The Role of Selenium on the Formation of Spheroidal Graphite in Cast Iron
Abstract
:1. Introduction
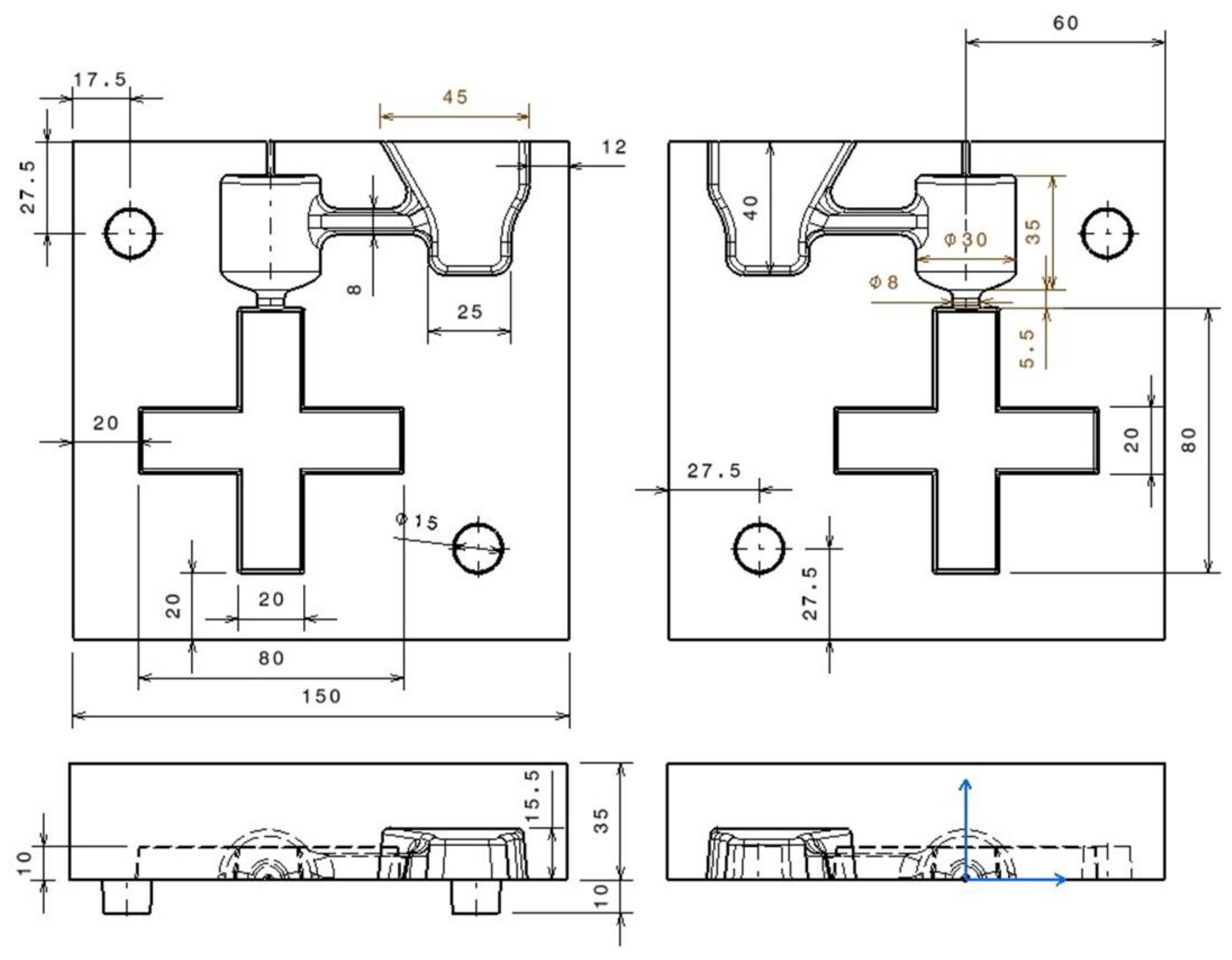
2. Materials and Methods
3. Results and Discussion
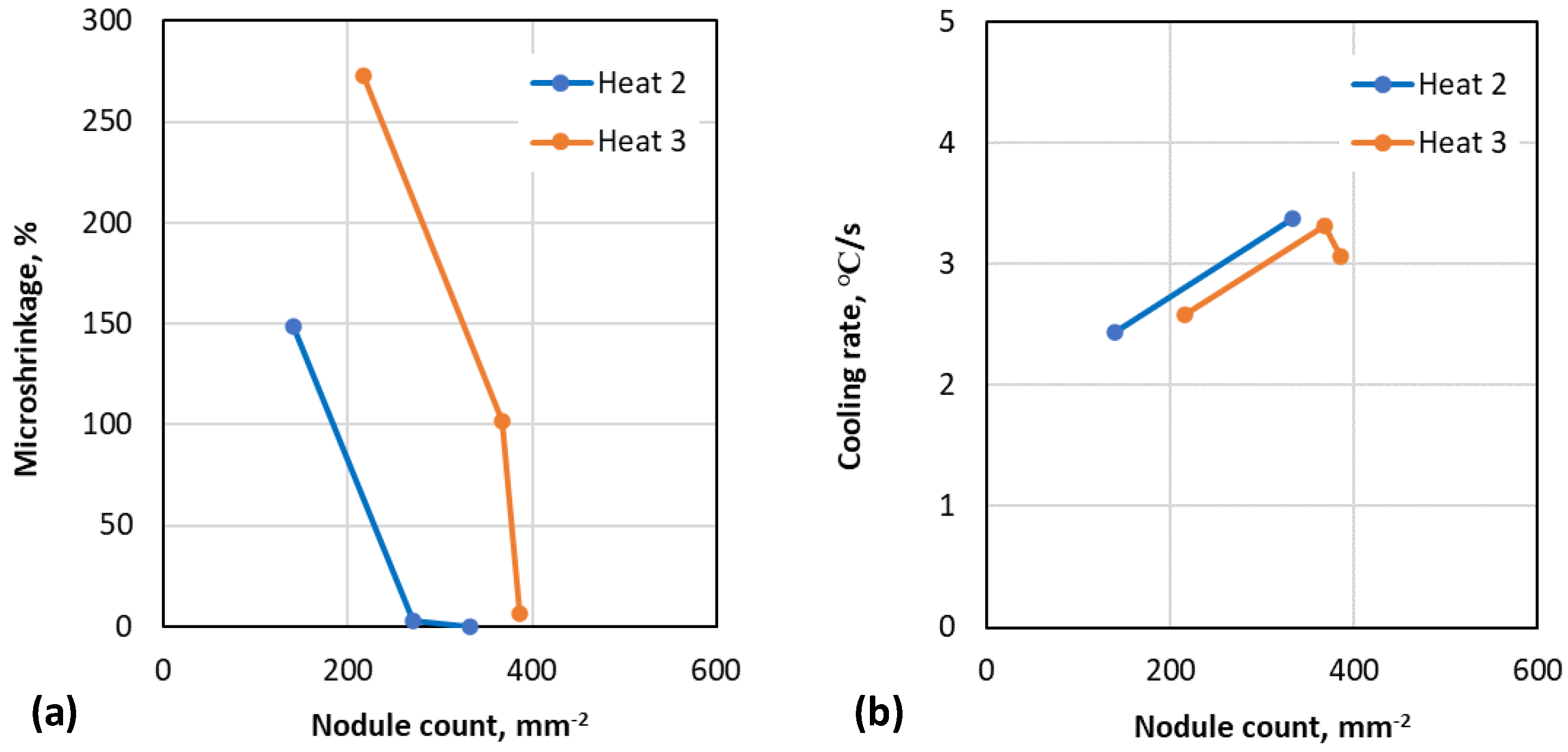
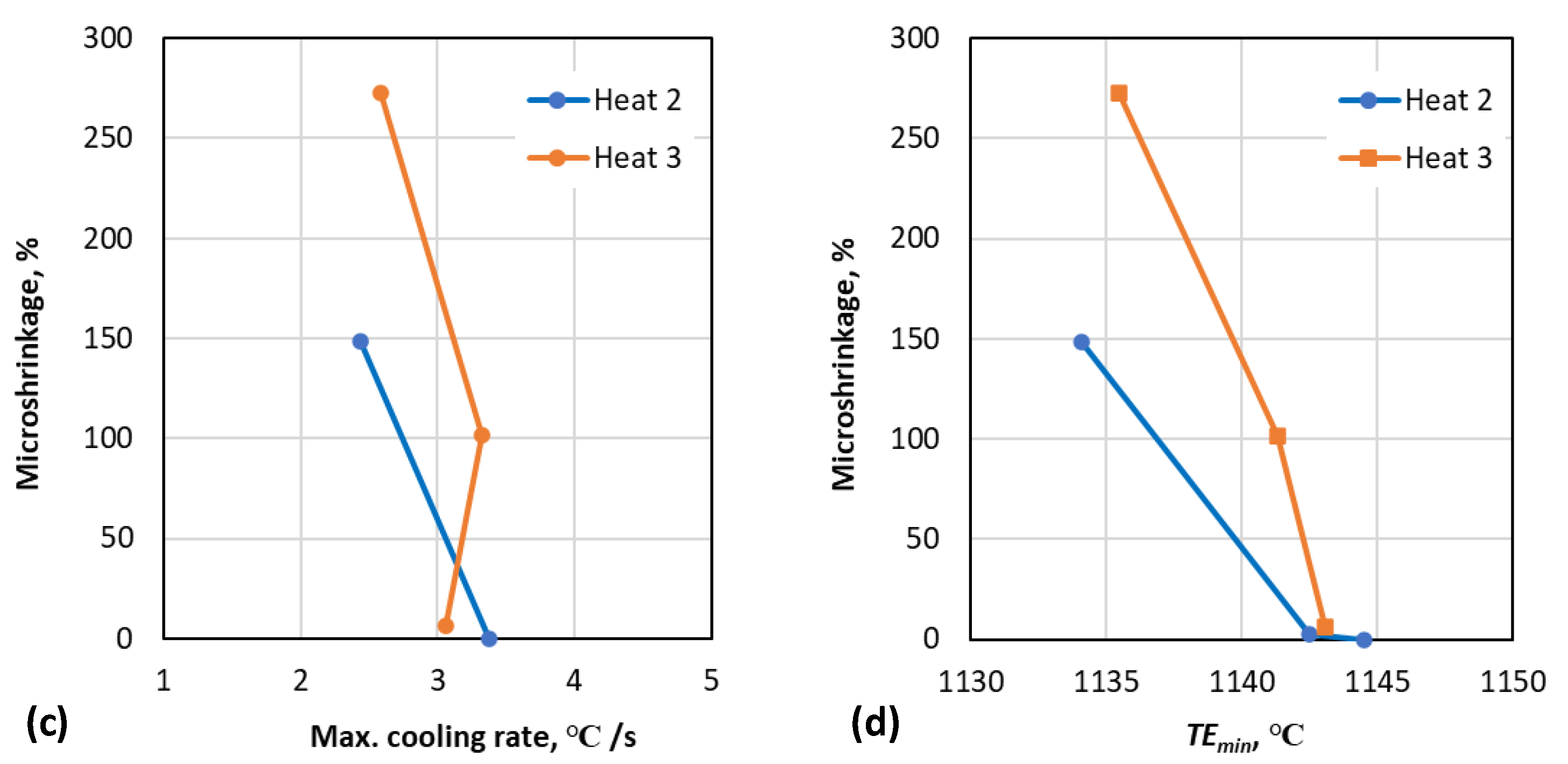
3.1. Correlation between Cooling Curve Parameters, Nodule Count and Porosity
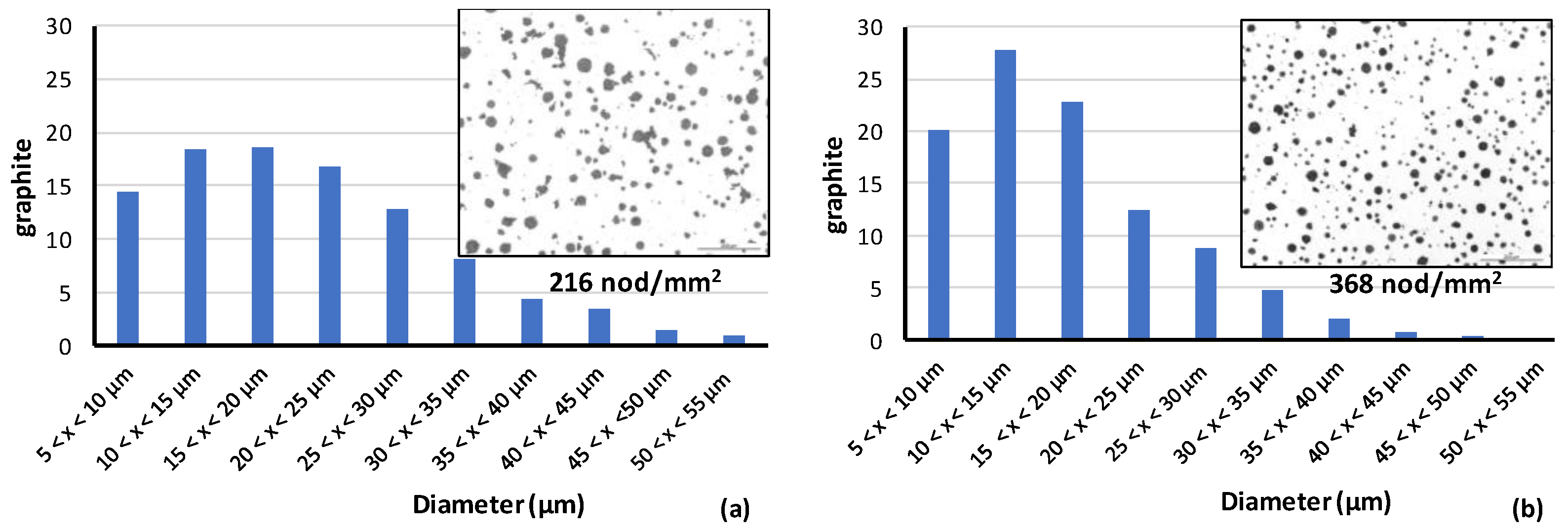
3.2. Size Distribution of Graphite
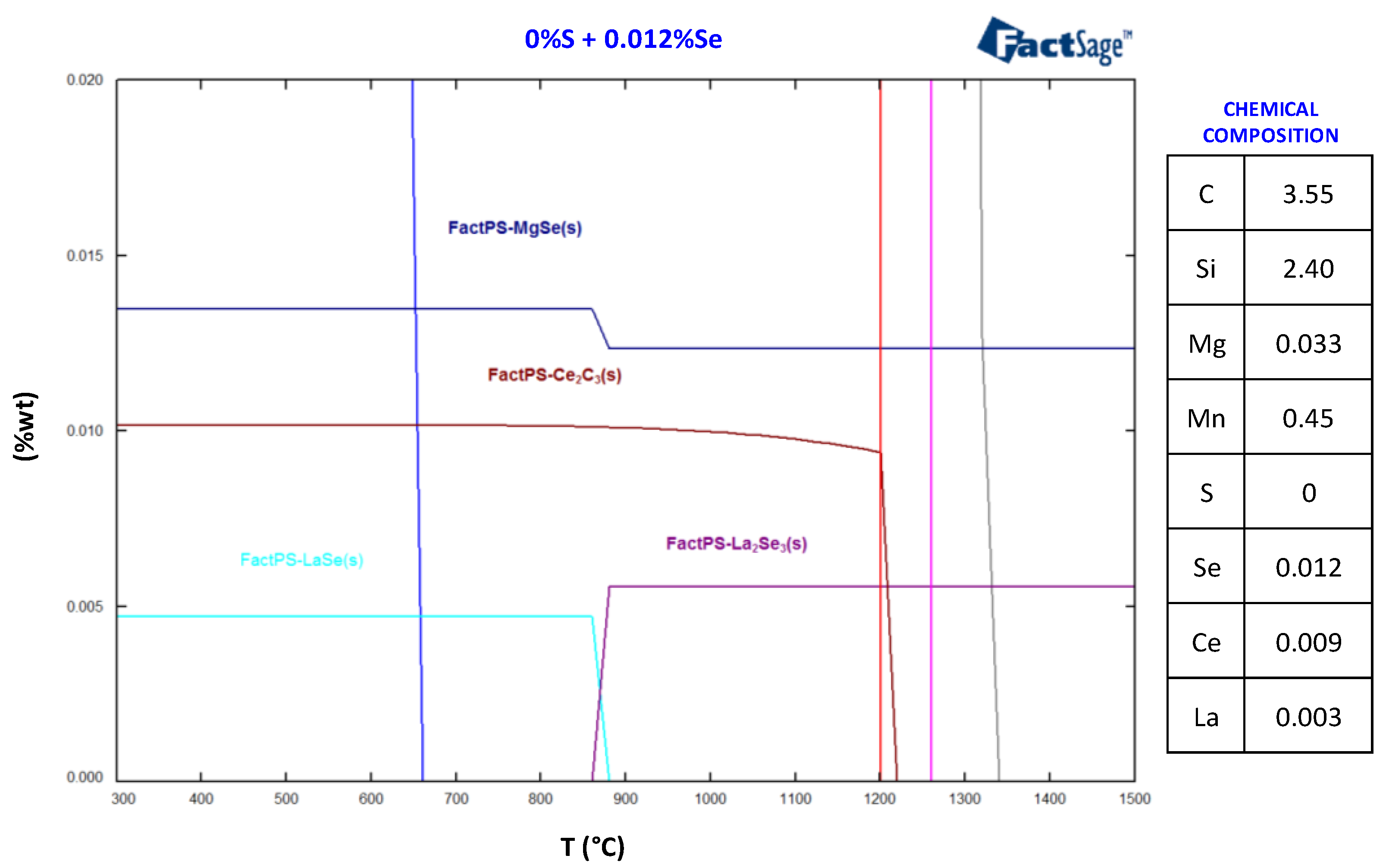
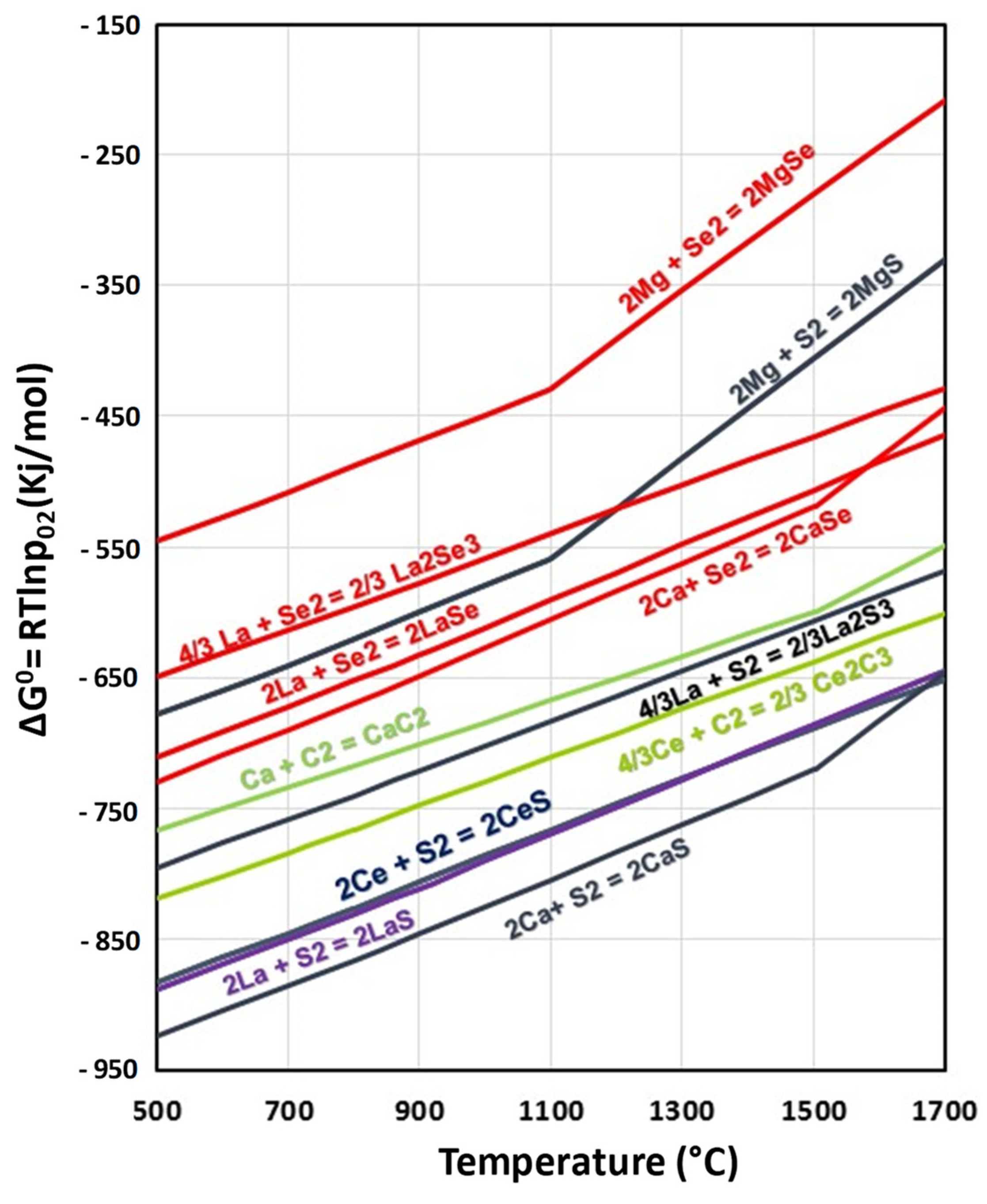
3.3. Thermodynamics Calculations
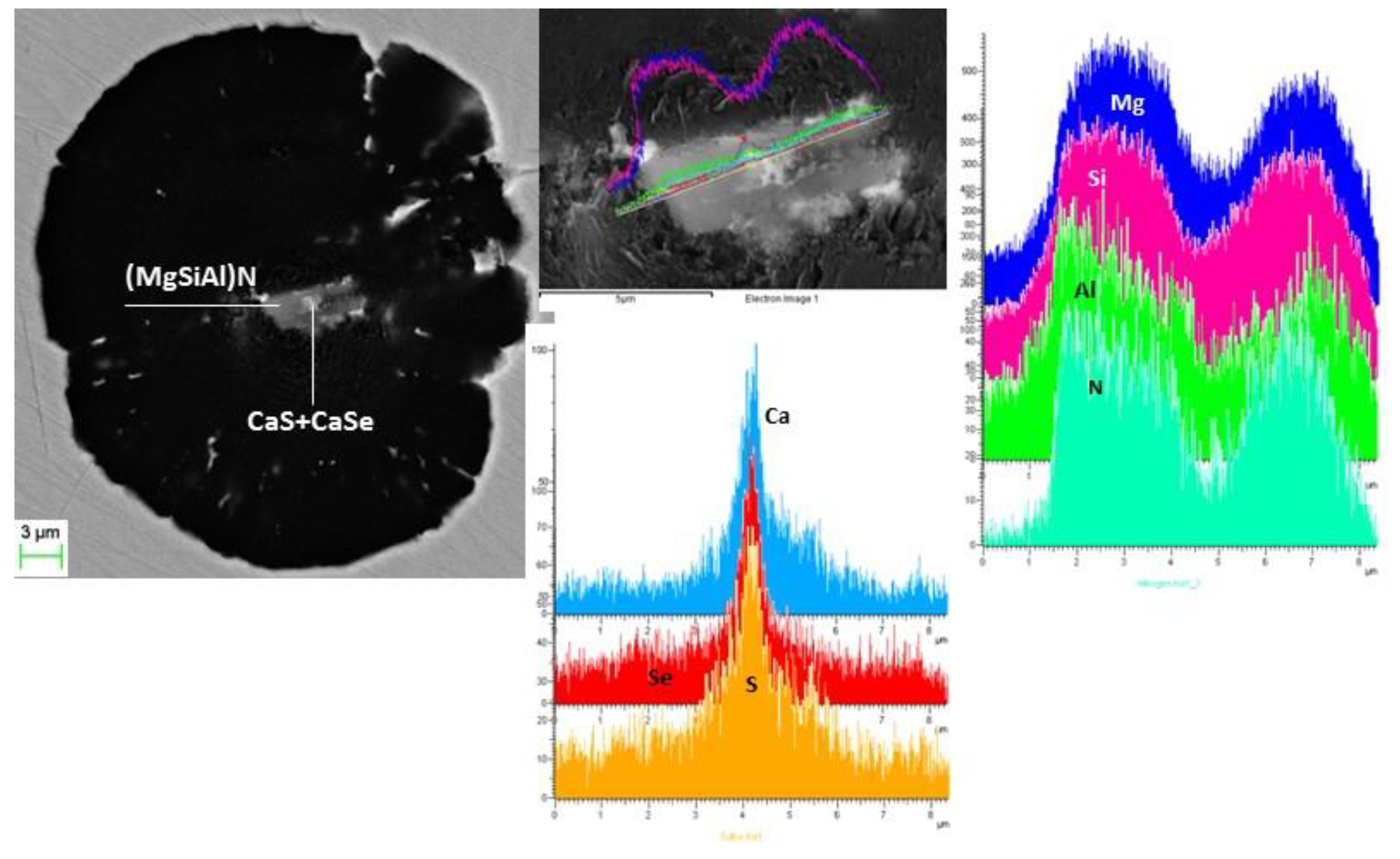
3.4. Nature of Nuclei
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warrick, R. Spheroidal Graphite Nuclei in Rare Earth and Magnesium Inoculated Irons. AFS Cast Met. Res. J. 1966, 2, 97–108. [Google Scholar]
- Lalich, M.; Hitchings, J.R. Characterization of inclusions as nuclei for spheroidal graphite in ductile cast iron. AFS Trans. 1976, 84, 653–664. [Google Scholar]
- Alonso, G.; Stefanescu, D.M.; De la Fuente, E.; Larrañaga, P.; Suárez, R. The Influence of Trace Elements on the Nature of the Nuclei of the Graphite in Ductile Iron. In Proceedings of the SPCI_XI, Jönköping, Sweden, 3–6 September 2017. [Google Scholar]
- Skaland, T. Nucleation Mechanism in Ductile Iron. In Proceedings of the AFS Cast Iron Inoculation Conference, Schaumburg, IL, USA, 29–30 September 2005. [Google Scholar]
- Jacobs, M.; Law, T.J.; Melford, D.A.; Stowell, M.J. Identification of heterogeneous nuclei for graphite spheroids in chill-cast iron. Met. Technol. 1976, 3, 98–108. [Google Scholar]
- Jacobs, M.; Law, T.J.; Melford, D.A.; Stowell, M.J. Basic Processes Controlling the Nucleation of Graphite Nodules in Chill Cast Iron. Met. Technol. 1974, 1, 490–500. [Google Scholar] [CrossRef]
- Askeland, D.; Trojan, P.K. Microstructure control in molybdenum ductile irons. AFS Trans. 1969, 77, 344–352. [Google Scholar]
- Askeland, D.; Trojan, P.K.; Flinn, R.A. Investigation of Mechanism of Dross Formation in Ductile Iron. AFS Trans. 1970, 78, 125–132. [Google Scholar]
- Stransky, K.; Rek, A. Contribution to the Microanalysis of Graphite Nuclei in Spheroidal Graphite Iron. Giess. Rundsch. 1969, 6, 32. [Google Scholar]
- Mercier, J.; Paton, R.; Margerie, J.C.; Mascré, C. Inclusions in Spheroidal Graphite. Fonderie 1969, 191. [Google Scholar]
- Feest, G.A.; McHugh, G.; Morton, D.O.; Welch, L.S.; Cook, I.A. Proc. Sol. Tech. in the Foundry and Casthouse; The Metals Society: London, UK, 1983; pp. 232–239. [Google Scholar]
- Muzumdar, K.; Wallace, J.F. Effect of Sulfur in Cast Iron. AFS Trans. 1973, 81, 412–423. [Google Scholar]
- Riposan, I.; Chisamera, M.; Kelley, R.; Barstow, M.; Naro, R.L. Magnesium-Sulfur Relationships in Ductile and Compacted Graphite Cast Irons as Influenced by Late Sulfur Additions. AFS Trans. 2003, 93, 869–883. [Google Scholar]
- Suárez, O.M.; Kendrick, R.D.; Loper, C.R., Jr. Late sulfur inoculation of spheroidal graphite cast irons. Int. J. Cast Met. Res. 2003, 16, 1–6. [Google Scholar] [CrossRef]
- Suárez, O.M.; Kendrick, R.D.; Loper, C.R., Jr. A study of sulphur effect in high silicon ductile irons. Int. J. Cast Met. Res. 2000, 13, 135–145. [Google Scholar] [CrossRef]
- Nakae, H.; Igarashi, Y. Influence of Sulfur on Heterogeneous Nucleus of Spheroidal Graphite. Mater. Trans. 2002, 43, 2826–2831. [Google Scholar] [CrossRef]
- George, M.W. Selenium and Tellurium. In U.S. Geological Survey Minerals Yearbook; U.S. Geological Survey: Liston, VA, USA, 2004. [Google Scholar]
- Naumov, A.V. Selenium and Tellurium:State of the Markets, the Crisis and its Consequences. Metallurgist 2010, 54, 197. [Google Scholar] [CrossRef]
- Kurka, V.; Pindor, J.; Hudzieczek, Z.; Adolf, Z.; Cienciala, J. Effect of Selenium on Metallographic Purity and Formability. Metal 2013, 5, 15–17. [Google Scholar]
- Aborn, R.H. The Role of Selenium and Tellurium in Ferrous Metals. In Symposium on Metallurgy of substitute Ferrous & Non-Ferrous Alloys; NML: Jamshedpur, India, 27–30 April 2011. [Google Scholar]
- Horie, H. Inhibitory Effects of Tellurium and Selenium on the Formation of Spheroidal Graphite in Cast Iron. J. Jpn. Foundrymen Soc. 1975, 47, 836–840. [Google Scholar]
- Borgs, S.; Stets, W. Porosity in Ductile Cast Iron and its Influence on the Mechanical Performance, under Cyclic Loading. Mater.-Cast. Plant Technol. 2015, 3, 8–21. [Google Scholar]
- Chisamera, M.; Riposan, I.; Stan, S.; Toboc, P.; Skaland, T.; White, D. Influence of residual aluminium on solidification pattern of ductile iron. Int. J. Cast Met. Res. 2009, 22, 401–410. [Google Scholar] [CrossRef]
- Lekakh, S.N.; Hrebec, B. Solidification Kinetics of Graphite Nodules in Cast Iron and Shrinkage Porosity. Inter Met. 2016, 10, 389–400. [Google Scholar] [CrossRef]
- Skaland, T. Ductile iron shrinkage control through graphite nucleation and growth. Int. J. Cast Met. Res. 2003, 16, 11–16. [Google Scholar] [CrossRef]
- Ellingham, H. Transactions and Communications. J. Soc. Chem. Ind. 1944.
- Alonso, G.; Stefanescu, D.M.; Larrañaga, P.; De la Fuente, E.; Suarez, R. The Role of Titanium and Nitrogen in Graphite Nucleation of Ductile Iron. In Proceedings of the 5th Decennial International Conference on Solidification Processing, SP17 2017, Old Windsor, UK, 25–28 July 2017. [Google Scholar]
- Alonso, G.; Crisan, A.; Stefanescu, D.M.; Suarez, R. Effect of Titanium in the Nucleation Process of Spheroidal and Compacted Graphite Cast Iron. In Proceedings of the 11th International Conference on Materials Science & Engineering, BRAMAT 2019, Poiana Brasov, Romania, 13–16 March 2019. [Google Scholar]




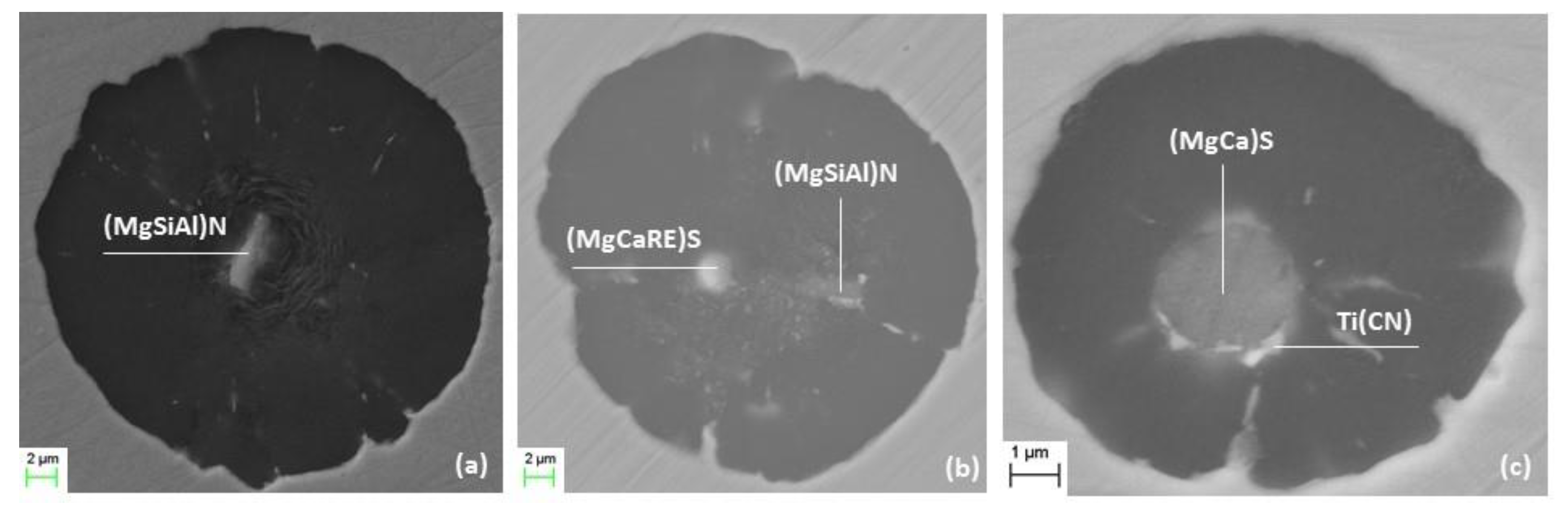
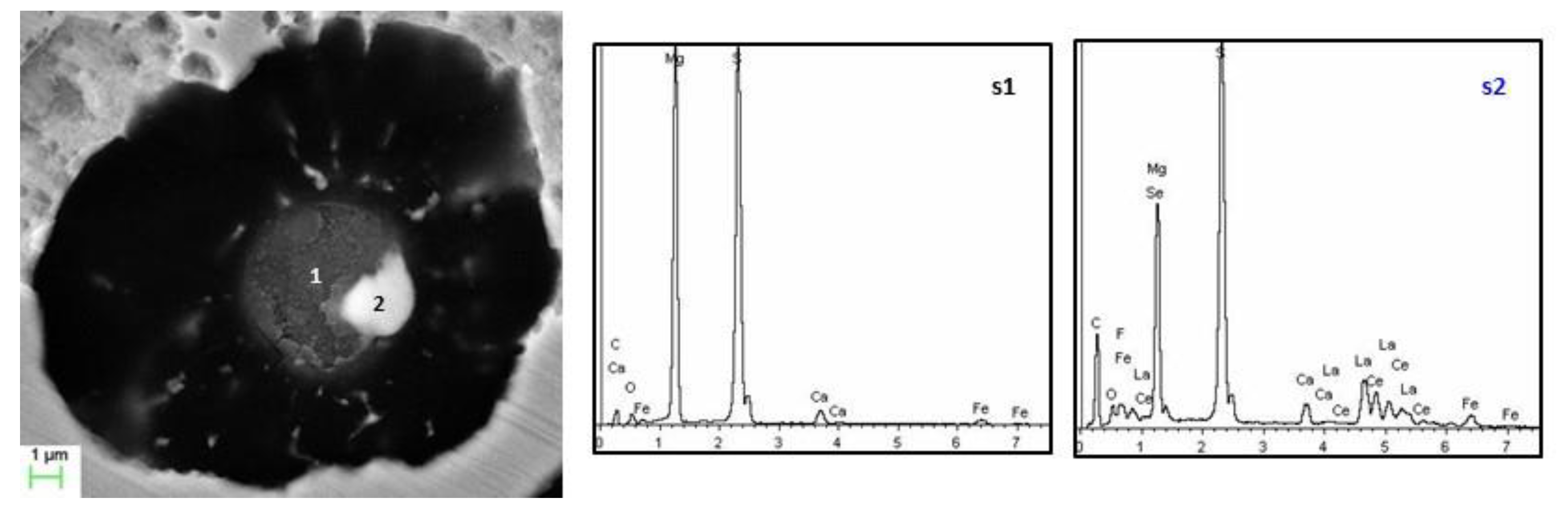
| %S in Base Iron | Nucleus Shape | dia. (µm) | Main Compounds | Other Compounds |
|---|---|---|---|---|
| 0.0022 | rectangular | 0.5–1.0 | (Mg,Si,Al) N | MgS, MgO, (Ca Mg) S |
| 0.0052 | spherical | 0.5–1.0 | (Mg,Ca) S | MgO, (Mg,Si,Al) N |
| 0.013 | spherical | 0.5–1.0 | (Mg,Ca) S | MgO, (Mg,Si,Al) ON |
| 0.050 | spherical | 1.0–2.0 | (Mg,Ca) S | MgO, (La,Ce,Nd) S |
| 0.072 | spherical | 1.5–5.0 | (Mg,Ca) S | MgO, (La,Ce,Nd) S |
| 0.083 | spherical/faceted | 1.5–5.0 | (Mg,Ca,Mn) S | MgO, (La,Ce,Nd) S |
| Properties | Sulfur | Selenium | Tellurium |
|---|---|---|---|
| Density (kg/m3) | 1960 | 4790 | 6240 |
| Structure | orthorhombic | hexagonal | hexagonal |
| Melting Point (K) | 388.36 | 494 | 722.66 |
| Boling Point (K) | 717.87 | 957.8 | 1261 |
| Specific Heat (J/Kg·K) | 710 | 320 | 202 |
| Thermal Conductivity (W/k·m) | 0.269 | 2.04 | 2.35 |
| Heat | C | Si | P | S | Mg | Mn | Cu | Ti |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.55 | 2.39 | 0.014 | 0.003 | 0.034 | 0.55 | 0.14 | 0.020 |
| 2 | 3.54 | 2.42 | 0.016 | 0.005 | 0.032 | 0.4 | 0.19 | 0.022 |
| 3 | 3.58 | 2.40 | 0.015 | 0.005 | 0.032 | 0.4 | 0.21 | 0.021 |
| Heat | Inoc. | Nod/mm2 | TL °C | TEmin °C | ∆Trecal °C | TS °C | CRmax °C/s | Microshrinkage mm3 |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.8 Ce | 255 | 1146.8 | 1141.3 | 5.6 | 1118.6 | 3.80 | 11.22 |
| 1.8 Ce + Se | 377 | 1149 | 1147.4 | 2.9 | 1125 | 3.88 | 22.26 | |
| 2 | none | 140 | 1135.9 | 1134.1 | 3.5 | 1104.6 | 2.44 | 148.46 |
| 1.8 Ce | 270 | 1148.2 | 1142.5 | 5.7 | 1125.1 | - | 2.85 | |
| 1.8 Ce + Se | 333 | 1152.2 | 1144.5 | 7.8 | 1127.3 | 3.38 | 0 | |
| 3 | none | 216 | 1139.7 | 1135.5 | 4.1 | 1106.1 | 2.58 | 272.82 |
| 1.8 Ce | 368 | 1148.5 | 1141.3 | 7.2 | 1125.4 | 3.32 | 101.82 | |
| 1.8 Ce + Se | 386 | 1149.6 | 1143.1 | 6.5 | 1124.5 | 3.06 | 6.50 |
| Heat | Inoc. | No. Nuclei | Oxides | Sulfides | (MgSiAl) N | Ti (CN) | Graph. with RE | Graph. with Se |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.8 Ce | 20 | 4% | 40% | 33% | 25% | 25% | 0% |
| 1.8 Ce + Se | 20 | 0% | 57% | 30% | 13% | 50% | 60% | |
| 2 | 1.8 Ce | 20 | 7% | 46% | 36% | 11% | 35% | 0% |
| 1.8 Ce + Se | 20 | 3% | 47% | 29% | 21% | 50% | 50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso, G.; Stefanescu, D.M.; Aguado, E.; Suarez, R. The Role of Selenium on the Formation of Spheroidal Graphite in Cast Iron. Metals 2021, 11, 1600. https://doi.org/10.3390/met11101600
Alonso G, Stefanescu DM, Aguado E, Suarez R. The Role of Selenium on the Formation of Spheroidal Graphite in Cast Iron. Metals. 2021; 11(10):1600. https://doi.org/10.3390/met11101600
Chicago/Turabian StyleAlonso, Gorka, Doru Michael Stefanescu, Edurne Aguado, and Ramon Suarez. 2021. "The Role of Selenium on the Formation of Spheroidal Graphite in Cast Iron" Metals 11, no. 10: 1600. https://doi.org/10.3390/met11101600






