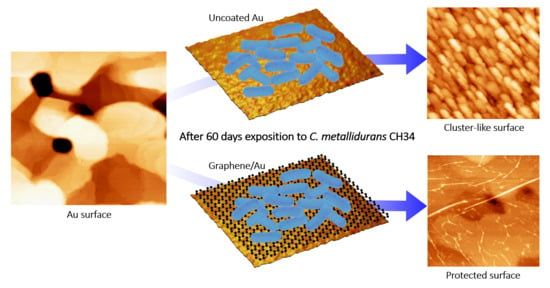
Graphene Coating as an Effective Barrier to Prevent Bacteria-Mediated Dissolution of Gold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Au Films Growth
2.3. Synthesis of Single-Layer Graphene
2.4. Graphene Transference onto Gold Samples
2.5. Preparation of C. metallidurans Culture
2.6. Characterization of Samples Prior and Post Biological Tests
3. Results and Discussion
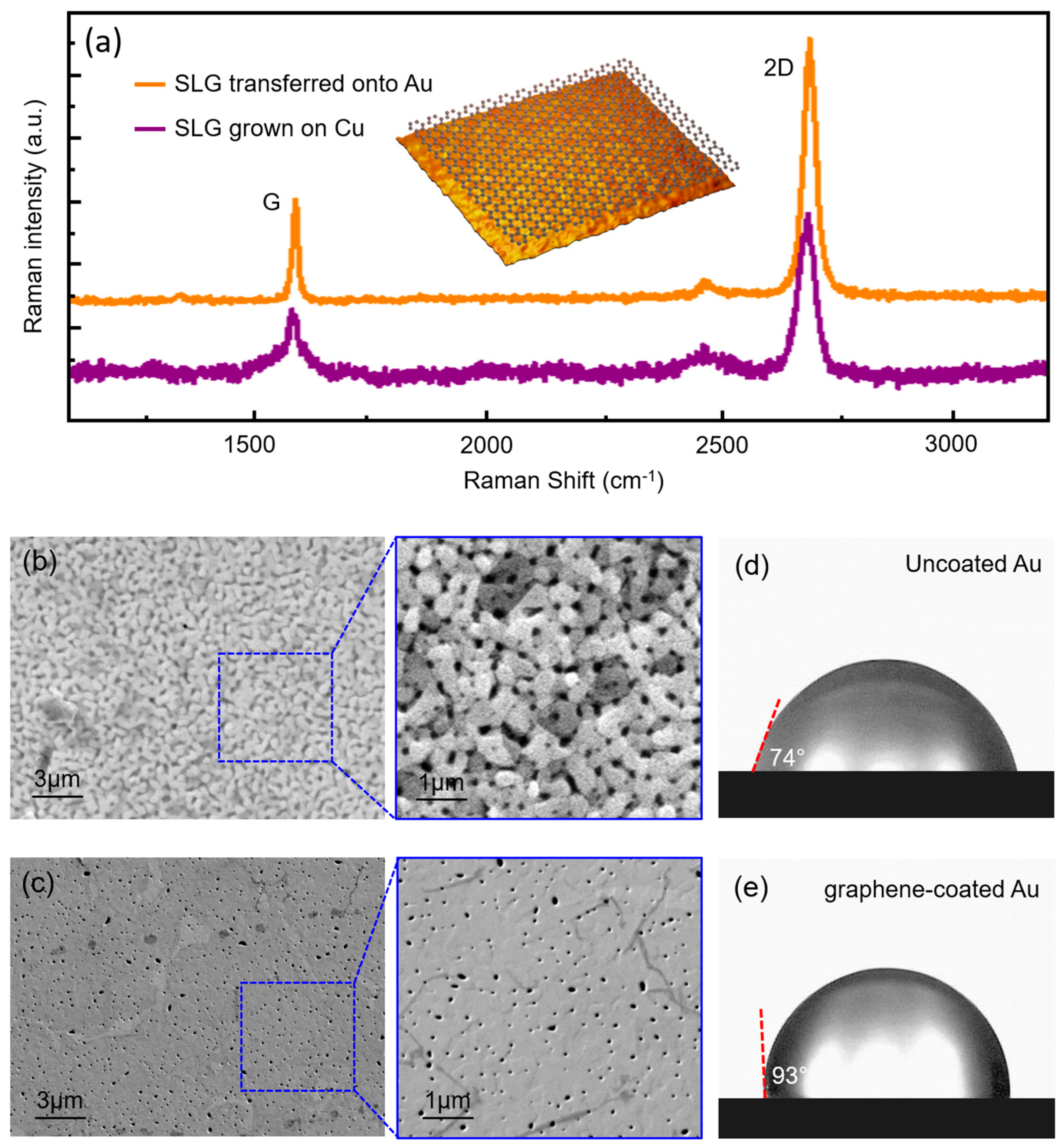
3.1. Characterization of Graphene-Coated and Uncoated Au Samples
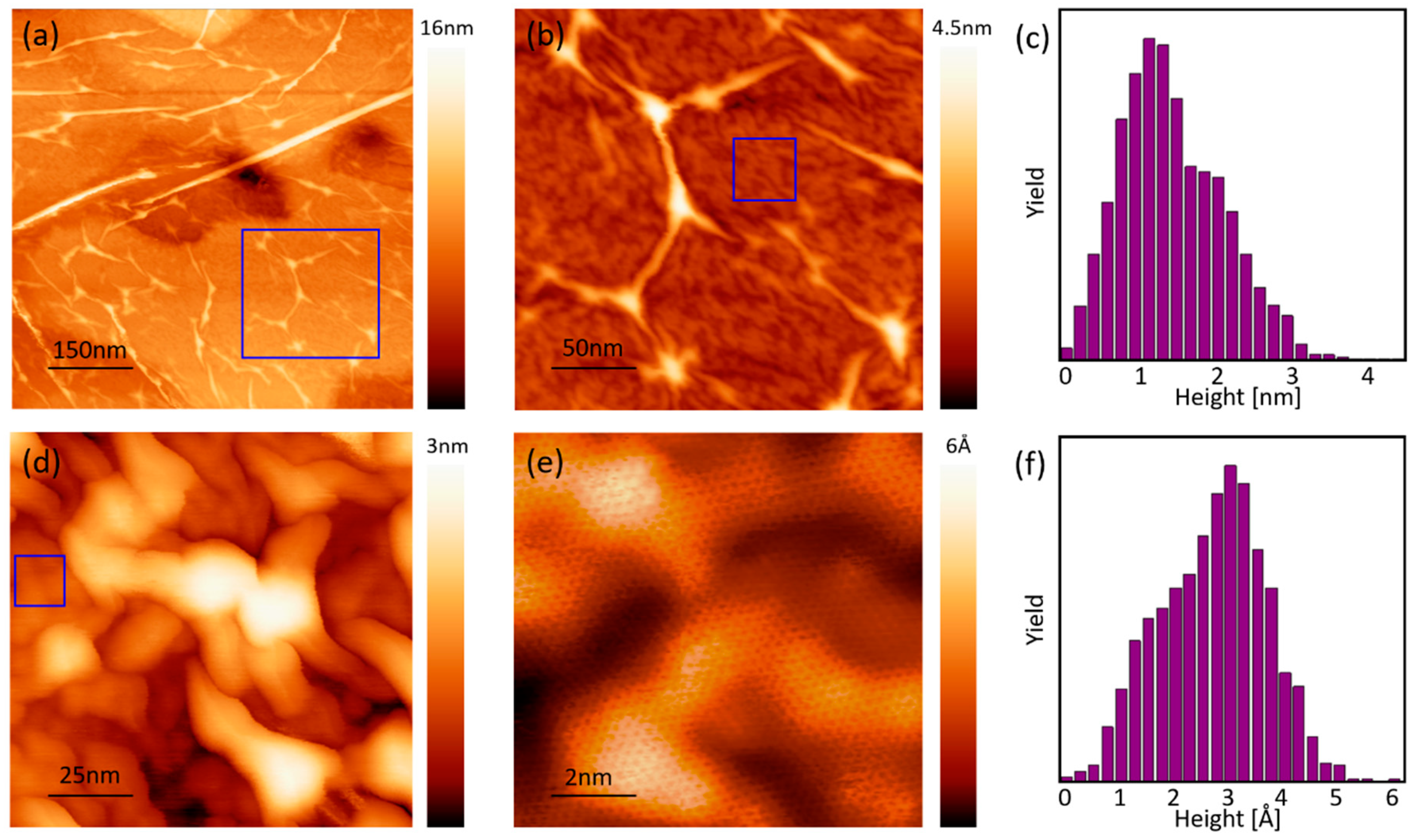
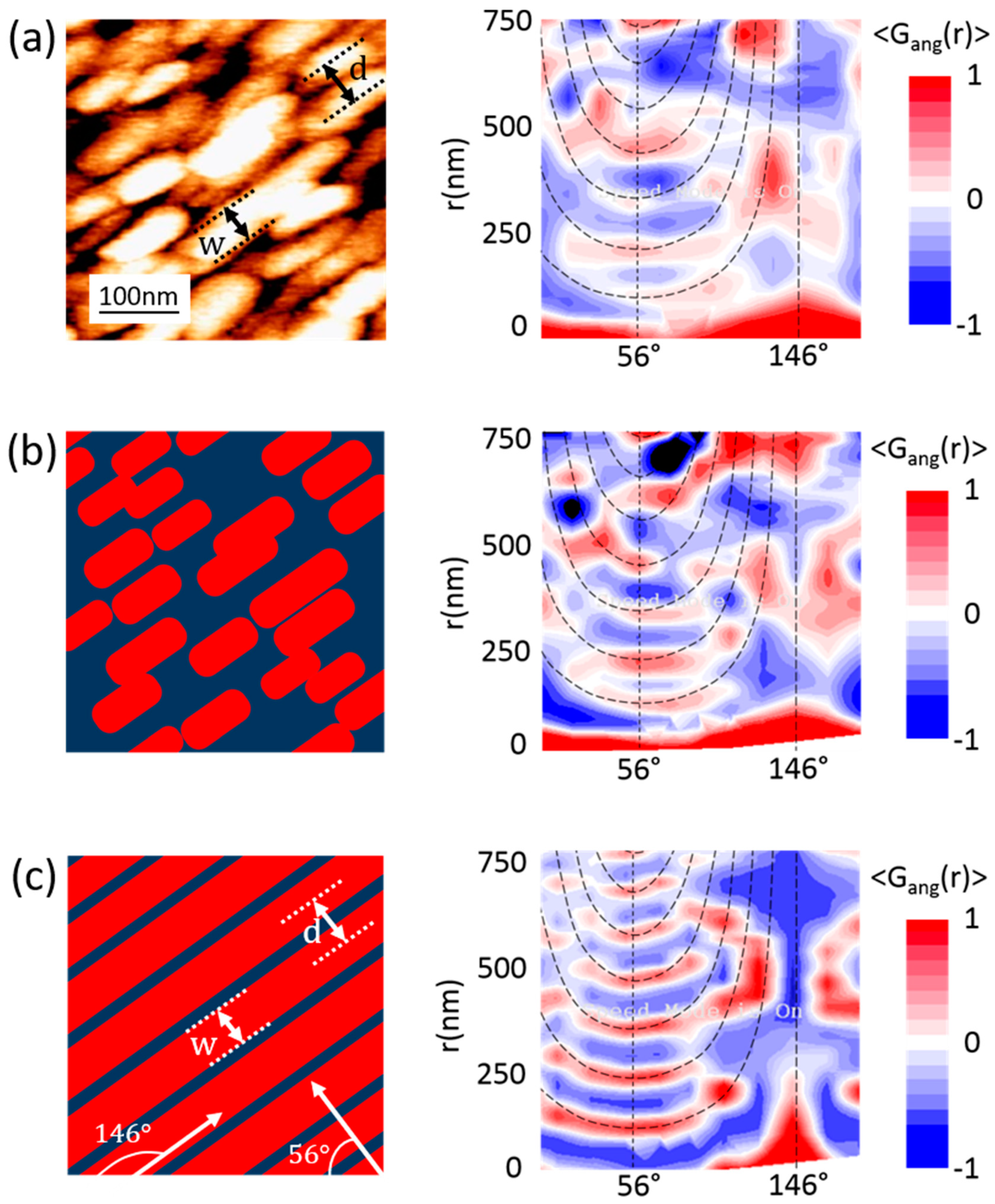
3.2. Nanoscale Topography and Roughness by STM
3.3. Characterization after C. metallidurans Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kanematsu, H.; Barry, D.M. Biofilm and Materials Science; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319145655. [Google Scholar]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation-part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Wikieł, A.J.; Datsenko, I.; Vera, M.; Sand, W. Impact of Desulfovibrio alaskensis biofilms on corrosion behaviour of carbon steel in marine environment. Bioelectrochemistry 2014, 97, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Yang, D.; Abd Rahman, H.B.; Gu, T. An enhanced oil recovery polymer promoted microbial growth and accelerated microbiologically influenced corrosion against carbon steel. Corros. Sci. 2018, 139, 301–308. [Google Scholar] [CrossRef]
- Rajala, P.; Bomberg, M.; Vepsäläinen, M.; Carpén, L. Microbial fouling and corrosion of carbon steel in deep anoxic alkaline groundwater. Biofouling 2017, 33, 195–209. [Google Scholar] [CrossRef]
- Jirón-Lazos, U.; Corvo, F.; De la Rosa, S.C.; García-Ochoa, E.M.; Bastidas, D.M.; Bastidas, J.M. Localized corrosion of aluminum alloy 6061 in the presence of Aspergillus niger. Int. Biodeterior. Biodegrad. 2018, 133, 17–25. [Google Scholar] [CrossRef]
- de Andrade, J.S.; Vieira, M.R.S.; Oliveira, S.H.; de Melo Santos, S.K.; Urtiga Filho, S.L. Study of microbiologically induced corrosion of 5052 aluminum alloy by sulfate-reducing bacteria in seawater. Mater. Chem. Phys. 2020, 241, 122296. [Google Scholar] [CrossRef]
- Pratikno, H.; Titah, H.S. Bio-corrosion on Aluminium 6063 by Escherichia coli in Marine Environment. Maj. IPTEK Inst. Teknol. Sci. 2017, 28, 55–58. [Google Scholar] [CrossRef]
- Narenkumar, J.; Elumalai, P.; Subashchandrabose, S.; Megharaj, M.; Balagurunathan, R.; Murugan, K.; Rajasekar, A. Role of 2-mercaptopyridine on control of microbial influenced corrosion of copper CW024A metal in cooling water system. Chemosphere 2019, 222, 611–618. [Google Scholar] [CrossRef]
- Swaroop, B.S.; Victoria, S.N.; Manivannan, R. Azadirachta indica leaves extract as inhibitor for microbial corrosion of copper by Arthrobacter sulfureus in neutral pH conditions-A remedy to blue green water problem. J. Taiwan Inst. Chem. Eng. 2016, 64, 269–278. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Rajala, P.; Bomberg, M.; Carpén, L. EIS study on aerobic corrosion of copper in ground water: Influence of micro-organisms. Electrochim. Acta 2017, 240, 163–174. [Google Scholar] [CrossRef]
- Kannan, P.; Su, S.S.; Mannan, M.S.; Castaneda, H.; Vaddiraju, S. A Review of Characterization and Quantification Tools for Microbiologically Influenced Corrosion in the Oil and Gas Industry: Current and Future Trends. Ind. Eng. Chem. Res. 2018, 57, 13895–13922. [Google Scholar] [CrossRef]
- Unsal, T.; Ilhan-Sungur, E.; Arkan, S.; Cansever, N. Effects of Ag and Cu ions on the microbial corrosion of 316L stainless steel in the presence of Desulfovibrio sp. Bioelectrochemistry 2016, 110, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Chen, T.E.; Lo, K.Y.; Lee, Y.L. Inhibitive properties of benzyldimethyldodecylammonium chloride on microbial corrosion of 304 stainless steel in a desulfovibrio desulfuricans-inoculated medium. Materials 2019, 12, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grengg, C.; Mittermayr, F.; Ukrainczyk, N.; Koraimann, G.; Kienesberger, S.; Dietzel, M. Advances in concrete materials for sewer systems affected by microbial induced concrete corrosion: A review. Water Res. 2018, 134, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Zhou, M.; Chiu, T.H.; Sun, X.; Keller, J.; Bond, P.L. Wastewater-Enhanced Microbial Corrosion of Concrete Sewers. Environ. Sci. Technol. 2016, 50, 8084–8092. [Google Scholar] [CrossRef] [PubMed]
- Grengg, C.; Mittermayr, F.; Koraimann, G.; Konrad, F.; Szabó, M.; Demeny, A.; Dietzel, M. The decisive role of acidophilic bacteria in concrete sewer networks: A new model for fast progressing microbial concrete corrosion. Cem. Concr. Res. 2017, 101, 93–101. [Google Scholar] [CrossRef]
- Guiamet, P.S.; Gomez de Saravia, S.G. Biofilms Formation and Microbiologically Influenced Corrosion (MIC) in Different Materials. Innov. Corros. Mater. Sci. Former. Recent Patents Corros. Sci. 2018, 7, 117–121. [Google Scholar] [CrossRef]
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Usher, K.M.; Kaksonen, A.H.; Cole, I.; Marney, D. Critical review: Microbially influenced corrosion of buried carbon steel pipes. Int. Biodeterior. Biodegrad. 2014, 93, 84–106. [Google Scholar] [CrossRef]
- Chilkoor, G.; Shrestha, N.; Karanam, S.P.; Upadhyayula, V.K.K.; Gadhamshetty, V. Graphene Coatings for Microbial Corrosion Applications. Encycl. Water 2019, 1–25. [Google Scholar] [CrossRef]
- Jogdeo, P.; Chai, R.; Shuyang, S.; Saballus, M.; Constancias, F.; Wijesinghe, S.L.; Thierry, D.; Blackwood, D.J.; McDougald, D.; Rice, S.A.; et al. Onset of Microbial Influenced Corrosion (MIC) in Stainless Steel Exposed to Mixed Species Biofilms from Equatorial Seawater. J. Electrochem. Soc. 2017, 164, C532–C538. [Google Scholar] [CrossRef] [Green Version]
- Kay, M.; Flitton, A.; Yoder, T.S. Twelve Year Study of Underground Corrosion of Activated Metals. In Proceedings of the NACE International Corrosion Conference and Expo 2012, Salt Lake City, UT, USA, 11–15 March 2012. [Google Scholar]
- Vaishampayan, A.; Grohmann, E. Multi-resistant biofilm-forming pathogens on the International Space Station. J. Biosci. 2019, 44, 125. [Google Scholar] [CrossRef] [PubMed]
- Zea, L.; McLean, R.J.C.; Rook, T.A.; Angle, G.; Carter, D.L.; Delegard, A.; Denvir, A.; Gerlach, R.; Gorti, S.; McIlwaine, D.; et al. Potential biofilm control strategies for extended spaceflight missions. Biofilm 2020, 2, 100026. [Google Scholar] [CrossRef]
- Liu, C. The theory and application of space microbiology: China’s experiences in space experiments and beyond. Environ. Microbiol. 2017, 19, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Sawada, H.; Borisenko, K.B.; Shima, M.; Ikita, K.; Hashiguchi, H.; Onishi, I.; Okunishi, E.; Kirkland, A.I. Corrosion of Gold by a Nanoscale Gold and Copper Beltlike Structure. J. Phys. Chem. C 2019, 123, 19920–19926. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.; Ahn, J.; Rhee, Y. Bioleaching: A microbial process of metal recovery; A review. Met. Mater. Int. 2005, 11, 249–256. [Google Scholar] [CrossRef]
- Tay, S.B.; Natarajan, G.; Rahim, M.N.B.A.; Tan, H.T.; Chung, M.C.M.; Ting, Y.P.; Yew, W.S. Enhancing gold recovery from electronic waste via lixiviant metabolic engineering in Chromobacterium violaceum. Sci. Rep. 2013, 3, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Cockell, C.S.; Santomartino, R.; Finster, K.; Waajen, A.C.; Eades, L.J.; Moeller, R.; Rettberg, P.; Fuchs, F.M.; Van Houdt, R.; Leys, N.; et al. Space station biomining experiment demonstrates rare earth element extraction in microgravity and Mars gravity. Nat. Commun. 2020, 11, 5523. [Google Scholar] [CrossRef]
- Brugger, J.; Etschmann, B.; Grosse, C.; Plumridge, C.; Kaminski, J.; Paterson, D.; Shar, S.S.; Ta, C.; Howard, D.L.; de Jonge, M.D.; et al. Can biological toxicity drive the contrasting behavior of platinum and gold in surface environments? Chem. Geol. 2013, 343, 99–110. [Google Scholar] [CrossRef]
- Li, J.; Wen, J.; Guo, Y.; An, N.; Liang, C.; Ge, Z. Bioleaching of gold from waste printed circuit boards by alkali-tolerant Pseudomonas fluorescens. Hydrometallurgy 2020, 194, 105260. [Google Scholar] [CrossRef]
- Khaing, S.Y.; Sugai, Y.; Sasaki, K. Gold Dissolution from Ore with Iodide-Oxidising Bacteria. Sci. Rep. 2019, 9, 4178. [Google Scholar] [CrossRef] [Green Version]
- Ober, J.A. Mineral commodity summaries 2018. In Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2018. [Google Scholar] [CrossRef]
- Gentina, J.C.; Acevedo, F. Application of bioleaching to copper mining in Chile. Electron. J. Biotechnol. 2013, 16, 16. [Google Scholar] [CrossRef]
- Yin, S.; Wang, L.; Kabwe, E.; Chen, X.; Yan, R.; An, K.; Zhang, L.; Wu, A. Copper bioleaching in China: Review and prospect. Minerals 2018, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M. A comparison study of heap bioleaching sites in Chile and Finland for further development of biotechnology for mining. Evergreen 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Yuan, Z.; Huang, Z.; Ruan, J.; Li, Y.; Hu, J.; Qiu, R. Contact Behavior between Cells and Particles in Bioleaching of Precious Metals from Waste Printed Circuit Boards. ACS Sustain. Chem. Eng. 2018, 6, 11570–11577. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, H.S.; Kumar, S. Bioleaching of Gold and Silver from Waste Printed Circuit Boards by Pseudomonas balearica SAE1 Isolated from an e-Waste Recycling Facility. Curr. Microbiol. 2018, 75, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Maryatt, B.W.; Smith, M.J. Microbial Growth Control in the International Space Station. In Proceedings of the 47th International Conference on Environmental Systems, Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Mijnendonckx, K.; Provoost, A.; Ott, C.M.; Venkateswaran, K.; Mahillon, J.; Leys, N.; van Houdt, R. Characterization of the Survival Ability of Cupriavidus metallidurans and Ralstonia pickettii from Space-Related Environments. Microb. Ecol. 2013, 65, 347–360. [Google Scholar] [CrossRef]
- Klintworth, R.; Reher, H.J.; Viktorov, A.N.; Bohle, D. Biological induced corrosion of materials: New test methods and experiences from MIR station. Eur. Sp. Agency Spec. Publ. ESA SP 1997, 44, 513–522. [Google Scholar] [CrossRef]
- de Rooij, A. Corrosion in Space. Encycl. Aerosp. Eng. 2010, 1–10. [Google Scholar] [CrossRef]
- Taghavi Kalajahi, S.; Rasekh, B.; Yazdian, F.; Neshati, J.; Taghavi, L. Graphene oxide/silver nanostructure as a green anti-biofouling composite toward controlling the microbial corrosion. Int. J. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Gadhamshetty, V.; Mukherjee, R.; Chen, Z.; Ren, W.; Cheng, H.M.; Koratkar, N. Passivation of microbial corrosion using a graphene coating. Carbon N. Y. 2013, 56, 45–49. [Google Scholar] [CrossRef]
- Otter, J.A.; Vickery, K.; Walker, J.T.; de Lancey Pulcini, E.; Stoodley, P.; Goldenberg, S.D.; Salkeld, J.A.G.; Chewins, J.; Yezli, S.; Edgeworth, J.D. Surface-attached cells, biofilms and biocide susceptibility: Implications for hospital cleaning anddisinfection. J. Hosp. Infect. 2015, 89, 16–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Lee, S.; Mcnear, K.L.; Chung, T.F.; Lee, S.; Lee, K.; Crist, S.A.; Ratliff, T.L.; Zhong, Z.; Chen, Y.P.; et al. Use of graphene as protection film in biological environments. Sci. Rep. 2014, 4, 4097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.J.; Gong, L.X.; Tang, L.C.; Wu, L.B.; Jiang, J.X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Ituen, E.; Ekemini, E.; Yuanhua, L.; Li, R.; Singh, A. Mitigation of microbial biodeterioration and acid corrosion of pipework steel using Citrus reticulata peels extract mediated copper nanoparticles composite. Int. Biodeterior. Biodegrad. 2020, 149, 104935. [Google Scholar] [CrossRef]
- Okeniyi, J.O.; John, G.S.; Owoeye, T.F.; Okeniyi, E.T.; Akinlabu, D.K.; Taiwo, O.S.; Awotoye, O.A.; Ige, O.J.; Obafemi, Y.D. Effects of Dialium Guineense Based Zinc Nanoparticle Material on the Inhibition of Microbes Inducing Microbiologically Influenced Corrosion; Meyers, M.A., Benavides, H.A.C., Brühl, S.P., Colorado, H.A., Dalgaard, E., Elias, C.N., Figueiredo, R.B., Garcia-Rincon, O., Kawasaki, M., Langdon, T.G., et al., Eds.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2017; pp. 21–31. ISBN 978-3-319-52131-2. [Google Scholar]
- Kalajahi, S.T.; Rasekh, B.; Yazdian, F.; Neshati, J.; Taghavi, L. Green mitigation of microbial corrosion by copper nanoparticles doped carbon quantum dots nanohybrid. Environ. Sci. Pollut. Res. 2020, 27, 40537–40551. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Jabbar, K.A.; Mackey, H.R.; Mahmoud, K.A. Recent advancements of nanomaterials as coatings and biocides for the inhibition of sulfate reducing bacteria induced corrosion. Curr. Opin. Chem. Eng. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Parra, C.; Montero-Silva, F.; Gentil, D.; del Campo, V.; da Cunha, T.H.R.; Henríquez, R.; Häberle, P.; Garín, C.; Ramírez, C.; Fuentes, R.; et al. The many faces of graphene as protection barrier. Performance under microbial corrosion and Ni allergy conditions. Materials 2017, 10, 1406. [Google Scholar] [CrossRef] [Green Version]
- Parra, C.; Montero-Silva, F.; Henríquez, R.; Flores, M.; Garín, C.; Ramírez, C.; Moreno, M.; Correa, J.; Seeger, M.; Häberle, P. Suppressing bacterial interaction with copper surfaces through graphene and hexagonal-boron nitride coatings. ACS Appl. Mater. Interfaces 2015, 7, 6430–6437. [Google Scholar] [CrossRef]
- Parra, C.; Dorta, F.; Jimenez, E.; Henríquez, R.; Ramírez, C.; Rojas, R.; Villalobos, P. A nanomolecular approach to decrease adhesion of biofouling-producing bacteria to graphene-coated material. J. Nanobiotechnol. 2015, 13, 82. [Google Scholar] [CrossRef] [Green Version]
- Zurob, E.; Dennett, G.; Gentil, D.; Montero-Silva, F.; Gerber, U.; Naulín, P.; Gómez, A.; Fuentes, R.; Lascano, S.; Henrique Rodrigues da Cunha, T.; et al. Inhibition of wild Enterobacter cloacae biofilm formation by nanostructured graphene-and hexagonal boron nitride-coated surfaces. Nanomaterials 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellieu, L.; Lawarée, E.; Reckinger, N.; Didembourg, C.; Letesson, J.J.; Sarrazin, M.; Deparis, O.; Matroule, J.Y.; Colomer, J.F. Do CVD grown graphene films have antibacterial activity on metallic substrates? Carbon N. Y. 2015, 84, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Pandit, S.; Cao, Z.; Mokkapati, V.R.S.S.; Celauro, E.; Yurgens, A.; Lovmar, M.; Westerlund, F.; Sun, J.; Mijakovic, I. Vertically Aligned Graphene Coating is Bactericidal and Prevents the Formation of Bacterial Biofilms. Adv. Mater. Interfaces 2018, 5, 1701331. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, J.; Hou, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Graphene-based antimicrobial polymeric membranes: A review. J. Mater. Chem. A 2017, 5, 6776–6793. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y., II; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reith, F.; Rogers, S.L.; McPhail, D.C.; Webb, D. Biomineralization of gold: Biofilms on bacterioform gold. Science 2006, 313, 233–236. [Google Scholar] [CrossRef] [Green Version]
- Montero-Silva, F.; Durán, N.; Seeger, M. Synthesis of extracellular gold nanoparticles using Cupriavidus metallidurans CH34 cells. IET Nanobiotechnol. 2018, 12, 40–46. [Google Scholar] [CrossRef]
- Monsieurs, P.; Moors, H.; Van Houdt, R.; Janssen, P.J.; Janssen, A.; Coninx, I.; Mergeay, M.; Leys, N. Heavy metal resistance in Cupriavidus metallidurans CH34 is governed by an intricate transcriptional network. BioMetals 2011, 24, 1133–1151. [Google Scholar] [CrossRef]
- Fairbrother, L.; Etschmann, B.; Brugger, J.; Shapter, J.; Southam, G.; Reith, F. Biomineralization of gold in biofilms of Cupriavidus metallidurans. Environ. Sci. Technol. 2013, 47, 2628–2635. [Google Scholar] [CrossRef]
- Mora, M.; Wink, L.; Kögler, I.; Mahnert, A.; Rettberg, P.; Schwendner, P.; Demets, R.; Cockell, C.; Alekhova, T.; Klingl, A.; et al. Space Station conditions are selective but do not alter microbial characteristics relevant to human health. Nat. Commun. 2019, 10, 3990. [Google Scholar] [CrossRef] [Green Version]
- Mergeay, M.; Nies, D.; Schlegel, H.G.; Gerits, J.; Charles, P.; Van Gijsegem, F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J. Bacteriol. 1985, 162, 328–334. [Google Scholar] [CrossRef] [Green Version]
- No, Y.S.; Choi, H.K.; Kim, J.S.; Kim, H.; Yu, Y.J.; Choi, C.G.; Choi, J.S. Layer number identification of CVD-grown multilayer graphene using Si peak analysis. Sci. Rep. 2018, 8, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Her, M.; Beams, R.; Novotny, L. Graphene transfer with reduced residue. Phys. Lett. Sect. A 7Gen. At. Solid State Phys. 2013, 377, 1455–1458. [Google Scholar] [CrossRef] [Green Version]
- Verguts, K.; Schouteden, K.; Wu, C.H.; Peters, L.; Vrancken, N.; Wu, X.; Li, Z.; Erkens, M.; Porret, C.; Huyghebaert, C.; et al. Controlling Water Intercalation Is Key to a Direct Graphene Transfer. ACS Appl. Mater. Interfaces 2017, 9, 37484–37492. [Google Scholar] [CrossRef] [PubMed]
- Correa-Puerta, J.; Del Campo, V.; Henríquez, R.; Häberle, P. Resistivity of thiol-modified gold thin films. Thin Solid Films 2014, 570, 150–154. [Google Scholar] [CrossRef]
- Elbourne, A.; Truong, V.K.; Cheeseman, S.; Rajapaksha, P.; Gangadoo, S.; Chapman, J.; Crawford, R.J. The Use of Nanomaterials for the Mitigation of Pathogenic Biofilm Formation, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; Volume 46, ISBN 9780128149928. [Google Scholar]
- Barin, G.B.; Song, Y.; Gimenez, I.D.F.; Filho, A.G.S.; Barreto, L.S.; Kong, J. Optimized graphene transfer: Influence of polymethylmethacrylate (PMMA) layer concentration and baking time on grapheme final performance. Carbon N. Y. 2015, 84, 82–90. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Christian, M.; Morandi, V.; Favaro, G.; Bongiorno, C.; Mio, A.M.; Russo, M.; Sitta, A.; Calabretta, M.; Sciuto, A.; et al. Mechanical and electrical characterization of CVD-grown graphene transferred on chalcogenide Ge2Sb2Te5 layers. Carbon N. Y. 2018, 132, 141–151. [Google Scholar] [CrossRef]
- Calado, V.E.; Schneider, G.F.; Theulings, A.M.M.G.; Dekker, C.; Vandersypen, L.M.K. Formation and control of wrinkles in graphene by the wedging transfer method. Appl. Phys. Lett. 2012, 101, 103116. [Google Scholar] [CrossRef] [Green Version]
- Preedy, E.; Perni, S.; Nipiĉ, D.; Bohinc, K.; Prokopovich, P. Surface roughness mediated adhesion forces between borosilicate glass and gram-positive bacteria. Langmuir 2014, 30, 9466–9476. [Google Scholar] [CrossRef] [Green Version]
- Gharechahi, M.; Moosavi, H.; Forghani, M. Effect of Surface Roughness and Materials Composition. J. Biomater. Nanobiotechnol. 2012, 3, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, R.; Hu, Y.C.; Chao, Y.C.; Li, R.J.; Tzeng, Y.R.; Li, L.J.; Liou, S.C.; Lin, K.C.; Chen, C.W.; Pai, W.W. Flipping nanoscale ripples of free-standing graphene using a scanning tunneling microscope tip. Carbon N. Y. 2014, 77, 236–243. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamim, S.; Rehman, A. Physicochemical surface properties of Cupriavidus metallidurans CH34 and Pseudomonas putida mt2 under cadmium stress. J. Basic Microbiol. 2014, 54, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Tegou, E.; Magana, M.; Katsogridaki, A.E.; Ioannidis, A.; Raptis, V.; Jordan, S.; Chatzipanagiotou, S.; Chatzandroulis, S.; Ornelas, C.; Tegos, G.P. Terms of endearment: Bacteria meet graphene nanosurfaces. Biomaterials 2016, 89, 38–55. [Google Scholar] [CrossRef]
- Reith, F.; Etschmann, B.; Grosse, C.; Moors, H.; Benotmane, M.A.; Monsieurs, P.; Grass, G.; Doonan, C.; Vogt, S.; Lai, B.; et al. Mechanisms of gold biomineralization in the bacterium Cupriavidus metallidurans. Proc. Natl. Acad. Sci. USA 2009, 106, 17757–17762. [Google Scholar] [CrossRef] [Green Version]
- Kaksonen, A.H.; Mudunuru, B.M.; Hackl, R. The role of microorganisms in gold processing and recovery—A review. Hydrometallurgy 2014, 142, 70–83. [Google Scholar] [CrossRef]
- Berry, V. Impermeability of graphene and its applications. Carbon N. Y. 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Tsetseris, L.; Pantelides, S.T. Graphene: An impermeable or selectively permeable membrane for atomic species? Carbon N. Y. 2014, 67, 58–63. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Sreeprasad, T.S.; Berry, V. How do the electrical properties of graphene change with its functionalization? Small 2013, 9, 341–350. [Google Scholar] [CrossRef]
- Klein, W. Crystal structure of strontium thiosulfate monohydrate Klein Wilhelm. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Lijk, L.J.; Torfs, C.A.; Kalk, K.H.; De Maeyer, M.C.H.; Hol, W.G.J. Differences in the binding of sulfate, selenate and thiosulfate ions to bovine liver rhodanese, and a description of a binding site for ammonium and sodium ions: An X-ray diffraction study. Eur. J. Biochem. 1984, 142, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Hesse, W.; Leutner, B.; Böhn, K.H.; Walker, N.P.C. Structure of a new sodium thiosulfate hydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1993, 49, 363–365. [Google Scholar] [CrossRef]
- Morris, D.F.C. Crystal radius of the cyanide ion. Acta Crystallogr. 1961, 14, 547–548. [Google Scholar] [CrossRef]
- Ching, C.B.; Hidajat, K.; Uddin, M.S. Evaluation of Equilibrium and Kinetic Parameters of Smaller Molecular Size Amino Acids on KX Zeolite Crystals via Liquid Chromatographic Techniques. Sep. Sci. Technol. 1989, 24, 581–597. [Google Scholar] [CrossRef]
- Tyagi, A.; Sharma, V.; Srivastava, A. Electronic Properties of Graphene Based Hydrogen Cyanide Sensor. Adv. Sci. Lett. 2014, 20, 1570–1573. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra, C.; Aristizabal, J.; Arce, B.; Montero-Silva, F.; Lascano, S.; Henriquez, R.; Lazcano, P.; Giraldo-Gallo, P.; Ramírez, C.; Henrique Rodrigues da Cunha, T.; et al. Graphene Coating as an Effective Barrier to Prevent Bacteria-Mediated Dissolution of Gold. Metals 2021, 11, 147. https://doi.org/10.3390/met11010147
Parra C, Aristizabal J, Arce B, Montero-Silva F, Lascano S, Henriquez R, Lazcano P, Giraldo-Gallo P, Ramírez C, Henrique Rodrigues da Cunha T, et al. Graphene Coating as an Effective Barrier to Prevent Bacteria-Mediated Dissolution of Gold. Metals. 2021; 11(1):147. https://doi.org/10.3390/met11010147
Chicago/Turabian StyleParra, Carolina, Juliet Aristizabal, Bárbara Arce, Francisco Montero-Silva, Sheila Lascano, Ricardo Henriquez, Paola Lazcano, Paula Giraldo-Gallo, Cristian Ramírez, Thiago Henrique Rodrigues da Cunha, and et al. 2021. "Graphene Coating as an Effective Barrier to Prevent Bacteria-Mediated Dissolution of Gold" Metals 11, no. 1: 147. https://doi.org/10.3390/met11010147
APA StyleParra, C., Aristizabal, J., Arce, B., Montero-Silva, F., Lascano, S., Henriquez, R., Lazcano, P., Giraldo-Gallo, P., Ramírez, C., Henrique Rodrigues da Cunha, T., & Barrera de Brito, A. (2021). Graphene Coating as an Effective Barrier to Prevent Bacteria-Mediated Dissolution of Gold. Metals, 11(1), 147. https://doi.org/10.3390/met11010147