Effect of Mechanical Surface Treatments on the Surface State and Passive Behavior of 304L Stainless Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Preparations
2.2. Surfaces and Subsurface Analysis
2.3. Characterization of the Passive Film Properties
- Polarization curve attended to characterize the surface’s electrochemical behavior (film formation, passivation ability) in the potential range between −0.8 V to 1.2 V vs. MSE. The scan rate was fixed to 0.5 mV·s−1.
Semiconductive Properties Determination
3. Results
3.1. Surface State Characterizations
3.1.1. Top Surface Characterizations
3.1.2. Microstructural Subsurface Modifications
3.1.3. Residual Stresses Generated through Mechanical Surface Modification
3.2. Corrosion Behavior in Borate Buffer Solution
3.2.1. Electrochemical Characterizations in Borate Buffer Solution
3.2.2. Mott–Schottky Analysis
4. Discussions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, M.G.S.; Da Cunha Belo, M.; Hakiki, N.E.; Goodlet, G.; Montemor, M.F.; Simõesa, A.M.P. Semiconducting properties of oxide and passive films formed on AISI 304 stainless steel and Alloy 600. J. Braz. Chem. Soc. 2002, 13, 433–440. [Google Scholar] [CrossRef]
- Montemor, M.F.; Ferreira, M.G.S.; Hakiki, N.E.; Da Cunha Belo, M. Chemical composition and electronic structure of the oxide films formed on 316L stainless steel and nickel based alloys in high temperature aqueous environments. Corros. Sci. 2000, 42, 1635–1650. [Google Scholar] [CrossRef]
- Normand, B.; Pierre, A.; Pagetti, J. Electrochemical and surface studies of the passive layers grown on sputter-deposited nitrogen-stainless steel alloys in 1M H2SO4 solution. Corros. Sci. 1995, 37, 1537–1549. [Google Scholar] [CrossRef]
- Wallinder, D.; Pan, J.; Leygraf, C.; Delblanc-Bauer, A. EIS and XPS study of surface modification of 316LVM stainless steel after passivation. Corros. Sci. 1998, 41, 275–289. [Google Scholar] [CrossRef]
- Sudesh, T.L.; Wijesighe, L.; Blackwood, D.J. Electrochemical & optical characterisation of passive films on stainless steels. J. Phys. Conf. Ser. 2006, 28, 74–78. [Google Scholar] [CrossRef]
- Jargelius-Pettersson, R.F.A. Examination of the Role of Molybdenum in Passivation of Stainless Steels Using AC Impedance Spectroscopy. J. Electrochem. Soc. 1998, 145, 1462. [Google Scholar] [CrossRef]
- BenSalah, M.; Sabot, R.; Triki, E.; Dhouibi, L.; Refait, P.; Jeannin, M. Passivity of Sanicro28 (UNS N-08028) stainless steel in polluted phosphoric acid at different temperatures studied by electrochemical impedance spectroscopy and Mott-Schottky analysis. Corros. Sci. 2014, 86, 61–70. [Google Scholar] [CrossRef]
- Olsson, C.O.A.; Landolt, D. Passive films on stainless steels—Chemistry, structure and growth. Electrochim. Acta 2003, 48, 1093–1104. [Google Scholar] [CrossRef]
- Teramoto, K.; Asami, K.; Hashimoto, K. Composition of passive films on ferritic 30Cr stainless steels in H2SO4. Corros. Eng. 1978, 27, 57–61. [Google Scholar] [CrossRef]
- Lopes, J.C.; Fragoso, K.M.; Garcia, M.V.; Ribeiro, F.S.F.; Francelin, A.P.; de Angelo Sanchez, L.E.; Rodrigues, A.R.; de Mello, H.J.; Aguiar, P.R.; Blanchi, E.C. Behavior of hardened steel grinding using MQL under cold air and MQL CBN wheel cleaning. Int. J. Adv. Manuf. Technol. 2019, 105, 4373–4387. [Google Scholar] [CrossRef]
- Bianchi, E.C.; Sato, B.K.; Sales, A.R.; Lopes, J.C.; Sanchez, H.J.D.; Diniz, A.E.; Aguiar, P.R. Evaluating the effect of the compressed air wheel cleaning in grinding the AISI 4340 steel with CBN and MQL with water. Int. J. Adv. Manuf. Technol. 2018, 95, 2855–2864. [Google Scholar] [CrossRef]
- Silva, L.R.; Corrêa, E.C.S.; Brandão, J.R.; de Ávila, R.F. Environmentally friendly manufacturing: Behavior analysis of minimum quantity of lubricant—MQL in grinding process. J. Clean. Prod. 2020, 256, 103287. [Google Scholar] [CrossRef]
- Lou, S.; Li, Y.; Zhou, L.; Nie, X.; He, G.; He, W. Surface nanocrystallization of metallic alloys with different stacking fault energy induced by laser shock processing. Mater. Des. 2016, 104, 320–326. [Google Scholar] [CrossRef]
- Fredj, N.B.; Nasr, M.B.; Rhouma, A.B.; Braham, C.; Sidhom, H. Fatigue life improvements of the AISI 304 stainless steel ground surfaces by wire brushing. J. Mater. Eng. Perform. 2004, 13, 564–574. [Google Scholar] [CrossRef]
- Rhouma, A.B.; Sidhom, N.; Makhlouf, K.; Sidhom, H.; Braham, C.; Gonzalez, G. Effect of machining processes on the residual stress distribution heterogeneities and their consequences on the stress corrosion cracking resistance of AISI 316L SS in chloride medium. Int. J. Adv. Manuf. Technol. 2019, 105, 1699–1711. [Google Scholar] [CrossRef]
- Abe, H.; Watanabe, Y.; Miyazaki, T. Characteristics of work hardened surface layer on austenitic stainless steels and its relation to SCC susceptibility in high temperature water. In Proceedings of the 19th International Conference on Environmental Degradation of Materials in Nuclear Power Systems—Water Reactors, EnvDeg, Boston, MA, USA, 18–22 August 2019; pp. 165–173. [Google Scholar]
- Lyon, K.N.; Marrow, T.J.; Lyon, S.B. Influence of milling on the development of stress corrosion cracks in austenitic stainless steel. J. Mater. Process. Technol. 2015, 218, 32–37. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Microstructural changes in AISI 304L stainless steel due to surface machining: Effect on its susceptibility to chloride stress corrosion cracking. J. Nuclear Mater. 2010, 403, 62–67. [Google Scholar] [CrossRef]
- MacDonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar] [CrossRef]
- Macdonald, D.D. The point defect model for the passive state. J. Electrochem. Soc. 1992, 139, 3434. [Google Scholar] [CrossRef]
- Marcelin, S.; Ter-Ovanessian, B.; Normand, B. Electronic properties of passive films from the multi-frequency Mott-Schottky and power-law coupled approach. Electrochem. Commun. 2016, 66, 62–65. [Google Scholar] [CrossRef]
- Ter-Ovanessian, B.; Alemany-Dumont, C.; Normand, B. Electronic and transport properties of passive films grown on different Ni-Cr binary alloys in relation to the pitting susceptibility. Electrochim. Acta 2014, 133, 373–381. [Google Scholar] [CrossRef]
- Toor, I.H.; Ejaz, M.; Kwon, H.S. Mott–Schottky analysis of passive films on Cu containing Fe–20Cr–xCu (x = 0, 4) alloys. Corros. Eng. Sci. Technol. 2014, 49, 390–395. [Google Scholar] [CrossRef]
- Coelho, L.B.; Kossman, S.; Mejias, A.; Noirfalise, X.; Montagne, A.; van Gorp, A.; Poortemana, M.; Olivier, M.-G. Mechanical and corrosion characterization of industrially treated 316L stainless steel surfaces. Surf. Coat. Technol. 2020, 382, 125175. [Google Scholar] [CrossRef]
- Matsuda, M.; Okita, K.; Nakagawa, T.; Sasaki, T. Application of X-ray stress measurement for residual stress analysis by inherent strain method—Comparison of cosα and sin2Ψ method. Mech. Eng. J. 2017, 4, 17-00022. [Google Scholar] [CrossRef][Green Version]
- Luo, H.; Wang, X.; Dong, C.; Xiao, K.; Li, X. Effect of cold deformation on the corrosion behaviour of UNS S31803 duplex stainless steel in simulated concrete pore solution. Corros. Sci. 2017, 124, 178–192. [Google Scholar] [CrossRef]
- Hakiki, N.B.; Boudin, S.; Rondot, B.; Da Cunha Belo, M. The electronic structure of passive films formed on stainless steels. Corros. Sci. 1995, 37, 1809–1822. [Google Scholar] [CrossRef]
- Dewald, J.F. The charge distribution at the zinc oxide-electrolyte interface. J. Phys. Chem. Solids 1960, 14, 155–161. [Google Scholar] [CrossRef]
- Permittivity of Inorganic Solids. In CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014; pp. 12–48.
- Lovreček, B.; Sefaja, J. Semiconducting aspects of the passive layer on chromium. Electrochim. Acta 1972, 17, 1151–1155. [Google Scholar] [CrossRef]
- Chakri, S.; Frateur, I.; Orazem, M.E.; Sutter, E.M.M.; Tran, T.T.M.; Tribollet, B.; Vivier, V. Improved EIS Analysis of the Electrochemical Behaviour of Carbon Steel in Alkaline Solution. Electrochim. Acta 2017, 246, 924–930. [Google Scholar] [CrossRef]
- Jonscher, A.K. Admittance spectroscopy of systems showing low-frequency dispersion. Electrochim. Acta 1990, 35, 1595–1600. [Google Scholar] [CrossRef]
- Benoit, M.; Bataillon, C.; Gwinner, B.; Miserque, F.; Orazem, M.E.; Sánchez-Sánchez, C.M.; Tribollet, B.; Vivier, V. Comparison of different methods for measuring the passive film thickness on metals. Electrochim. Acta 2016, 201, 340–347. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Effect of surface machining and cold working on the ambient temperature chloride stress corrosion cracking susceptibility of AISI 304L stainless steel. Mater. Sci. Eng. A 2010, 527, 679–683. [Google Scholar] [CrossRef]
- Vignal, V.; Mary, N.; Valot, C.; Oltra, R.; Coudreuse, L. Influence of Elastic Deformation on Initiation of Pits on Duplex Stainless Steels. Electrochem. Solid State Lett. 2004, 7, 39–42. [Google Scholar] [CrossRef]
- Cho, E.A.; Kwon, H.S.; Macdonald, D.D. Photoelectrochemical analysis on the passive film formed on Fe-20Cr in pH 8.5 buffer solution. Electrochim. Acta 2002, 47, 1661–1668. [Google Scholar] [CrossRef]
- Da Cunha Belo, M.; Hakiki, N.E.; Ferreira, M.G.S. Semiconducting properties of passive films formed on nickel-base alloys type Alloy 600: Influence of the alloying elements. Electrochim. Acta 1999, 44, 2473–2481. [Google Scholar] [CrossRef]
- Toor, I.-H. Mott-Schottky Analysis of Passive Films on Si Containing Stainless Steel Alloys. J. Electrochem. Soc. 2011, 158, C391. [Google Scholar] [CrossRef]
- Navaï, F. Effects of tensile and compressive stresses on the passive layers formed on a type 302 stainless steel in a normal sulphuric acid bath. J. Mater. Sci. 1995, 30, 1166–1172. [Google Scholar] [CrossRef]
- Hakiki, N.E. Influence of surface roughness on the semiconducting properties of oxide films formed on 304 stainless steel. J. Appl. Electrochem. 2008, 38, 679–687. [Google Scholar] [CrossRef]
- Kloppers, M.J.; Bellucci, F.; Latanision, R.M. Electronic properties and defect structure of Fe and Fe-Cr passive films. Corrosion 1992, 48, 229–238. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Impedance investigation of thermally formed oxide films on AISI 304L stainless steel. Corros. Sci. 2010, 52, 859–864. [Google Scholar] [CrossRef]
- Lavigne, O.; Alemany-Dumont, C.; Normand, B.; Delichère, P.; Descamps, A. Cerium insertion in 316L passive film: Effect on conductivity and corrosion resistance performances of metallic bipolar plates for PEM fuel cell application. Surf. Coat. Technol. 2010, 205, 1870–1877. [Google Scholar] [CrossRef]
- Azumi, K. Mott-Schottky Plot of the Passive Film Formed on Iron in Neutral Borate and Phosphate Solutions. J. Electrochem. Soc. 1987, 134, 1352. [Google Scholar] [CrossRef]




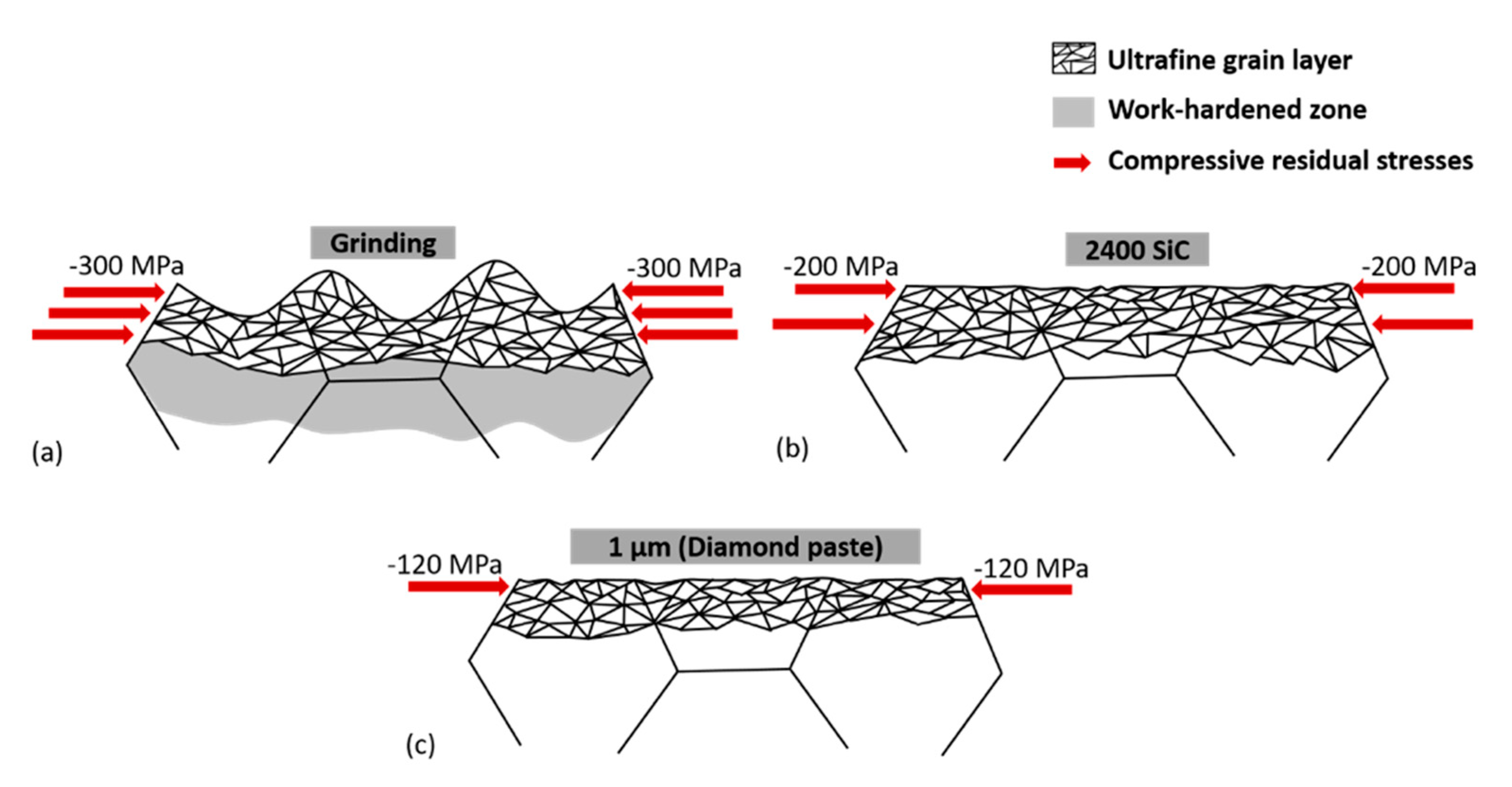
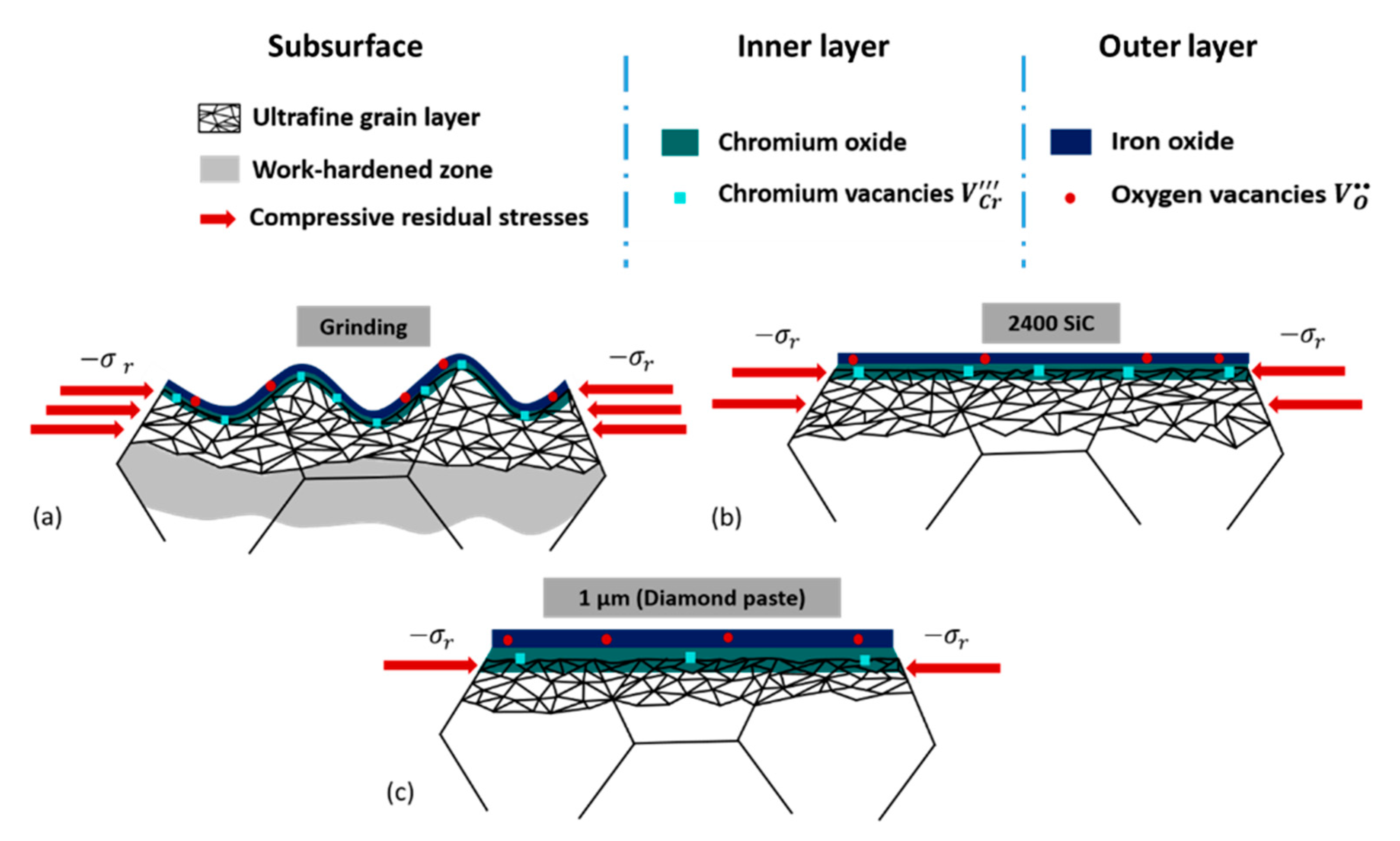

| Specimens | RMS (nm) | Thickness of the Ultrafine-Grained Layer (nm) | Residual Stresses (MPa) |
|---|---|---|---|
| Grinding | 1300 ± 200 | 150–280 | −432 ± 17 (Rolling direction) |
| 2400 SiC | 12 ± 2 | 250 | −231 ± 49 |
| 1 µm | 16 ± 4 | 150 | −110 ± 84 |
| Specimens | Grinding | 2400 SiC | 1 µm |
|---|---|---|---|
| NA × 1020 cm−3 | 8.0 | 4.4 | 2.2 |
| ND × 1020 cm−3 | 6.0 | 3.9 | 3.8 |
| Thickness δ (nm) | 1.4~1.6 | 2.6~2.9 | 3~3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffré, K.; Ter-Ovanessian, B.; Abe, H.; Mary, N.; Normand, B.; Watanabe, Y. Effect of Mechanical Surface Treatments on the Surface State and Passive Behavior of 304L Stainless Steel. Metals 2021, 11, 135. https://doi.org/10.3390/met11010135
Jaffré K, Ter-Ovanessian B, Abe H, Mary N, Normand B, Watanabe Y. Effect of Mechanical Surface Treatments on the Surface State and Passive Behavior of 304L Stainless Steel. Metals. 2021; 11(1):135. https://doi.org/10.3390/met11010135
Chicago/Turabian StyleJaffré, Kathleen, Benoît Ter-Ovanessian, Hiroshi Abe, Nicolas Mary, Bernard Normand, and Yutaka Watanabe. 2021. "Effect of Mechanical Surface Treatments on the Surface State and Passive Behavior of 304L Stainless Steel" Metals 11, no. 1: 135. https://doi.org/10.3390/met11010135
APA StyleJaffré, K., Ter-Ovanessian, B., Abe, H., Mary, N., Normand, B., & Watanabe, Y. (2021). Effect of Mechanical Surface Treatments on the Surface State and Passive Behavior of 304L Stainless Steel. Metals, 11(1), 135. https://doi.org/10.3390/met11010135







