Role of Electrode Rotation on Improvement of Metal Pool Profile in Electroslag Remelting Process
Abstract
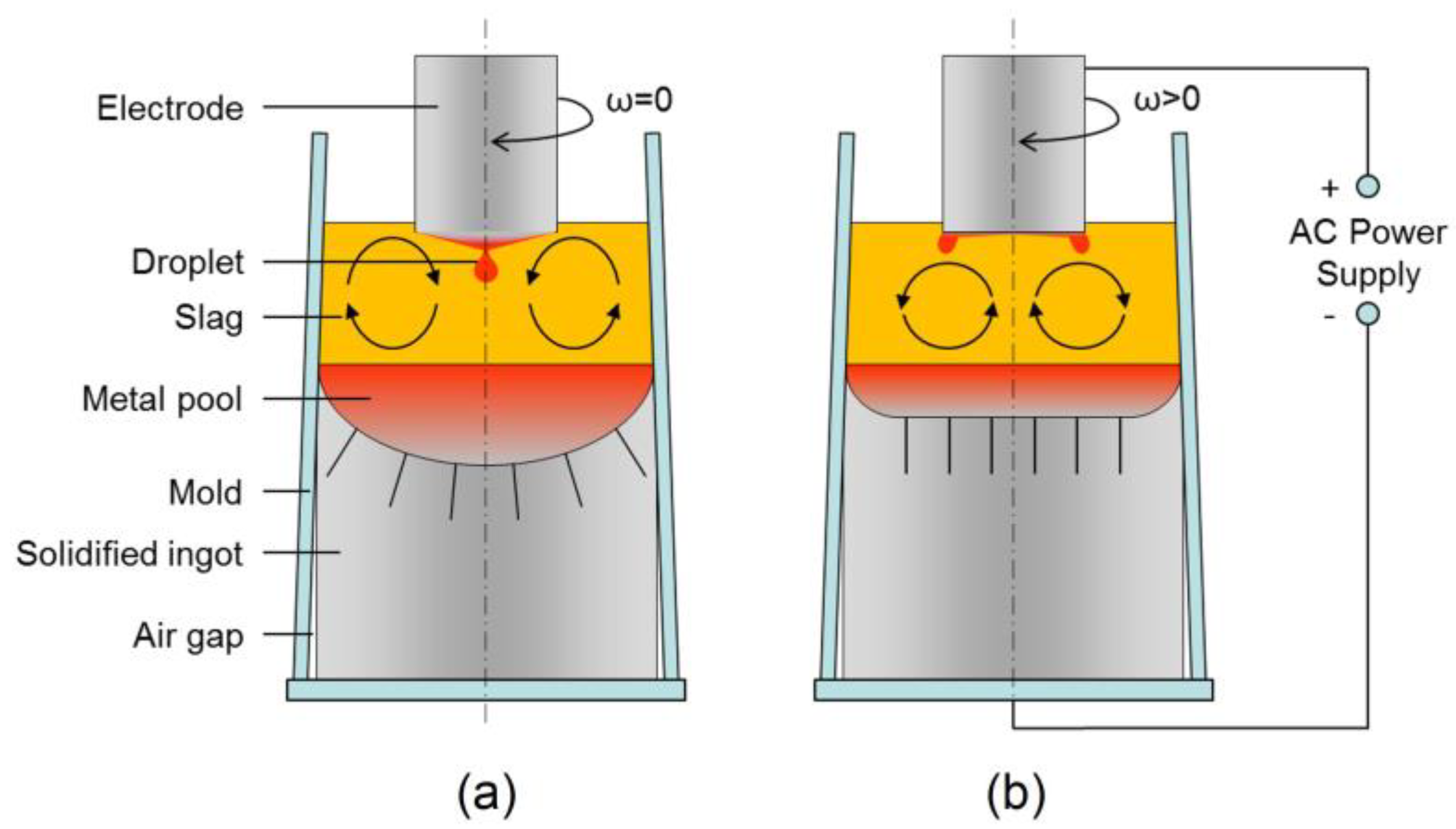
:1. Introduction
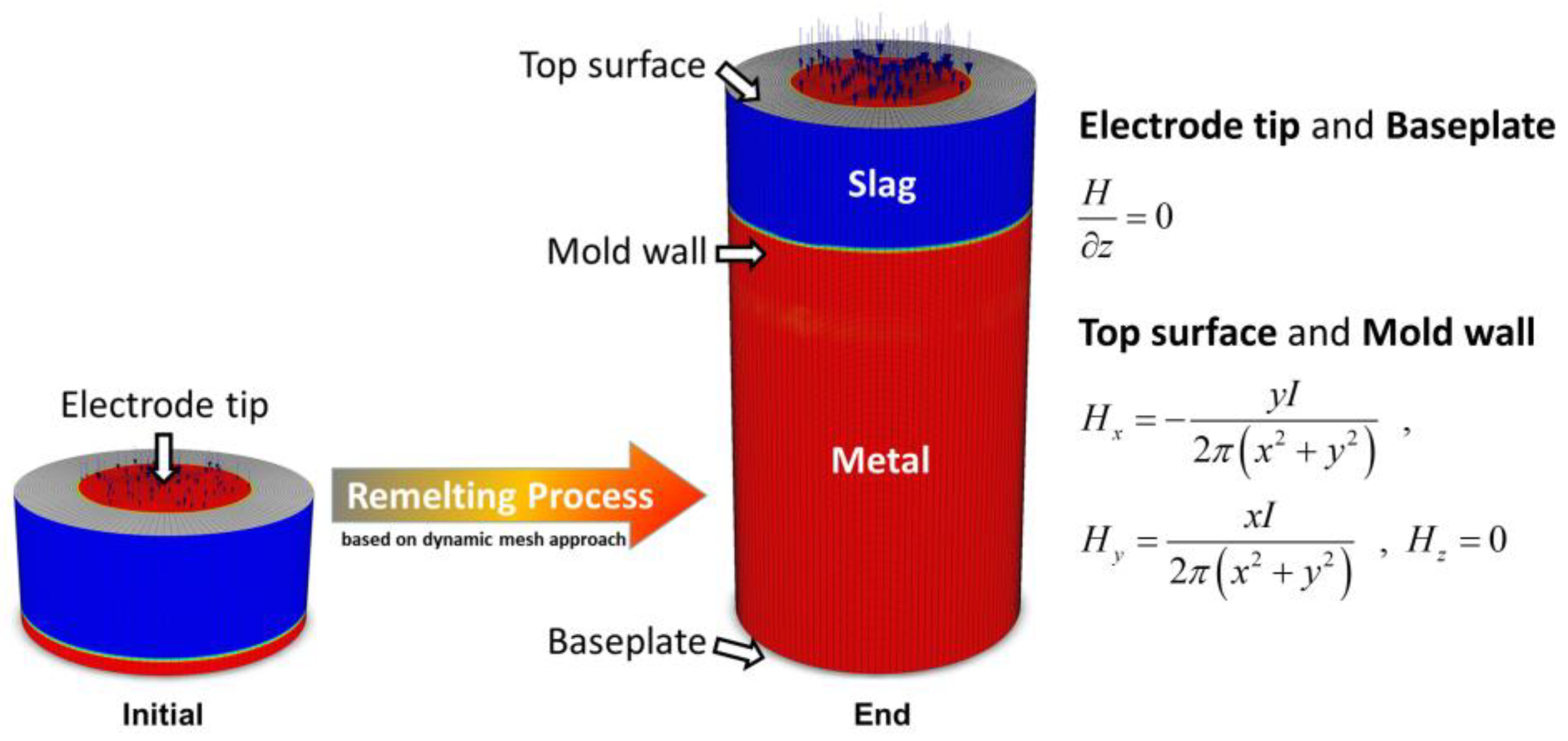
2. Model Description
2.1. Governing Equations
- VOF approach. The volume of fluid (VOF) approach is adopted to solve the redistribution of the metal and slag phases and accurately track the slag/metal interface [17]. The local volume fractions determine the properties such as density and electrical conductivity.
- Electromagnetism. For sinusoidal AC, the induced magnetic field can be expressed in complex notation, of which the amplitude is dependent on the position of AC [22]. With Maxwell’s equations and proper boundary conditions, the time-averaged electromagnetic force and Joule heating density could be calculated [23].
- All the governing equations are given in Table 1.
2.2. Melting Rate Calculation
- (a)
- The initial value of the ratio of sensible heat to latent heat is assumed to be 10.
- (b)
- Combining Equations (15)–(17), can be deduced.
- (c)
- Using Equation (19) and from step (b), the temperature profile in the solid electrode can be calculated.
- (d)
- is updated using Equation (18), and then is updated using Equations (15) and (16).
- (e)
- The ratio is updated and used as the initial value for the next iteration.
- (f)
- Repeat steps (b) through (e) until the ratio changes less than 0.01% in one iteration.
2.3. Evaluation of Solidification Quality
3. Process Parameters and Numerical Details
4. Results and Discussions
4.1. Model Validation
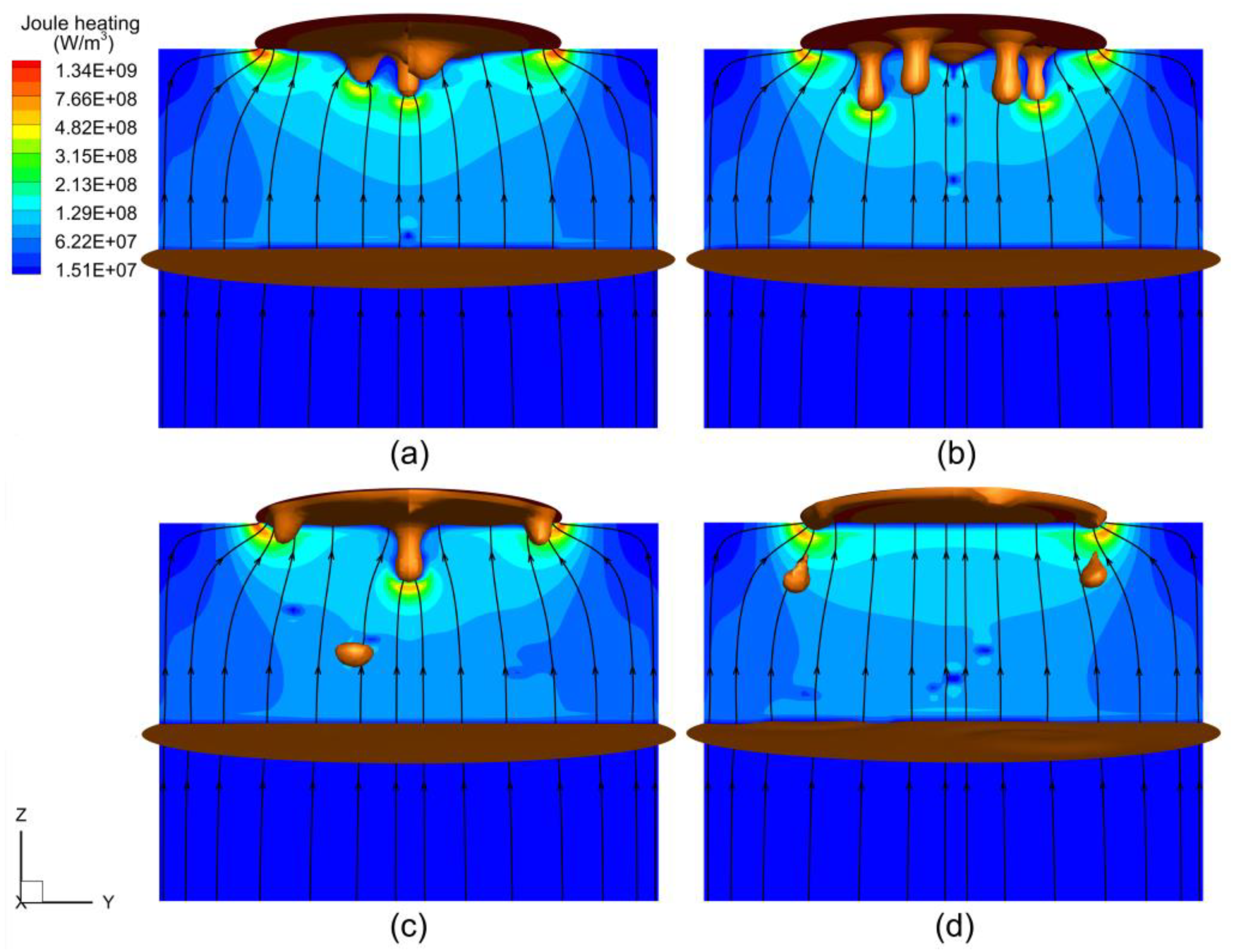
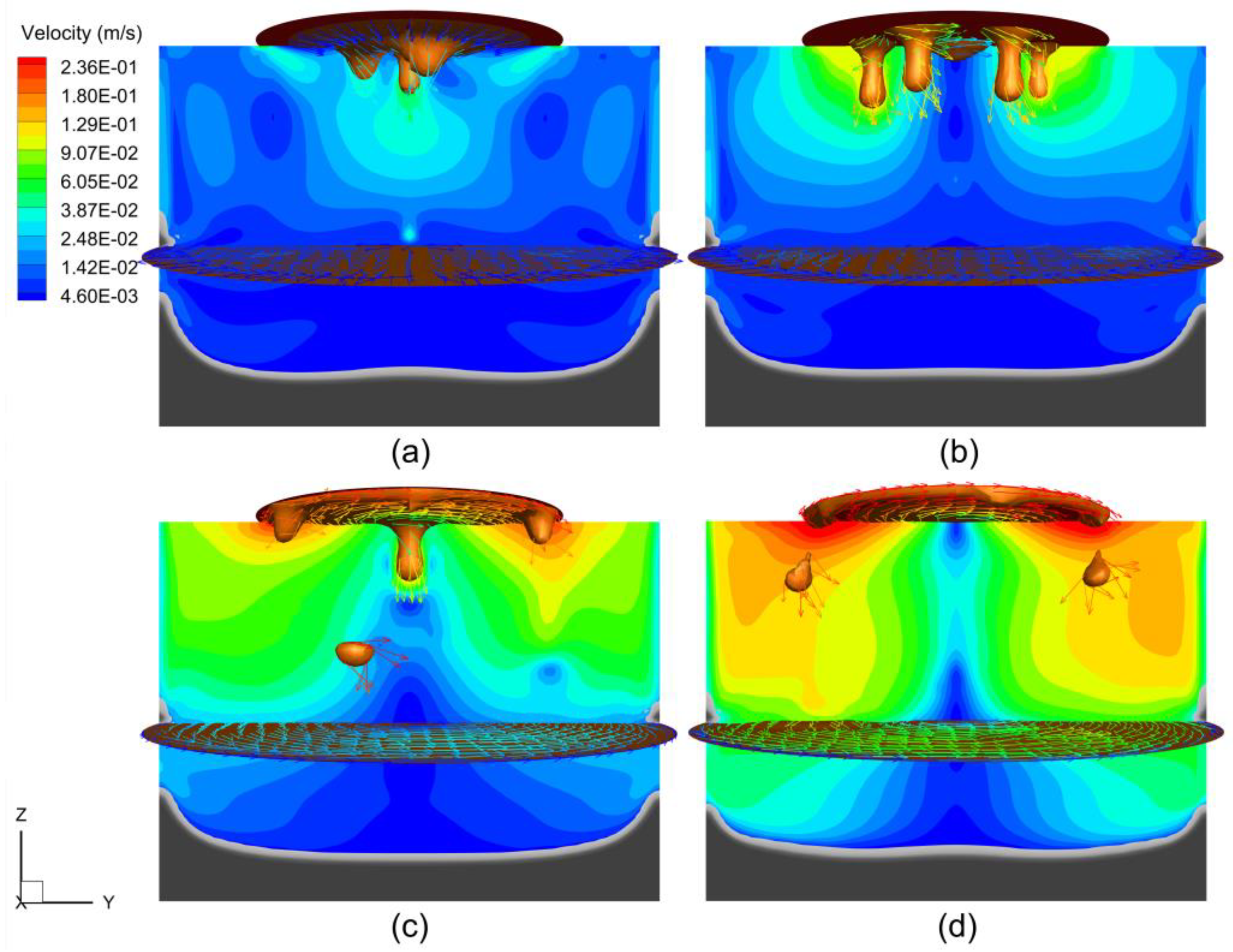
4.2. Magnetohydrodynamic Flow
4.3. Evolution of Metal Pool Profile
4.4. Solidification Quality
5. Conclusions
- The averaged melting rates were predicted as 0.0238, 0.0247, 0.0276, and 0.0261 kg/s in the cases of rotation at 0, 20, 40, and 60 rpm, respectively. Productivity reached a maximum of 15.97% at the rotating speed of 40 rpm, without increasing power.
- As the electrode rotated, the droplets detached at the periphery of the electrode tip rather than at the center, resulting in a flow structure reversed from that in the static case. The electrode rotation drove the melt, creating a strong swirling flow in the slag layer and liquid metal pool, which weakened with decreasing altitude.
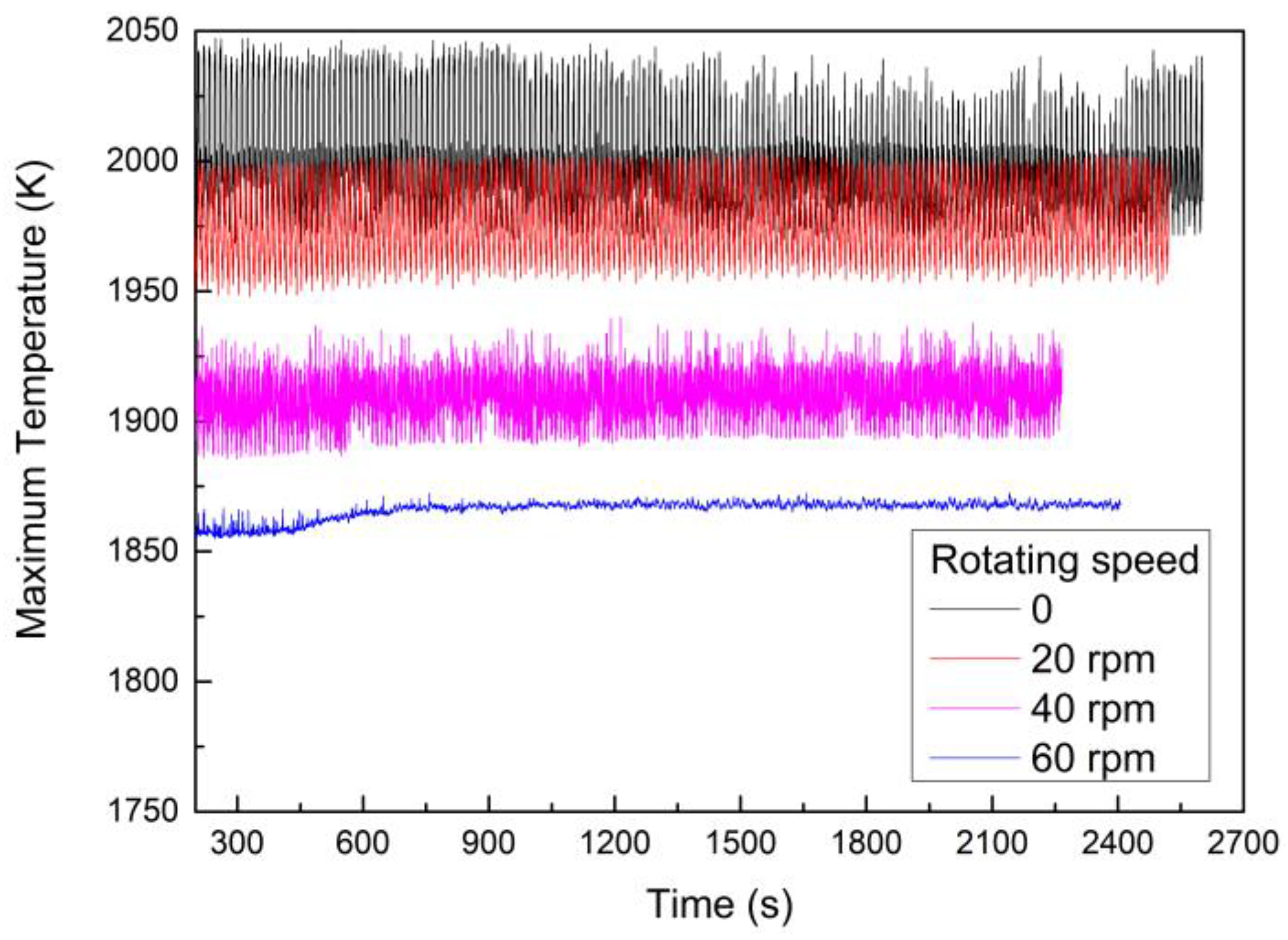
- With rotating speeds ranging from 0 to 60 rpm, the maximum temperature of the melt decreased from 1999.0 to 1866.0 K. Meanwhile, the temperature distribution in the slag layer became more uniform, and the thermal environment more stable.
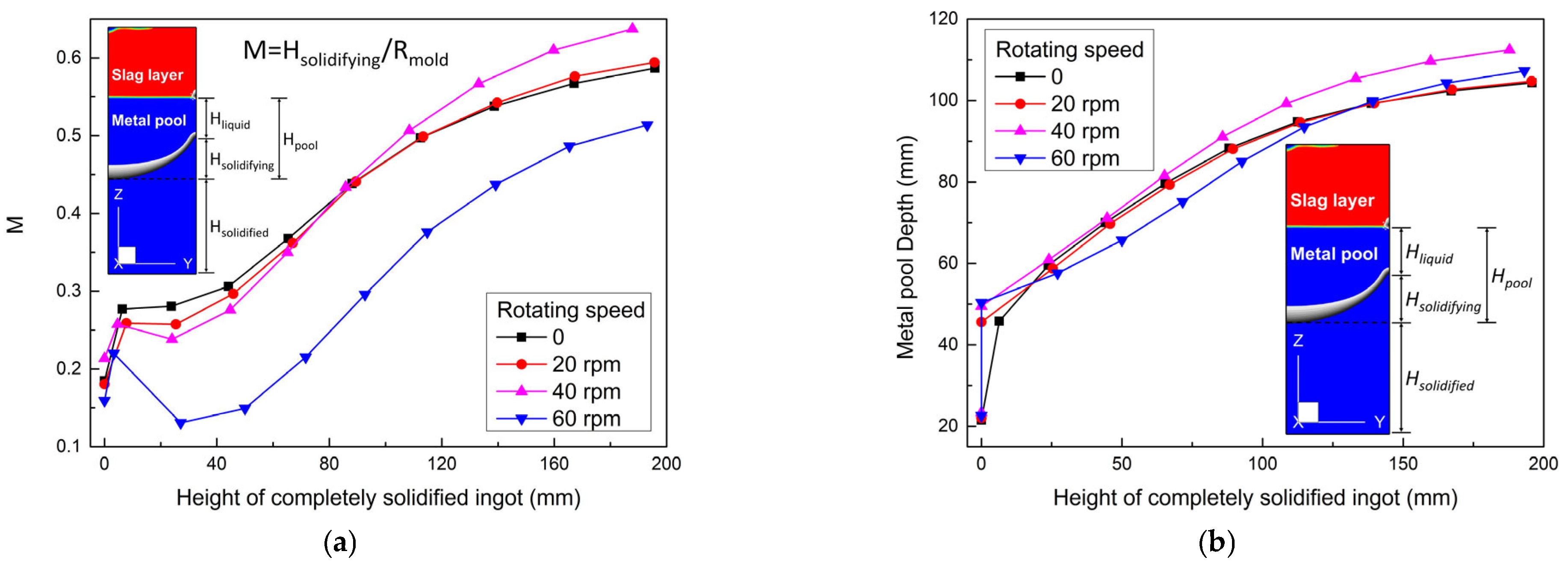
- The metal pool profile was improved with increasing rotating speed in the early stage. At the end stage, compared with the static case, the pool profiles were flattened by −1.19%, −8.52%, and 12.44% at the rotating speeds of 20, 40, and 60 rpm, respectively. Only at a higher speed was the metal pool profile is improved, and remarkably. The effect of rotating speed on the metal pool profile depends on the competition between the melting rate and slag temperature.
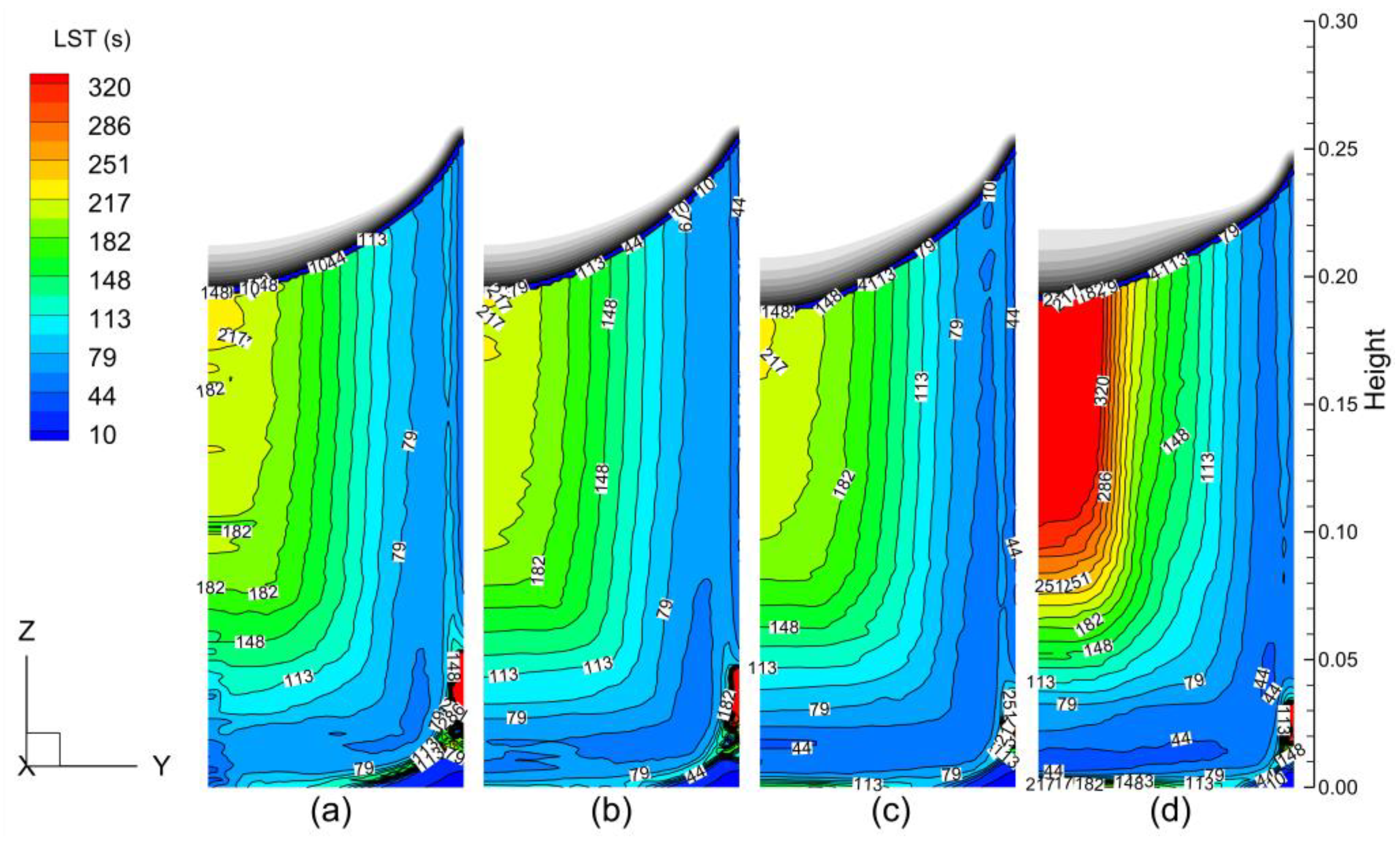
- The LST and SDAS increased layer by layer from the surface to the center of ESR ingot. Compared to the static case, the SDAS was slightly improved at lower rotating speeds (i.e., 20 and 40 rpm) but was significantly worse at a higher rotating speed (i.e., 60 rpm).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kharicha, A.; Karimi-Sibaki, E.; Wu, M.; Ludwig, A.; Bohacek, J. Review on modeling and simulation of electroslag remelting. Steel Res. Int. 2018, 89, 1700100. [Google Scholar] [CrossRef]
- Hernandez-Morales, B.; Mitchell, A. Review of mathematical models of fluid flow, heat transfer, and mass transfer in electroslag remelting process. Ironmak. Steelmak. 1999, 26, 423–438. [Google Scholar] [CrossRef]
- Huang, X.C.; Li, B.K.; Liu, Z.Q. Three-dimensional mathematical model of oxygen transport behavior in electroslag remelting process. Metall. Mater. Trans. B 2018, 49, 709–722. [Google Scholar] [CrossRef]
- Huang, X.C.; Li, B.K.; Liu, Z.Q.; Li, X.L.; Sun, M.J. Numerical investigation and experimental validation of motion and distribution of nonmetallic inclusions in argon protection electroslag remelting process. Metals 2018, 8, 392. [Google Scholar] [CrossRef] [Green Version]
- Li, B.K.; Wang, Q.; Wang, F.; Chen, M.Q. A coupled cellular automaton-finite-element mathematical model for the multiscale phenomena of electroslag remelting H13 die steel ingot. JOM 2014, 66, 1153–1165. [Google Scholar] [CrossRef]
- Fezi, K.; Yanke, J.; Krane, M.J.M. Macrosegregation during electroslag remelting of alloy 625. Metall. Mater. Trans. B 2015, 46, 766–779. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Li, B.K.; Tsukihashi, F. A three-dimensional comprehensive model for prediction of macrosegregation in electroslag remelting ingot. ISIJ Int. 2015, 55, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Viswanathan, S.; Melgaard, D.K.; Patel, A.D.; Evans, D.G. Effect of processing parameters on temperature profiles, fluid flow, and pool shape in the ESR process. In Proceedings of the Liquid Metal Processing and Casting, Santa Fe, NM, USA, 18–21 September 2005. [Google Scholar]
- Patel, A.D.; Gierula, M.; Tallman, D.J. Bounds on model parameters for computational analysis of the ESR process. In Proceedings of the Liquid Metal Processing and Casting, Santa Fe, NM, USA, 20–23 September 2009. [Google Scholar]
- Wang, Q.; Yan, H.G.; Ren, N.; Li, B.K. Effect of slag thickness on macrosegregation and transition zone width of electroslag remelting dual alloy ingot. JOM 2016, 68, 397–400. [Google Scholar] [CrossRef]
- Chumanov, I.V.; Chumanov, V.I. Technology for electroslag remelting with rotating of the consumable electrode. Metallurgist 2001, 45, 125–128. [Google Scholar] [CrossRef]
- Schwenk, M.; Friedrich, B. Enhancing the electroslag remelting process: Development and implementation of a rotating electrode set-up. In Proceedings of the Liquid Metal Processing and Casting, Philadelphia, PA, USA, 10–13 September 2017. [Google Scholar]
- Chumanov, V.I.; Chumanov, I.V. Increasing the efficiency of the electroslag process and improving the metal quality by rotating a consumable electrode: Part 1. Russ. Metal. 2010, 6, 499–504. [Google Scholar] [CrossRef]
- Demirci, C.; Mellinghoff, B.; Schlüter, J.; Wissen, C.; Schwenk, M.; Friedrich, B. Results of the new generation ESR (electroslag remelting) unit with rotating electrode, designed by SMS Mevac GmBH. In Proceedings of the Liquid Metal Processing and Casting, Birmingham, UK, 8–11 September 2019. [Google Scholar]
- Deng, N.Y.; Shi, X.F.; Chen, J.S.; Chang, K.H.; Yu, W.C.; Wang, J.J.; Chang, L.Z. Numerical simulation of mold rotation and its effect on carbides in HSS ESR ingot. Chin. J. Eng. 2020, 42, 516–526. [Google Scholar]
- Huang, X.C.; Li, B.K.; Liu, Z.Q.; Li, M.Z.; Qi, F.S. Modeling of fluid flow, heat transfer and inclusion removal in electroslag remelting process with a rotating electrode. Int. J. Heat Mass Tran. 2020, 163, 120473. [Google Scholar] [CrossRef]
- Hirt, C.W.; Nichols, B.D. Volume of fluid (VOF) method for the dynamics of free boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Wang, Q.; He, Z.; Li, G.Q.; Li, B.K.; Zhu, C.Y.; Chen, P.J. Numerical investigation of desulfurization behavior in electroslag remelting process. Int. J. Heat Mass Tran. 2017, 104, 943–951. [Google Scholar] [CrossRef]
- Jardy, A.; Ablitzer, D.; Wadier, J.F. Magnetohydrodynamic and thermal behavior of electroslag remelting slags. Metall. Trans. B 1991, 22, 111–120. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Niu, R.; Wu, Y.D.; Li, B.K.; Gan, Y.; Wu, M.H. Physical and numerical simulation of mixed columnar-equiaxed solidification during cold strip feeding in continuous casting. Int. J. Heat Mass Tran. 2021, 173, 121237. [Google Scholar] [CrossRef]
- Li, X.L.; Li, B.K.; Liu, Z.Q.; Niu, R.; Huang, X.C. Large eddy simulation of electromagnetic three-phase flow in a round bloom considering solidified shell. Steel Res. Int. 2019, 89, 1800133. [Google Scholar] [CrossRef]
- Kharicha, A.; Schützenhöfer, W.; Ludwig, A.; Tanzer, R.; Wu, M. On the importance of electric currents flowing directly into the mould during an ESR process. Steel Res. Int. 2008, 79, 632–636. [Google Scholar] [CrossRef]
- Karimi-Sibaki, E.; Kharicha, A.; Bohacek, J.; Wu, M.; Ludwig, A. A dynamic mesh-based approach to model melting and shape of an ESR electrode. Metall. Mater. Trans. B 2015, 46, 2049–2061. [Google Scholar] [CrossRef]
- Yanke, J.; Krane, M.J.M. A parametric study of slag skin formation in electroslag remelting. In Proceedings of the Liquid Metal Processing and Casting, Austin, TX, USA, 22–25 September 2013. [Google Scholar]
- Kharicha, A.; Ludwig, A.; Wu, M. 3D simulation of the melting during an electro-slag remelting process. In Proceedings of the TMS 2011 Annual meeting & Exhibition, San Diego, CA, USA, 27 February–3 March 2011. [Google Scholar]
- Yanke, J.; Fezi, K.; Trice, R.W.; Krane, M.J.M. Simulation of slag-skin formation in electroslag remelting using a volume of fluid method. Numer. Heat Trans. A Appl. 2015, 67, 268–292. [Google Scholar] [CrossRef]
- Ballantyne, A.S.; Kennedy, R.J.; Mitchell, A. The influence of melting rate on structure in VAR and ESR ingots. In Proceedings of the 5th International Conference on Vacuum Metallurgy and Electroslag Remelting Processes, Munich, Germany, 11–15 October 1976. [Google Scholar]
- Flemings, M.C. Solidification Processing; McGraw-Hill Book Co.: New York, NY, USA, 1974; p. 148. [Google Scholar]
- Choudhary, M.; Szekely, J. The effect of temperature dependent electrical conductivity on flow and temperature fields in slags in ESR systems. Metall. Trans. B 1981, 12, 418–421. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Li, G.Q.; Wu, Y.; Xu, D.M.; Li, B.K. Investigation of fluoride vaporization from CaF2-CaO-Al2O3 slag for vacuum electroslag remelting. Vacuum 2018, 158, 6–13. [Google Scholar] [CrossRef]
- Weber, V.; Jardy, A.; Dussoubs, B.; Ablitzer, D.; Ryberon, S.; Schmitt, V.; Hans, S.; Poisson, H. A comprehensive model of the electroslag remelting process: Description and validation. Metall. Mater. Trans. B 2009, 40, 271–280. [Google Scholar] [CrossRef]
- Dilawari, A.H.; Szekely, J. Heat transfer and fluid flow phenomena in electroslag refining. Metall. Trans. B 1978, 9, 77–87. [Google Scholar] [CrossRef]




| Governing Equations | Symbols |
|---|---|
| (1) VOF approach (1) (2) | magnetic flux density () specific heat () primary dendrite arm spacing () buoyancy force () Lorentz force () surface tension () damping force () liquid fraction () gravitational acceleration () magnetic field intensity () complex amplitude () enthalpy () sensible enthalpy () current density () effective thermal conductivity () latent heat of fusion () normal vector () pressure () Joule heating () temperature () reference temperature() time () velocity () volume fraction () thermal expansion coefficient () property () electrical conductivity () density () magnetic diffusivity () effective viscosity () turbulent viscosity () surface tension coefficient () curvature () |
| (2) fluid flow (3) (4) (5) (6) (7) (8) | |
| (3) heat transfer and solidification (9) (10) (11) (12) | |
| (4) electromagnetic field (13) (14) |
| Physical Properties | Metal | Slag |
|---|---|---|
| density, kg·m−3 | 7850 | 2600 |
| viscosity, Pa·s | 0.00441–6.384 | 0.0175–0.0768 |
| specific heat, J·kg−1·K−1 | 752 | 1255 |
| thermal conductivity, W·m−1·K−1 | 30.52 | 10.5 |
| electric conductivity, Ω−1·m−1 | 7.14 × 105 | |
| liquidus/solidus temperature, K | 1730/1636 | - |
| latent heat of fusion, kJ·kg−1 | 270 | - |
| thermal expansion coefficient, K−1 | 2 × 10−4 | 1 × 10−4 |
| Process parameters | Value | |
| electrode radius, mm | 60 | |
| mold radius, mm | 100 | |
| slag weight, kg | 6.5 | |
| current, kA | 3.2 | |
| frequency, Hz | 50 | |
| rotating speed of electrode, rpm | 0/20/40/60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Duan, Y.; Liu, Z.; Li, B.; Wang, F. Role of Electrode Rotation on Improvement of Metal Pool Profile in Electroslag Remelting Process. Metals 2021, 11, 1675. https://doi.org/10.3390/met11111675
Huang X, Duan Y, Liu Z, Li B, Wang F. Role of Electrode Rotation on Improvement of Metal Pool Profile in Electroslag Remelting Process. Metals. 2021; 11(11):1675. https://doi.org/10.3390/met11111675
Chicago/Turabian StyleHuang, Xuechi, Yiru Duan, Zhongqiu Liu, Baokuan Li, and Fang Wang. 2021. "Role of Electrode Rotation on Improvement of Metal Pool Profile in Electroslag Remelting Process" Metals 11, no. 11: 1675. https://doi.org/10.3390/met11111675
APA StyleHuang, X., Duan, Y., Liu, Z., Li, B., & Wang, F. (2021). Role of Electrode Rotation on Improvement of Metal Pool Profile in Electroslag Remelting Process. Metals, 11(11), 1675. https://doi.org/10.3390/met11111675






