substrate on the anodic oxidation in an environmentally-friendly electrolyte. Surf. Coat. Technol. 2018, 344, 680–688. [Google Scholar] [CrossRef]
- Chen, K.; Zeng, L.; Li, Z.; Chai, L.; Wang, Y.; Chen, L.-Y.; Yu, H. Effects of laser surface alloying with Cr on microstructure and hardness of commercial purity Zr. J. Alloys Compd. 2019, 784, 1106–1112. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Zhang, H. Microstructure and wear properties of self-lubricating TiB2-TiCxNy ceramic coatings on Ti-6Al-4V alloy fabricated by laser surface alloying. Surf. Coat. Technol. 2019, 369, 269–279. [Google Scholar] [CrossRef]
- Liu, S.; Han, S.; Zhang, L.; Chen, L.-Y.; Wang, L.; Zhang, L.; Tang, Y.; Liu, J.; Tang, H.; Zhang, L.-C. Strengthening mechanism and micropillar analysis of high-strength NiTi–Nb eutectic-type alloy prepared by laser powder bed fusion. Compos. Part B 2020, 200, 108358. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Qiao, L.; Zhao, R.; Zhang, S.; Zhang, R.; Chen, C.; Li, X.; Zhao, Y. Preparation and formation mechanism of copper incorporated micro-arc oxidation coatings developed on Ti-6Al-4V alloys. Surf. Coat. Technol. 2019, 375, 74–85. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Wang, H.; Zhao, C.; Lu, S.; Wang, Z.-X.; Sha, J.; Chen, S.; Zhang, L.-C. Automatic remelting and enhanced mechanical performance of a plasma sprayed NiCrBSi coating. Surf. Coat. Technol. 2019, 369, 31–43. [Google Scholar] [CrossRef]
- Bacci, T.; Bertamini, L.; Ferrari, F.; Galliano, F.P.; Galvanetto, E. Reactive plasma spraying of titanium in nitrogen containing plasma gas. Mater. Sci. Eng. A 2000, 283, 189–195. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Chin, W.H.; Chien, C.S.; Hsieh, Y.H. Mechanical and biological properties of graded porous tantalum coatings deposited on titanium alloy implants by vacuum plasma spraying. Surf. Coat. Technol. 2019, 372, 399–409. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Xu, T.; Wang, H.; Sang, P.; Lu, S.; Wang, Z.-X.; Chen, S.; Zhang, L.-C. Phase interaction induced texture in a plasma sprayed-remelted NiCrBSi coating during solidification: An electron backscatter diffraction study. Surf. Coat. Technol. 2019, 358, 467–480. [Google Scholar] [CrossRef]
- Li, L.-H.; Kong, Y.-M.; Kim, H.-W.; Kim, Y.-W.; Kim, H.-E.; Heo, S.-J.; Koak, J.-Y. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials 2004, 25, 2867–2875. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, K. On the pauling electronegativity scales-II. Polyhedron 1984, 3, 853–859. [Google Scholar] [CrossRef]
- Ohwada, K. On the Pauling electronegativity scales—I. Polyhedron 1983, 2, 423–424. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, L.; Wang, C.; Wang, Y. Theoretical and experimental investigations of the thermoelectric properties of Al-, Bi- and Sn-doped ZnO. Mater. Sci. Semicond. Process. 2017, 66, 247–252. [Google Scholar] [CrossRef]
- Bell, B.D.C.; Murphy, S.T.; Grimes, R.W.; Wenman, M.R. The effect of Nb on the corrosion and hydrogen pick-up of Zr alloys. Acta Mater. 2017, 132, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.P.; Mhamane, N.B.; Ghosalya, M.K.; Gopinath, C.S. Mapping Valence Band and Interface Electronic Structure Changes during the Oxidation of Mo to MoO3 via MoO2 and MoO3 Reduction to MoO2: A NAPPES Study. J. Phys. Chem. C 2018, 122, 23034–23044. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Zhang, L.C.; Lu, W.; Wang, L.; Zhang, L.; Zhang, D. Zr-Sn-Nb-Fe-Si-O alloy for fuel cladding candidate: Processing, microstructure, corrosion resistance and tensile behavior. Corros. Sci. 2015, 100, 332–340. [Google Scholar] [CrossRef]
- Zhang, L.C.; Jia, Z.; Lyu, F.; Liang, S.X.; Lu, J. A review of catalytic performance of metallic glasses in wastewater treatment: Recent progress and prospects. Prog. Mater. Sci. 2019, 105, 100576. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Xia, J.; Chen, Y. Effect of Hydrogen Precharging on Mechanical and Electrochemical Properties of Pure Titanium. Adv. Eng. Mater. 2020, 22, 1901182. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Wang, Z.; Yue, X.; Ji, P.-J.; Shu, S.-L. Simultaneously improved strength and toughness of in situ bi-phased TiB2–Ti(C,N)–Ni cermets by Mo addition. J. Alloys Compd. 2020, 820, 153068. [Google Scholar] [CrossRef]



| Ti | Mo | Zr | Sn | Nb | Al | V |
|---|---|---|---|---|---|---|
| Balance | 10.11 ± 0.31 | 5.71 ± 0.24 | 4.32 ± 0.18 | 2.83 ± 0.18 | - | - |
| Balance | - | - | - | - | 5.94 ± 0.21 | 4.28 ± 0.25 |
| Sample | Ecorr (VSCE) | Icorr (μA·cm−2) | Epp (VSCE) | Ipp (μA·cm−2) |
|---|---|---|---|---|
| Ti–10Mo–6Zr–4Sn–3Nb | −0.430 ± 0.012 | 0.007 ± 0.002 | 0.024 ± 0.014 | 3.664 ± 0.438 |
| Ti–6Al–4V | −0.504 ± 0.044 | 0.020 ± 0.008 | −0.133 ± 0.012 | 3.111 ± 0.172 |
| Sample | Rs (Ω cm2) | Rf (Ω cm2) | CPE1 × 10−6 (F·cm−2) | n1 | Rct (MΩ cm2) | CPEdl × 10−5 (F·cm−2) | n2 | χ2 |
|---|---|---|---|---|---|---|---|---|
| Ti–10Mo–6Zr–4Sn–3Nb | 18.10 ± 0.51 | 32.95 ± 2.12 | 24.41 ± 0.26 | 0.8995 ± 0.0018 | 1.3297 ± 0.0858 | 1.307 ± 0.08 | 0.8870 ± 0.0022 | 6.67 × 10−4 |
| Ti–6Al–4V | 13.77 ± 1.01 | 6.16 ± 0.47 | 8.35 ± 0.61 | 0.8784 ± 0.0117 | 0.7367 ± 0.0082 | 3.138 ± 0.13 | 0.9136 ± 0.0067 | 4.24 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Li, J.; Yu, S.; Du, Z.; Dong, F.; Zhang, J.; Zhang, X. Corrosion Behavior of As-Cast Ti–10Mo–6Zr–4Sn–3Nb and Ti–6Al–4V in Hank’s Solution: A Comparison Investigation. Metals 2021, 11, 11. https://doi.org/10.3390/met11010011
Cheng J, Li J, Yu S, Du Z, Dong F, Zhang J, Zhang X. Corrosion Behavior of As-Cast Ti–10Mo–6Zr–4Sn–3Nb and Ti–6Al–4V in Hank’s Solution: A Comparison Investigation. Metals. 2021; 11(1):11. https://doi.org/10.3390/met11010011
Chicago/Turabian StyleCheng, Jun, Jinshan Li, Sen Yu, Zhaoxin Du, Fuyu Dong, Jinyong Zhang, and Xiaoyong Zhang. 2021. "Corrosion Behavior of As-Cast Ti–10Mo–6Zr–4Sn–3Nb and Ti–6Al–4V in Hank’s Solution: A Comparison Investigation" Metals 11, no. 1: 11. https://doi.org/10.3390/met11010011






