Investigations into Ti-Based Metallic Alloys for Biomedical Purposes
Abstract
:1. Introduction
2. Materials and Methods
- Ti-10Nb-10Zr-5Ta.
- Ti-20Nb-20Zr-4Ta.
- Ti-29.3Nb-13.6Zr-1.9Fe.
- metallic Ti with controlled oxygen content (Ti grade 3), with a composition according to DIN 3.7055, containing 0.30% Fe, 0.05% N2, 0.25% O2, max. 0.013% H2, 0.10% C, balance Ti, provided by ZIROM Giurgiu, Romania.
- metallic Nb, 99.81% containing: 0.005% Fe; 0.005% Si; 0.010% Mo; 0.010% W; 0.002% Ti; 0.002% Cr; 0.1% Ta; 0.005% Ni; 0.02% O2; 0.02% C; 0.0015% H2; 0.015% N2; balance Nb (Sigma Aldrich, Burlington, MA, USA).
- metallic Zr, 99.6% with the following composition: 0.01% Fe; 0.035% Si; 0.03% Mo; 0.05% W; 0.01% Ti; 0.02% Ni; 0.02% O2; 0.01% C; 0.0015% H2; 0.01% N2; 0.2% Nb; balance Zr, provided by ZIROM Giurgiu, Romania.
- metallic Ta, 99.59% with the following composition: 0.01% Fe; 0.05% Si; 0.02% Mo; 0.05% W; 0.01% Ti; 0.01% Ni; 0.03% O2; 0.01% C; 0.0015% H2; 0.01% N2; 0.2% Nb; balance Ta (Alfa Aesar Chemicals, Ward Hill, MA, USA).
- Fe, with: 0.015% C; 0.01% Si; 0.02% Mn; 0.02% S; 0.01% P; 0.015% O2; balance Fe (Sigma Aldrich, Burlington, MA, USA).
- ingot diameter: 20 mm; -vacuum (primary and secondary).
- primary vine: 4 × 10−2 torr.
- secondary vine: 9 × 10−4 torr.
- inert working atmosphere (argon): −0.3 bar.
- electrical parameters of the levitation melting furnace.
- power: 23–24 kW.
- frequency: 105–110 Hz.
- cooling of the vacuum system, generator and melting module (crucible/ingot mold): continuous.
- MICRACUT 201 precision cutting machine, with diamond disc, with variable cutting speed, table with automatic movement on the X-axis with digital positioning, power 1 kW.
- Rolling mill (Mario di Maio, LQR120AS, laboratory mill) with a power of 4 kW, with variable working speed.
- Heat treatment furnace (Nabertherm High Temperature Furnace HTC 08/16/P330 LC080K6SN, Germany) with a maximum heating temperature of 1600 °C; volume 15 l, accuracy higher than 1 °C, with the possibility of programming the treatment diagram (programming was performed in steps of 1 °C or 1 min).
- cold rolling, with the alloy samples in the cast state, cut to the appropriate thickness, deformed with a maximum degree of 10% at each pass; the total degree of deformation was specific to each type of alloy.
- recrystallization treatment, which was necessary to finish the structure of the alloys after rolling, with the following parameters: recrystallization temperature; recrystallization time (maintenance at the highest temperature); cooling in water.
- cutting the ingot in a cast state to obtain the alloy strips.
- cold rolling of the cast alloy strips.
- recrystallization of laminated strips.
3. Results and Discussion
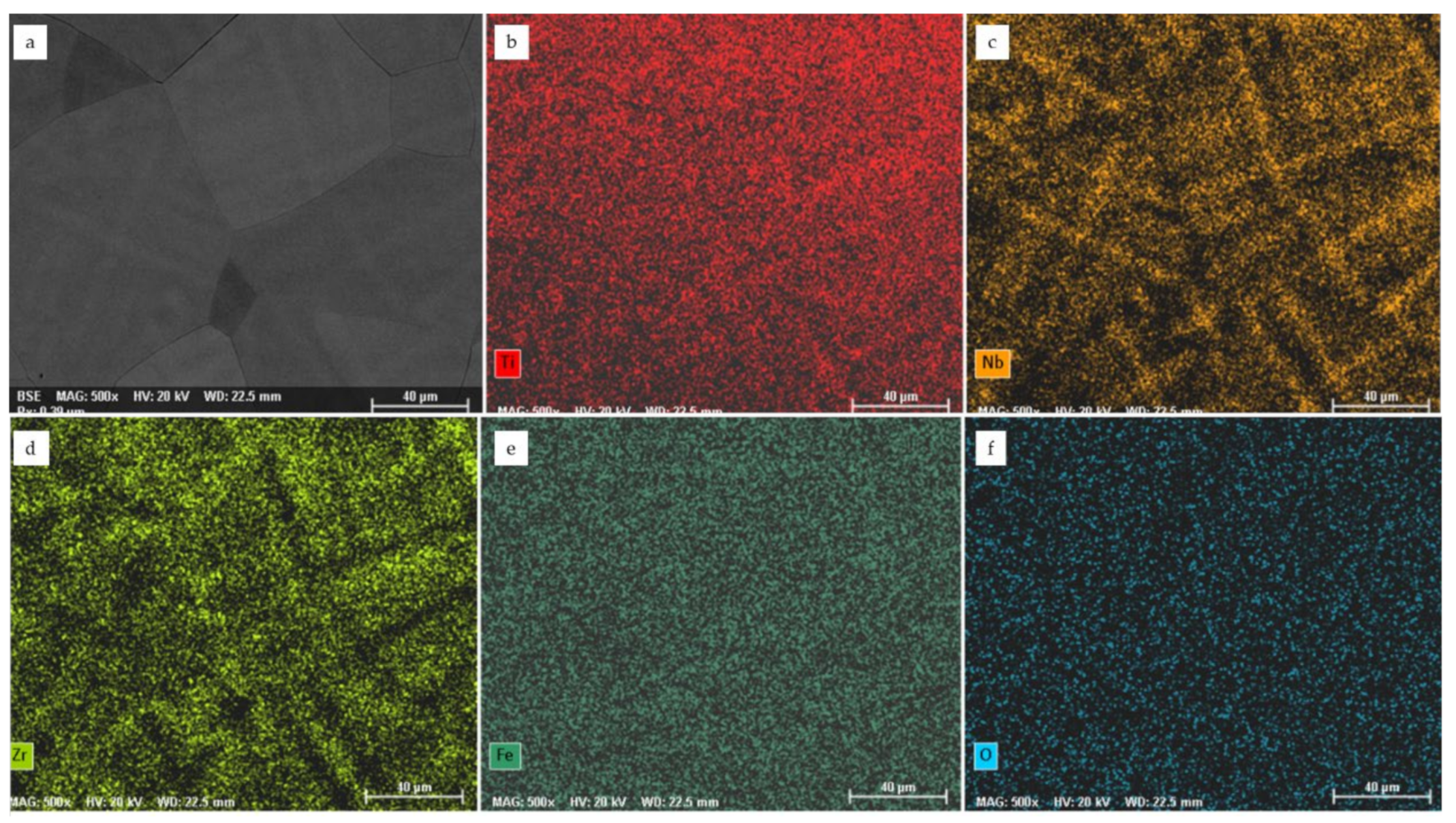
3.1. Chemical Characterization and Structural Analysis of the Cast Alloys
- -
- the elements in the alloys demonstrated great differences between their melting temperatures (from 1538 °C in the case of Fe to 1668 °C for Ti, 1855 °C for Zr, 2500 °C for Nb and about 3000 °C for Ta), which influenced the choice of synthesis process, because the temperature reached in the processing furnace needed to be high enough to obtain a homogeneous composition without un-melted metal after the solidification.
- -
- the alloys contained elements with densities with different ratios: 1:1.5, 1:1.7; 1:2 and 1:4, which made it difficult to create a uniform composition, which then required an intensive homogenization system.
- -
- the binary equilibrium diagrams of Ti with Nb, Zr, Ta and Fe highlighted their total liquid solubility with the formation of solid solutions.
- -
- heavy reactivity of the main elements in the composition of the alloys as the temperature increased towards the gases (oxygen, nitrogen, hydrogen), starting from 250 °C, with the formation of compounds that excessively hardened the alloys, developing centers of chemical and structural non-uniformity that affected the resistance corrosion.
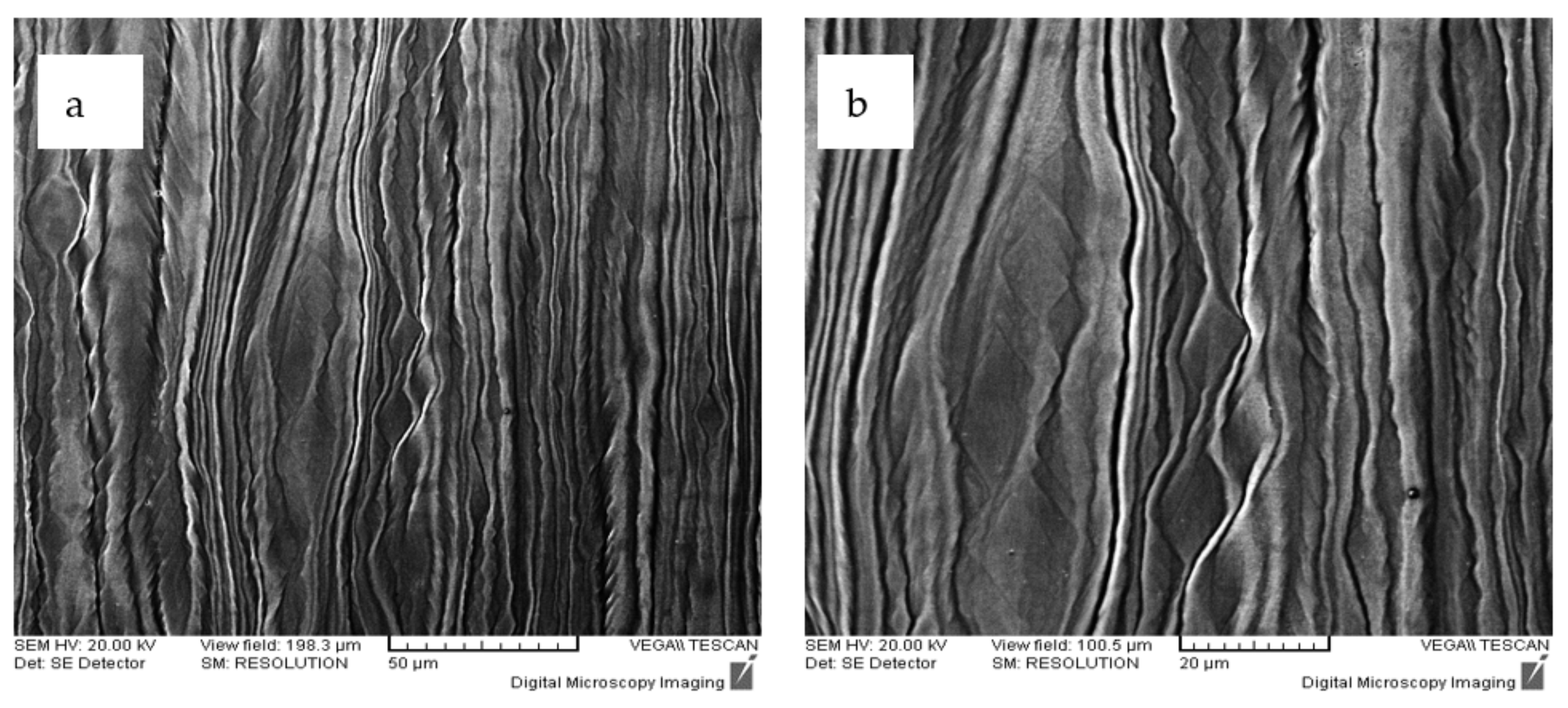
3.2. Design of the Method of Thermo-Mechanical Processing of Alloys
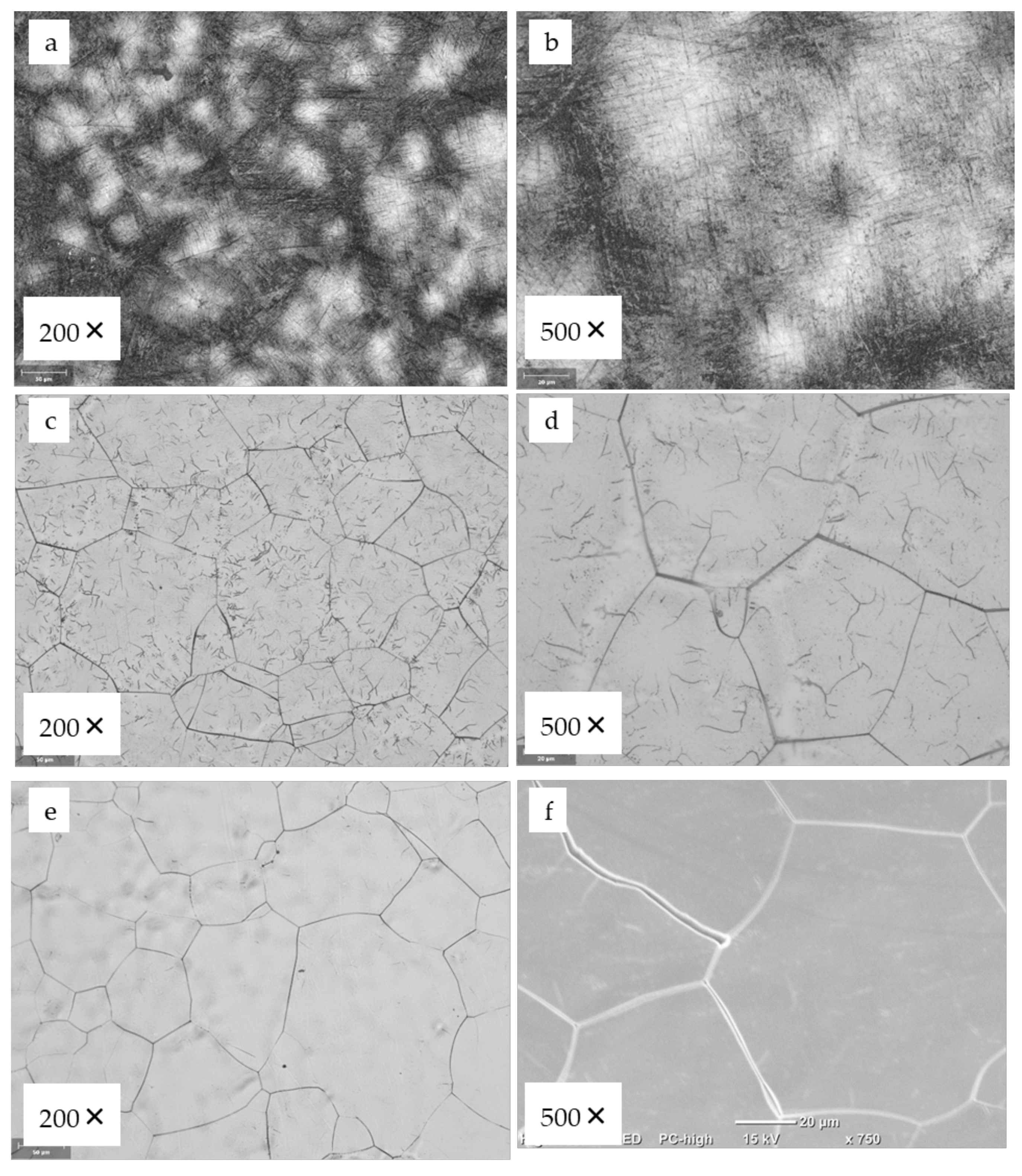
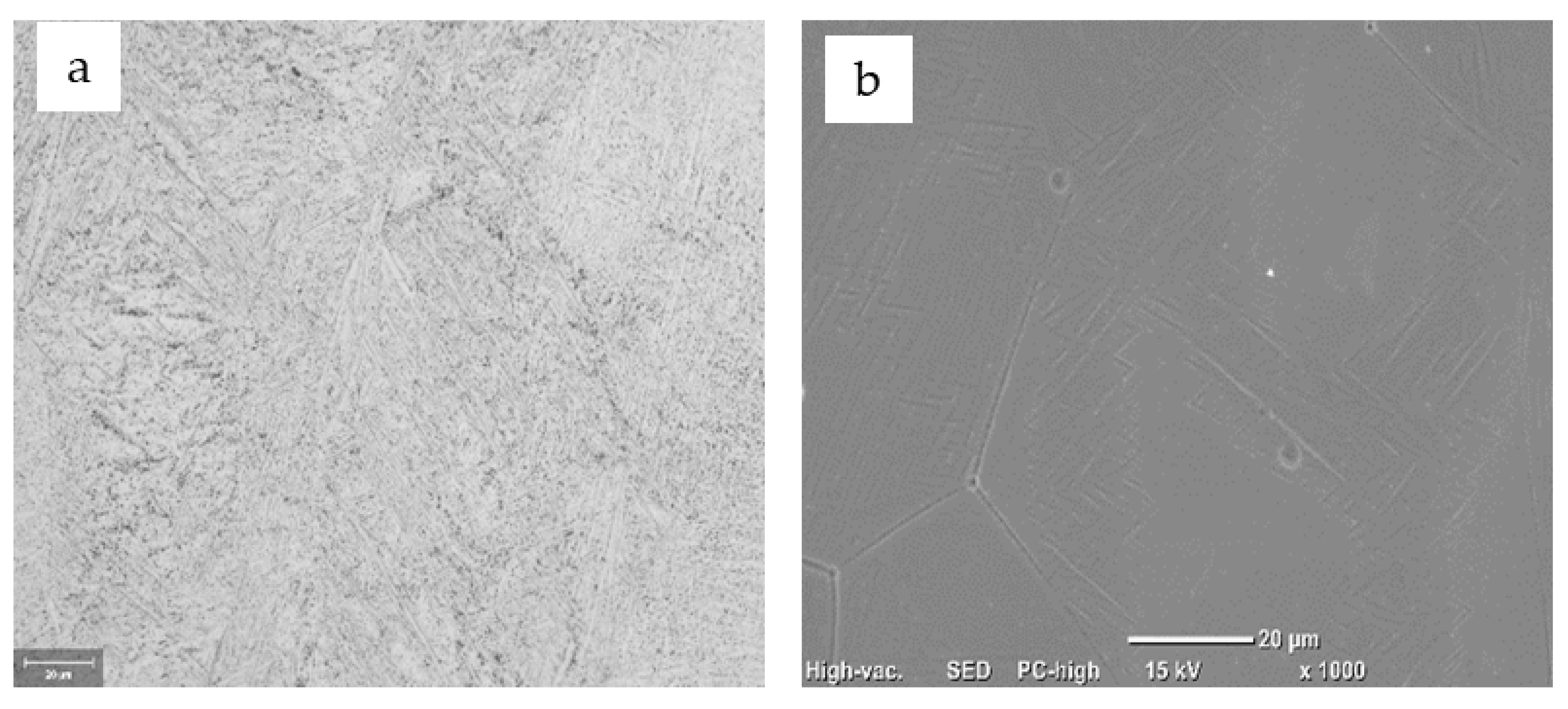
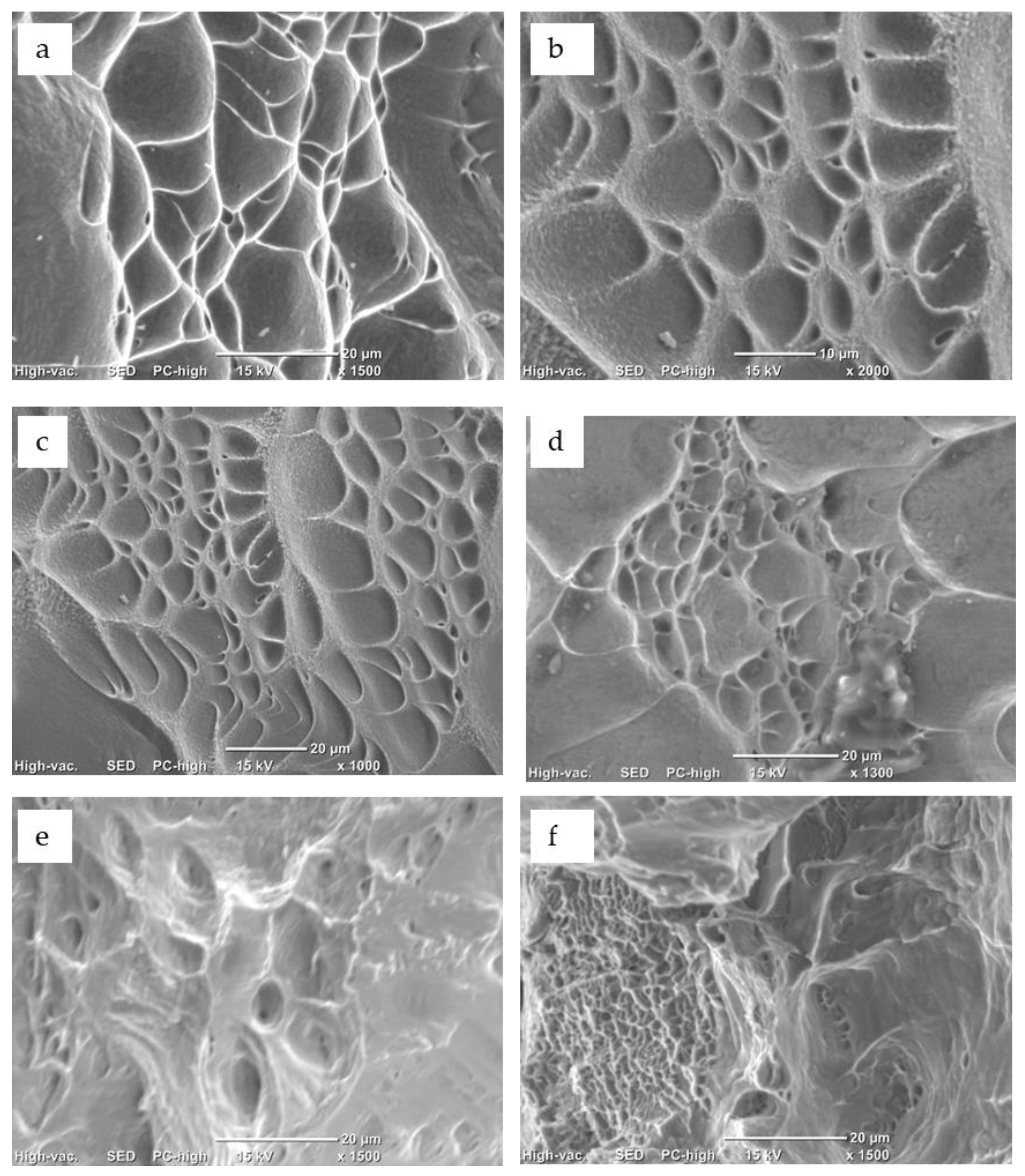
3.3. Structural Characterization of Alloys after Recrystallization Treatment
3.4. Mechanical Characterization of Processed Alloys
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials Mater. Sci. Eng. R. Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Adeosun, S.; Ilomuanya, M.; Gbenebor, O.; Dada, M.; Odili, C. Biomaterials for drug delivery: Sources, classification, synthesis, processing, and applications. In Advanced Functional Materials; IntechOpen: London, UK, 2020; pp. 141–167. [Google Scholar] [CrossRef]
- Sachot, N.; Mateos-Timoneda, M.A.; Planell, J.A.; Velders, A.H.; Lewandowska, M.; Engel, E.; Castaño, O. Towards 4th generation biomaterials: A covalent hybrid polymer–ormoglass architecture. Nanoscale 2015, 7, 15349–15361. [Google Scholar] [CrossRef] [Green Version]
- Walkowiak-Przybyło, M.; Klimek, L.; Okrój, W.; Jakubowski, W.; Chwiłka, M.; Czajka, A.; Walkowiak, B. Adhesion, activation, and aggregation of blood platelets and biofilm formation on the surfaces of titanium alloys Ti6Al4V and Ti6Al7Nb. J. Biomed. Mater. Res. Part A 2012, 100, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Dobrzański, L.A.; Dobrzański, L.B.; Achtelik-Franczak, A.; Dobrzańska, J. Application Solid Laser-Sintered or Machined Ti6Al4V Alloy in Manufacturing of Dental Implants and Dental Prosthetic Restorations According to Dentistry 4.0 Concept. Processes 2020, 8, 664. [Google Scholar] [CrossRef]
- Dobrzański, L.A. Introductory Chapter: Multi-Aspect Bibliographic Analysis of the Synergy of Technical, Biological and Medical Sciences Concerning Materials and Technologies Used for Medical and Dental Implantable Devices. In Biomaterials in Regenerative Medicine; Dobrzański, L.A., Ed.; IntechOpen: Rijeka, Croatia, 2018; Available online: https://www.intechopen.com/chapters/58850 (accessed on 25 September 2021). [CrossRef] [Green Version]
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006, 1, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.T.; Zhang, K.G. Influence of grain size on corrosion resistance of a HSLA steel. Adv. Mater. Res. 2012, 557–559, 143–146. [Google Scholar] [CrossRef]
- Anju, M.S.; Raj, D.K.; Madathil, B.K.; Kasoju, N.; Anil Kumar, P.R. Intelligent biomaterials for tissue engineering and biomedical applications: Current landscape and future prospects. In Biomaterials in Tissue Engineering and Regenerative Medicine: From Basic Concepts to State of the Art Approaches; Bhaskar, B., Sreenivasa Rao, P., Kasoju, N., Nagarjuna, V., Baadhe, R.R., Eds.; Springer: Singapore, 2021; pp. 535–560. [Google Scholar]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: Biomechanical and biocorrosion perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Zhang, S.; Liu, H. Role of TiO2 nanotubes on the surface of implants in osseointegration in animal models: A systematic review and meta-analysis. J. Prosthodont. 2020, 29, 501–551. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Mo, A. A review on the electrochemically self-organized titania nanotube arrays: Synthesis, modifications, and biomedical applications. Nanoscale Res. Lett. 2018, 13, 187–195. [Google Scholar] [CrossRef]
- Roguska, A.; Belcarz, A.; Suchecki, P.; Andrzejczuk, M.; Lewandows Ka, M. Antibacterial composite layers on Ti: Role of ZnO nanoparticles. Arch. Metall. Mater. 2016, 61, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cénédese, P.; Dubot, P. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Fattah-alhosseini, A.; Molaei, M.; Attarzadeh, N.; Babaei, K.; Attarzadeh, F. On the enhanced antibacterial activity of plasma electrolytic oxidation (PEO) coatings that incorporate particles: A review. Ceram. Int. 2020, 46, 20587–20607. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.; Andrei, V.; Coaca, E.; Mihailescu, C.N.; Lungu, C.P.; Radulescu, C.; Dulama, I.D. Obtaining and characterization of PEO layers prepared on CP-Ti in sodium dihydrogen phosphate dihydrate acidic electrolyte solution. Surf. Coat. Technol. 2019, 375, 621–636. [Google Scholar] [CrossRef]
- Abbasi, S.; Golestani-Fard, F.; Rezaie, H.R.; Mirhosseini, S.M.M. MAO-derived hydroxyapatite/TiO2 nanostructured multi-layer coatings on titanium substrate. Appl. Surf. Sci. 2012, 261, 37–42. [Google Scholar] [CrossRef]
- Barbînt, A.C.; Earar, K.; Crimu, C.-I.; Dragan, L.-A.; Munteanu, C. In vitro evaluation of the cytotoxicity of some new titanium alloys. Key Eng Mater. 2014, 587, 303–308. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Ma, J.U.N.; Gao, Y.; Yang, L.E.I. Cell sheet technology: A promising strategy in regenerative medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef] [PubMed]
- So, S.M.; Kim, K.Y.; Lee, S.J.; Yu, Y.J.; Lim, H.A.; Oh, M.S. Effects of Sn content and hot deformation on microstructure and mechanical properties of binary high Sn content Cu-Sn alloys. Mater. Sci. Eng. 2020, 796, 140–154. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, D.Z.; Jiang, J.; Fu, G.; Zhang, P. Processing optimisation, mechanical properties and microstructural evolution during selective laser melting of Cu-15Sn high-tin bronze. Mater. Sci. Eng. A 2018, 721, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Peter, I.; Rosso, M. Study of Ti-enriched CoCrMo alloy for dental application. IEEE Access. J. Rapid Open Access Publ. 2015, 3, 73–80. [Google Scholar] [CrossRef]
- Sykaras, N.; Iacopino, A.M.; Marker, V.A.; Triplett, R.G.; Woody, R.D. Implant materials, designs, and surface topographies: Their effect on osseointegration. A literature review. Int. J. Oral Maxillofac. Implant. 2000, 15, 675. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases WJCC 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloys Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Venezuela, J.; Dargusch, M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review. Acta Biomater. 2019, 87, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peuster, M.; Wohlsein, P.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal-Results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.F.; Qiu, H.; Tian, Y.; Hu, X.Y.; Luo, T.; Wu, C.; Tian, Y.; Tang, Y.; Song, L.F.; Li, L.; et al. Preclinical Evaluation of a Novel Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold in Porcine Coronary Artery at 6 Months. JACC Cardiovasc. Interv. 2019, 12, 245. [Google Scholar] [CrossRef]
- Zhang, D.; Lin, W.; Qi, H. Bioresorbable Scaffolds: From Basic Concept to Clinical Applications; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346. [Google Scholar] [CrossRef]
- Peter, I.; Matekovits, L. Multidisciplinary investigations on the use of TiNb alloy orthopedic device equipped with low profile antenna as smart sensor. Procedia Manuf. 2020, 46, 828–837. [Google Scholar] [CrossRef]
- Peter, I.; Rosso, M.; Toppi, A.; Dan, I.; Ghiban, B. Investigation on cobalt based alloy modified by titanium for dental application. Arch. Mater. Sci. Eng. 2013, 61, 62–68. [Google Scholar]
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical TiZrNbTaMo high entropy alloys. J. Alloys Compd. 2021, 861, 157997. [Google Scholar] [CrossRef]
- Yia, C.; Yuana, Y.; Zhangb, L.; Jianga, Y.; Hea, Z. Antibacterial Ti-35Nb-7Zr-xCu alloy with excellent mechanical properties generated with a spark plasma sintering method for biological applications. Mater. Sci. Eng. C 2017, 71, 512–519. [Google Scholar]
- Miai, J.; Liu, J.; Wang, H.; Yang, H.; Ruan, J. Preparation of porous Ta−10%Nb alloy scaffold and its in vitro biocompatibility evaluation using MC3T3-E1 cells. Trans. Nonferrous Met. Soc. China 2018, 28, 2053–2061. [Google Scholar] [CrossRef]
- Fashu, S.; Lototskyy, M.; Davids, M.W.; Pickering, L.; Linkov, V.; Tai, S.; Renheng, T.; Fangming, X.; Fursikov, P.V.; Tarasov, B.P. A review on crucibles for induction melting of titanium alloys. Mater. Des. 2020, 186, 108295. [Google Scholar] [CrossRef]
- Xu, X.; Ding, H.; Huang, H.; Liang, H.; Chen, R.; Guo, J.; Fu, H. Microstructure formation and columnar to equiaxed transition during cold crucible directional solidification of a high-Nb TiAl alloy. J. Mater. Res. Technol. 2021, 11, 2221–2234. [Google Scholar] [CrossRef]
- Moreno, J.G.; Bönisch, M.; Panagiotopoulos, N.T.; Calin, M.; Papageorgiou, D.G.; Gebert, A.; Eckert, J.; Evangelakis, G.A.; Lekka, C.E. Ab-initio and experimental study of phase stability of Ti-Nb alloys. J Alloys Compd. 2017, 696, 481–489. [Google Scholar] [CrossRef]
- Kolli, R.P.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef] [Green Version]
- Lemke, J.N.; Gallino, F.; Cresci, M.; Coda, A. Achieving improved workability and competitive high temperature shape memory performance by Nb addition to Ni-Ti-Hf alloys. Scr. Mater. 2021, 191, 161–166. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of Grain Size on Corrosion: A Review. Corrosion 2010, 66, 075005–075013. [Google Scholar] [CrossRef]
- Standard Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications; ASTM International: West Conshohocken, PA, USA, 2014; UNS R56400.





| Alloys | Composition | Total Charge | ||||||
|---|---|---|---|---|---|---|---|---|
| Ti | Nb | Zr | Ta | Fe | ||||
| 1 | Ti10Nb10Zr5Ta | (% mass) | 75.0 | 10.0 | 10.0 | 5.0 | – | |
| (g) | 112.5 | 15.0 | 15.0 | 7.5 | – | 150.0 | ||
| 2 | Ti20Nb20Zr4Ta | (% mass) | 56.0 | 20.0 | 20.0 | 4.0 | – | |
| (g) | 84.0 | 30.0 | 30.0 | 6.0 | – | 150.0 | ||
| 3 | Ti29.3Nb13,6Zr1.9Fe | (% mass) | 55.2 | 29.3 | 16.6 | – | 1.9 | |
| (g) | 82.8 | 43.9 | 20.4 | – | 2.9 | 150.0 | ||
| Alloys | Ultimate Tensile Strength σUTS [MPa] | 0.2 Yield Strength σ0.2 [MPa] | Elongation to Fracture ε [%] | Elastic Modulus E [GPa] |
|---|---|---|---|---|
| Ti10Nb10Zr5Ta | 742 ± 10 | 397 ± 8 | 13.9 ± 0.2 | 44 ± 2 |
| Ti20Nb20Zr4Ta | 716 ± 10 | 335 ± 5 | 14.8 ± 0.1 | 42 ± 3 |
| Ti29.3Nb13.6Zr1.9Fe | 995 ± 3 | 570 ± 7 | 7.6 ± 0.3 | 46 ± 5 |
| Ti29.3Nb13.6Zr1.9Fe laminated | 1355 ± 5 | 690 ± 5 | 8.7 ± 0.2 | 51 ± 2 |
| Ti29.3Nb13.6Zr1.9Fe recrystallized for 5 min | 933 ± 1 | 824 ± 3 | 14.2 ± 0.2 | 65 ± 5 |
| Ti29.3Nb13.6Zr1.9Fe recrystallized for 15 min | 909 ± 4 | 786 ± 5 | 8.8 ± 0.1 | 63 ± 5 |
| Ti6Al4V [33,37] | 895–1300 | 830–1100 | 10 | 100–120 |
| Alloys | Composition of the Alloys (% wt) | ||||
|---|---|---|---|---|---|
| Ti | Nb | Zr | Ta | Fe | |
| Ti-10Nb-10Zr-5Ta | 74.89 | 10.06 | 9.94 | 5.01 | |
| Ti-20Nb-20Zr-4Ta | 55.93 | 20.01 | 19.92 | 4.03 | |
| Ti-29,3Nb-13.6Zr-1.,9Fe | 55.10 | 29.39 | 13.55 | 1.91 | |
| Alloy | Initial Thickness of the Strips [mm] | Rolling Speed [m/min] | Degree of Deformation at One Pass [%] | Total Degree of Deformation [%] | Final Thickness of the Strips [mm] | Recrystallization Temperature [°C] | Holding Time at the Maximum Temperature [min] | Cooling Medium |
|---|---|---|---|---|---|---|---|---|
| Ti10Nb10Zr5Ta | 4 | 3 | 10 | 50 | 2 | 920 | 15 | Water |
| Ti20Nb20Zr4Ta | 4 | 3 | 10 | 50 | 2 | 920 | 15 | Water |
| Ti29.3Nb13.6Zr1.9Fe | 6.5 | 3 | About 10 | 87 | 0.84 | 950 | 5 and 15 | Water |
| Alloy | Time of the Recrystallization [min] | Number of the Gains [nr.] | The Average Size of the Grains [μm] |
|---|---|---|---|
| Ti29.3Nb13.6Zr1,9Fe | 5 | 375 | 41.8 |
| 15 | 154 | 64.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peter, I. Investigations into Ti-Based Metallic Alloys for Biomedical Purposes. Metals 2021, 11, 1626. https://doi.org/10.3390/met11101626
Peter I. Investigations into Ti-Based Metallic Alloys for Biomedical Purposes. Metals. 2021; 11(10):1626. https://doi.org/10.3390/met11101626
Chicago/Turabian StylePeter, Ildiko. 2021. "Investigations into Ti-Based Metallic Alloys for Biomedical Purposes" Metals 11, no. 10: 1626. https://doi.org/10.3390/met11101626






