Effect of Sodium-Containing Fluxes on the Residual Sodium Content and Distribution in Al–Mg Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussions
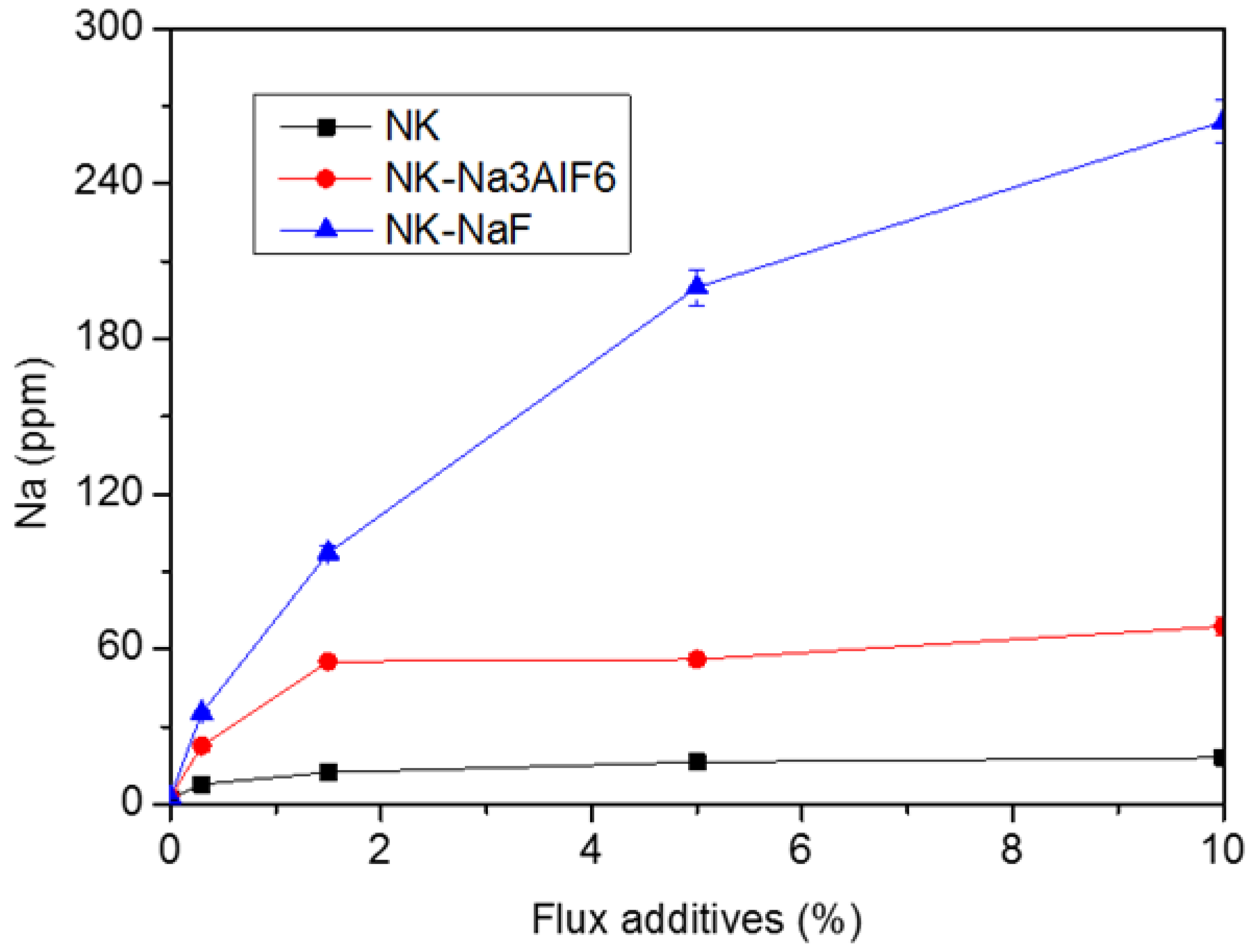
3.1. Effect of Different Sodium-Containing Fluxes on the Residual Sodium Content in Al–10Mg Alloys
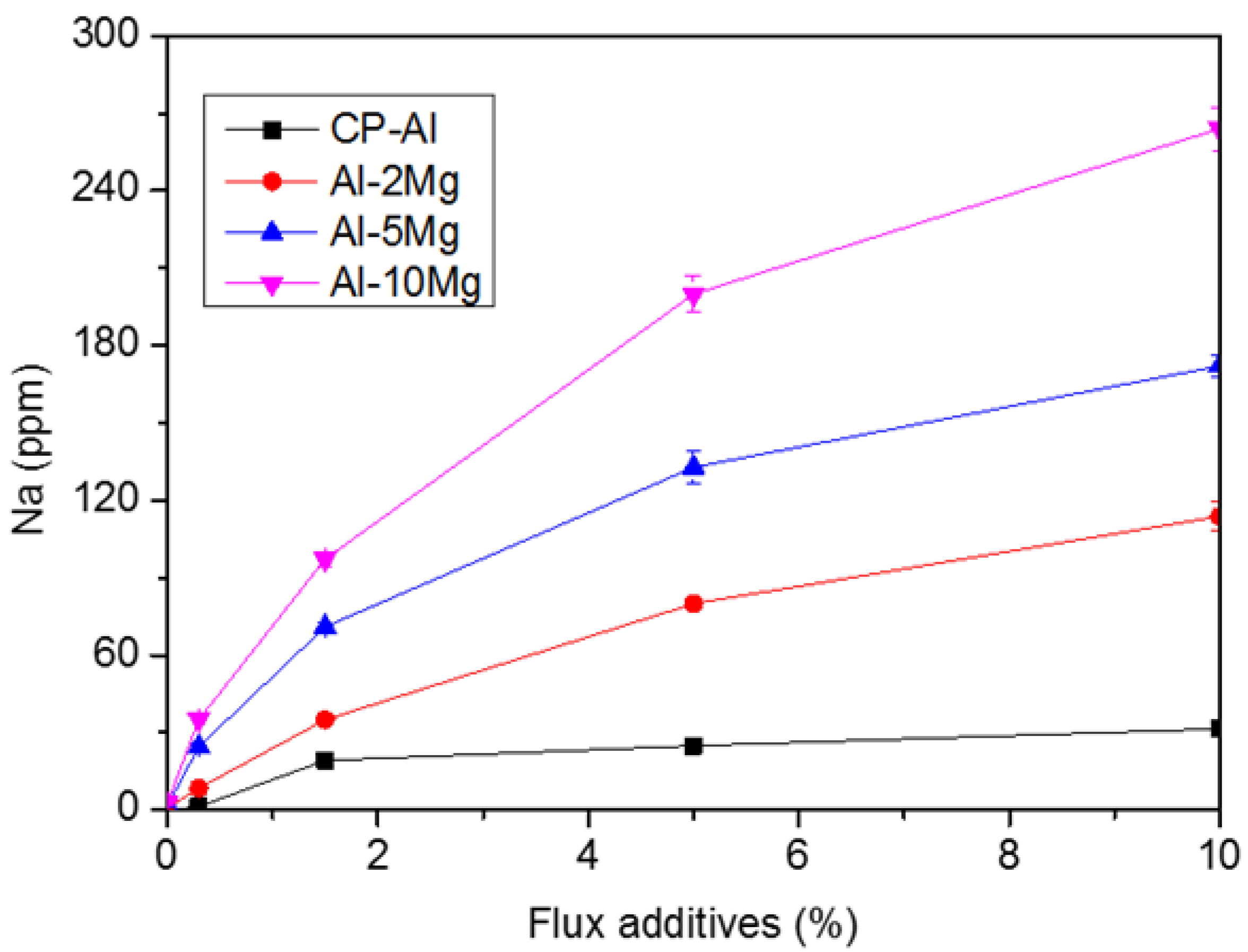
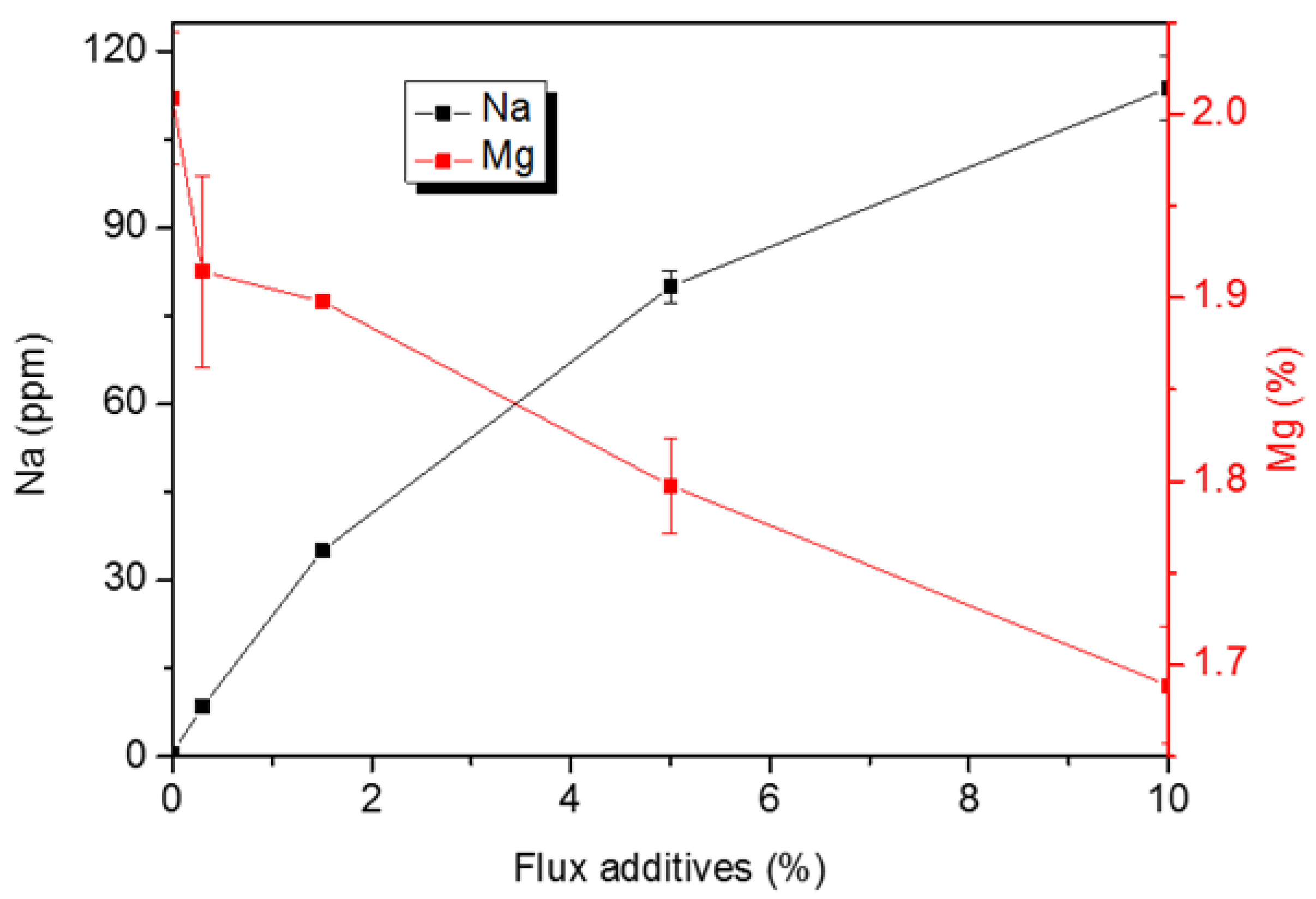
3.2. Effect of Mg Content on the Residual Content of Sodium in Al–Mg Alloys
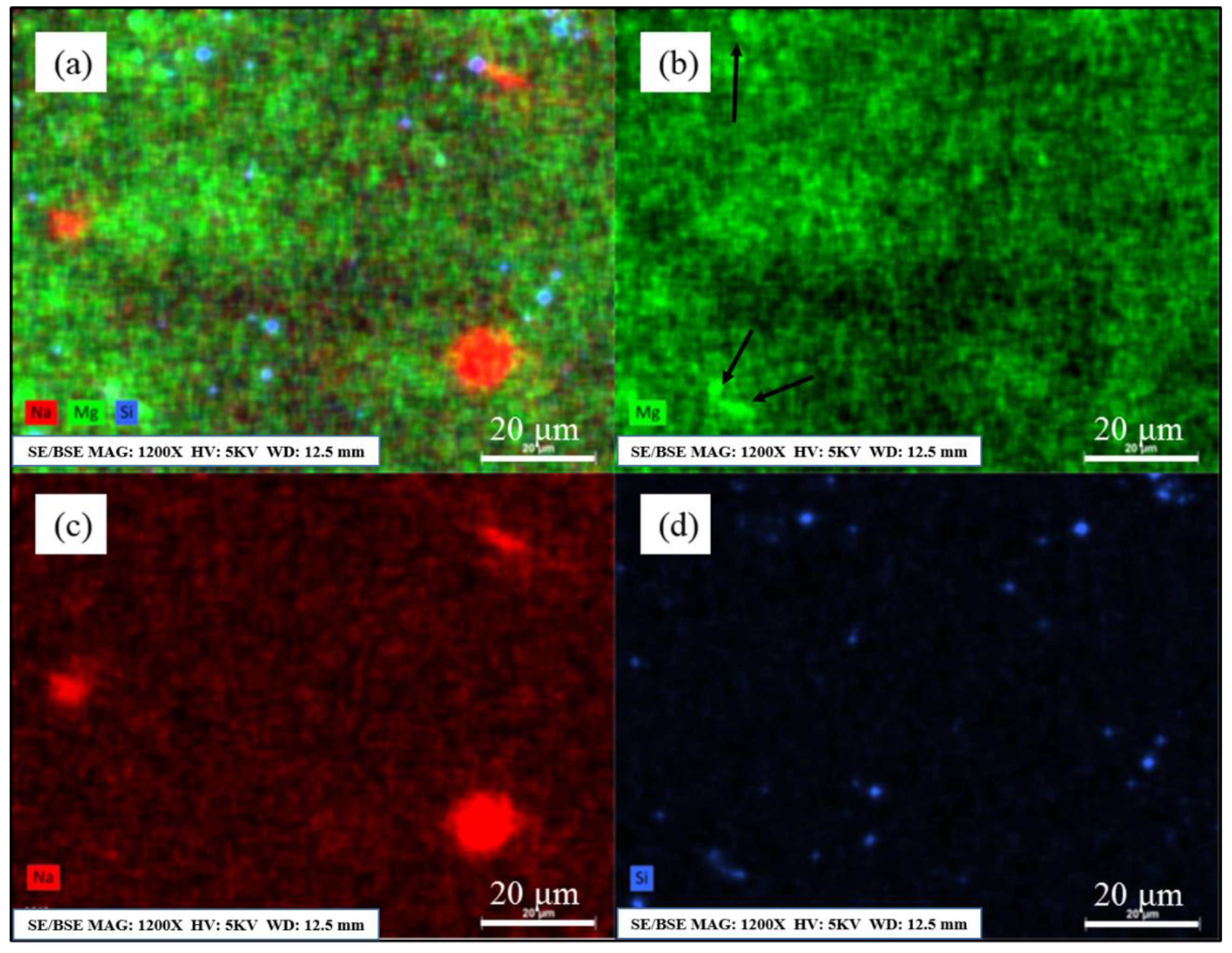
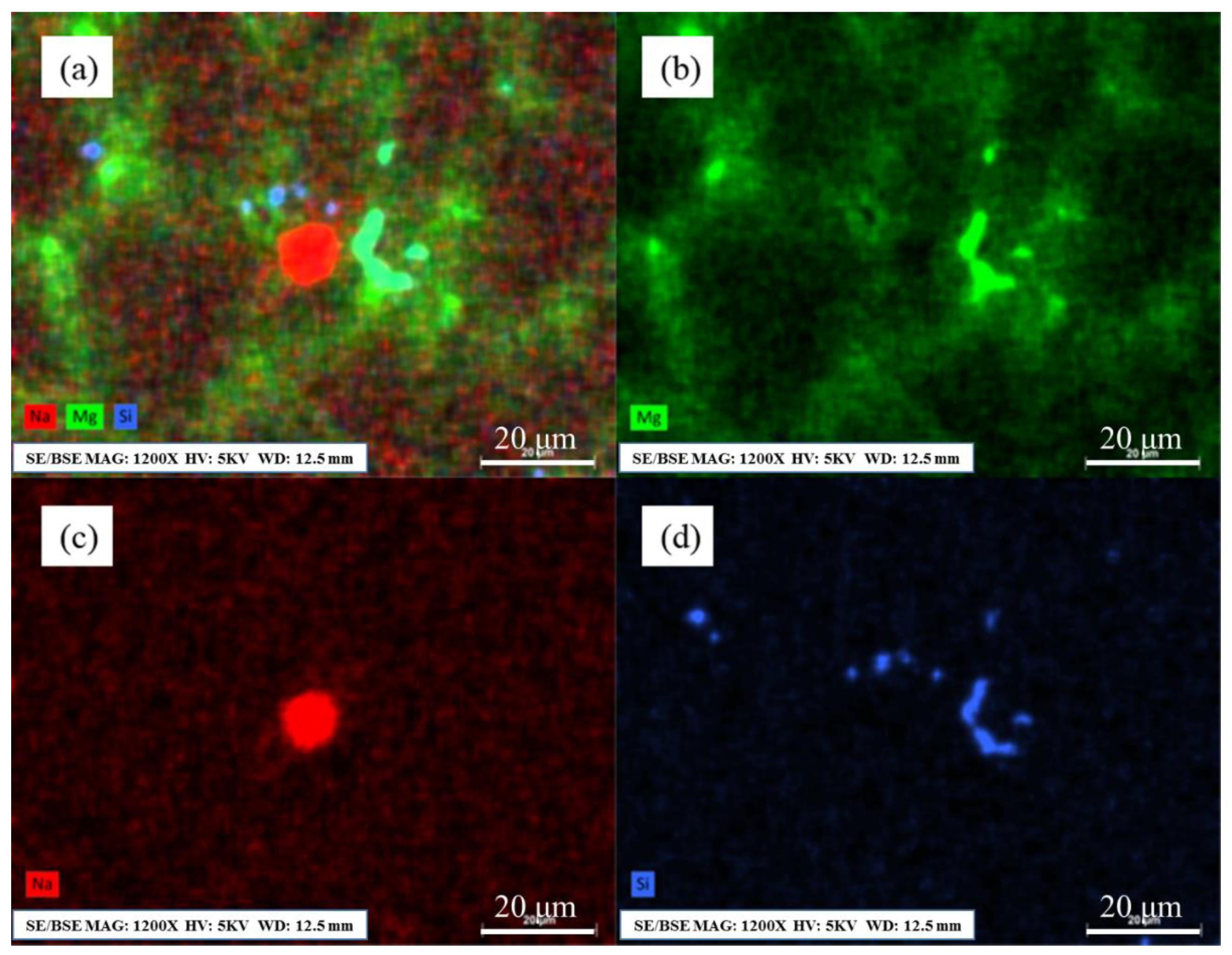
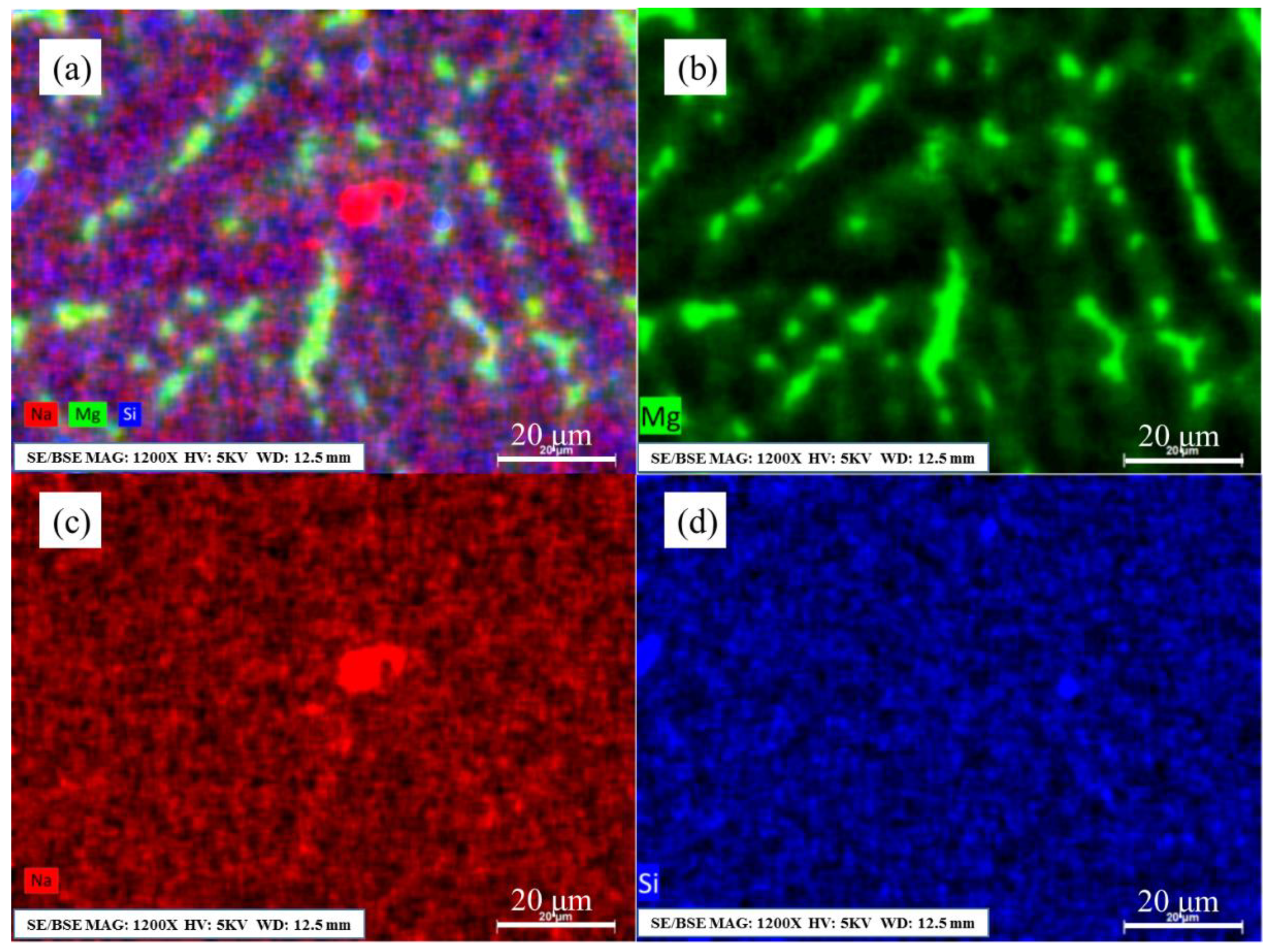
3.3. The Distribution of Residual Sodium in Al–Mg Alloys
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utigard, T.A.; Friesen, K.; Rory, R.R. The properties and uses of fluxes in molten aluminum processing. JOM 1998, 50, 38–43. [Google Scholar] [CrossRef]
- Luo, X.D.; Wu, X.F.; Zhang, Z.X.; Zhou, M.; Ding, Q.W.; Wang, J.C. Harmful Effect of Sodium and Calcium in Aluminum and Aluminum Alloys. Foundry Technol. 2015, 36, 2310–2314. [Google Scholar]
- Chi, F.Q.; Wang, L.J.; Peng, X.L. Effect of Na on casting property of Aluminum alloys. Light Alloys Fabr. Technol. 2002, 30, 14–15. [Google Scholar]
- Talbot, D.E.; Ransley, C.E. The addition of bismuth to aluminum-magnesium alloys to prevent embrittlement by sodium. Metall. Trans. A 1977, 8, 1149–1154. [Google Scholar] [CrossRef]
- Pascual, J.A. Emerging melt quality control solution technologies for aluminum melt. China Foundry 2009, 6, 358–365. [Google Scholar]
- Tremblay, S.; Desrosiers, L.; Levesque, D. Use of a binary salt flux of NaCl and MgCl2 for The Purification of Aluminum or Aluminum Alloys, And Method Thereof. U.S. Patent 7988763B2, 2 August 2011. [Google Scholar]
- Li, C.; Li, J.G.; Mao, Y.Z.; Ji, J.C. Mechanism to remove oxide inclusions from molten aluminum by method solid fluxes refining. China Foundry 2017, 14, 233–243. [Google Scholar] [CrossRef]
- Song, L.L.; Fu, G.S.; Chen, H.L.; Wang, H.S.; Lin, C.S. Purification of NaCl-KCl Based Inclusion Removal Flux on A356 Aluminum Alloy Melt and Structure and Properties after Purification. Mater. Mech. Eng. 2019, 43, 24–28. [Google Scholar]
- Horikawa, K.; Kuramoto, S.; Kanno, M. Intergranular fracture caused by trace impurities in an Al–5.5 mol.% Mg alloy. Acta Mater. 2001, 49, 3981–3989. [Google Scholar] [CrossRef]
- Lynch, S.P. Comments on “ntergranular fracture caused by trace impurities in an Al–5.5 mol% Mg alloy”. Scr. Mater. 2002, 47, 125–129. [Google Scholar] [CrossRef]
- Feng, L.; Sun, Y.M.; Zhao, X.L.; Wang, R.M.; Zhang, K.; Yang, J.Y. Existing form and harmful effects of sodium in Al–4.5%Cu alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3553–3559. [Google Scholar] [CrossRef]
- Sweet, E.D.; Lynch, S.P. Effects of alkali-metal impurities on fracture toughness of 2090 Al-Li-Cu extrusions. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1996, 27, 3530–3541. [Google Scholar] [CrossRef]
- Raja, R.R.; Torstein, A.; Utigard, T.A. Interfacial tension between aluminum and NaCl-KCl-based salt systems. Metall. Mater. Trans. B 1998, 29, 281–287. [Google Scholar]
- Friesen, K. Coalescence Behaviour and Inclusion Removal in Molten Aluminum Using Salt Fluxing; University of Toronto: Toronto, ON, Canada, 1996. [Google Scholar]
- Baxmann, K.; Bornand, J.; Leconte, B. Impact of purification methods on inclusion and melt loss. Light Metals TMS-AIME 1977, 2, 191–206. [Google Scholar]
- Friedrich, B.; Marcus, G.; Joachim, K.; Alexander, A. Improved Aluminium Recovery at Recycling Plants by integrated Slag Refining. In Proceedings of the European Metallurgical Conference, Friedrichshafen, Germany, 18–21 September 2001; Volume 2, pp. 121–139. [Google Scholar]
- Sydykov, A.; Friedrich, B.; Arnold, A. Impact of parameter changes on the aluminum recovery in a rotary kiln. In Proceedings of the Sessions, TMS Annual Meeting, Warrendale, PA, USA, 17-21 February 2002; pp. 1045–1052. [Google Scholar]
- Wang, X.; Peterson, R.D.; Richards, N.E. Dissolved metals in cryolitic melts. Essent. Read. Light Met. Alum. Reduct. Technol. 1991, 2, 49–56. [Google Scholar]
- Abdessameud, S.; Medraj, M. Understanding the hydrogen storage behavior of promising Al–Mg–Na compositions using thermodynamic modeling. Mater. Renew. Sustain. Energy 2016, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.J.; Han, Q.Y.; Liu, Z.K. Thermodynamic modeling of the Al-Mg-Na system. J. Alloys Compd. 2006, 419, 91–97. [Google Scholar] [CrossRef]
- Uhlmann, D.R.; Chalmers, B.; Jackson, K.A. Interaction Between Particles and a Solid-Liquid Interface. J. Appl. Phys. 1964, 35, 2986–2993. [Google Scholar] [CrossRef]

| Flux Name | Component |
|---|---|
| NK | 50 wt.%NaCl + 50 wt.%KCl |
| NK–Na3AlF6 | 47.5 wt.%NaCl + 47.5 wt.%KCl + 5 wt.% Na3AlF6 |
| NK–NaF | 47.5 wt.%NaCl + 47.5 wt.%KCl + 5 wt.%NaF |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Liu, Z.; Huang, J.; Liu, Q.; Li, J. Effect of Sodium-Containing Fluxes on the Residual Sodium Content and Distribution in Al–Mg Alloys. Metals 2021, 11, 1591. https://doi.org/10.3390/met11101591
Huang C, Liu Z, Huang J, Liu Q, Li J. Effect of Sodium-Containing Fluxes on the Residual Sodium Content and Distribution in Al–Mg Alloys. Metals. 2021; 11(10):1591. https://doi.org/10.3390/met11101591
Chicago/Turabian StyleHuang, Chunfa, Zhiguo Liu, Jianxian Huang, Qiwen Liu, and Jianguo Li. 2021. "Effect of Sodium-Containing Fluxes on the Residual Sodium Content and Distribution in Al–Mg Alloys" Metals 11, no. 10: 1591. https://doi.org/10.3390/met11101591






