Utilization of Rubber Tree Bark for Reduction of Mill Scale at 1550 °C: Implication for Sustainable Wastes Recycling in Steelmaking Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
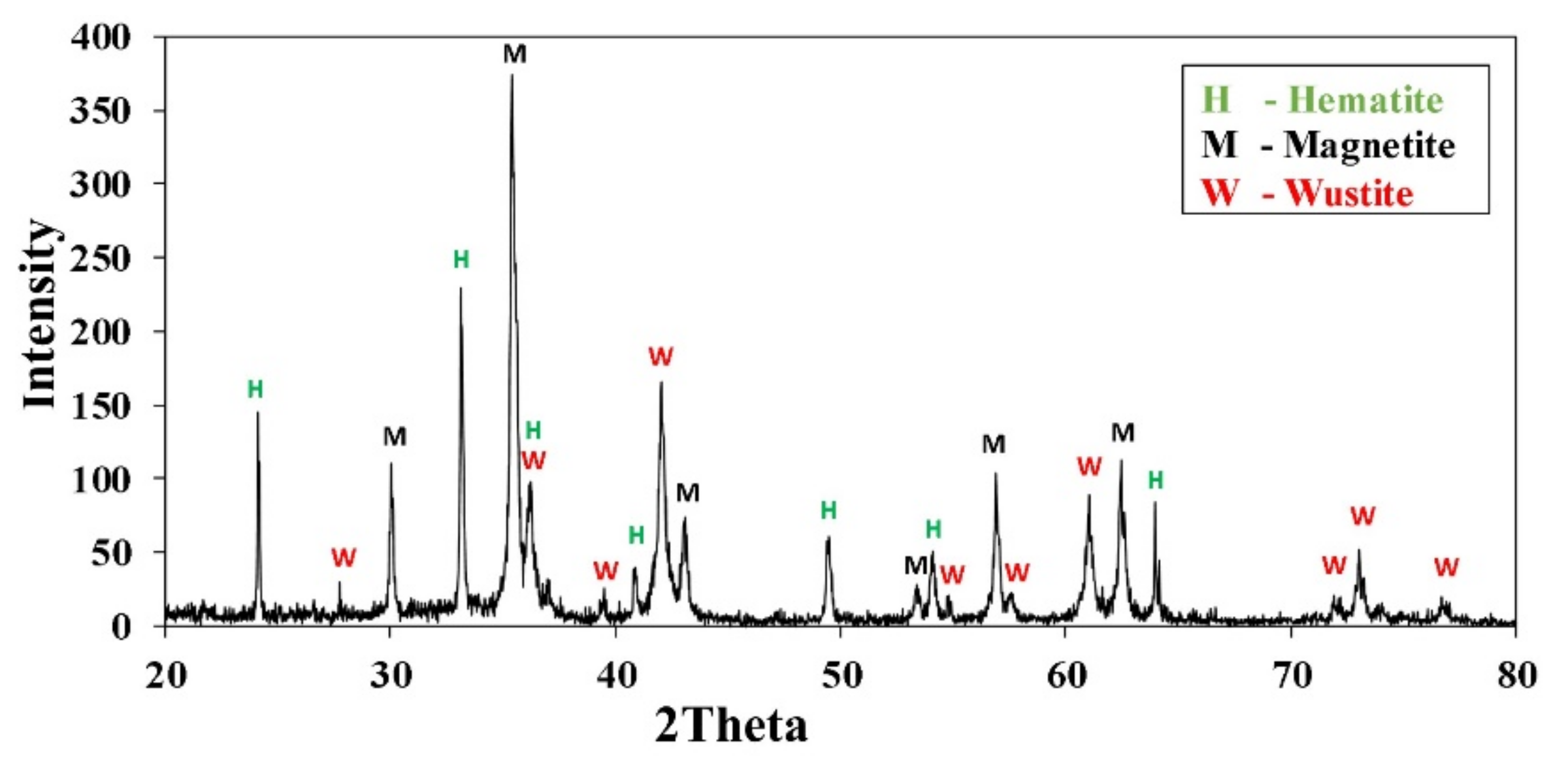
2.1.1. Mill Scale
2.1.2. Carbonaceous Samples
2.1.3. Carbon-Scale Composite Pellet (CCP)
2.2. High-Temperature Interaction
3. Results and Discussion
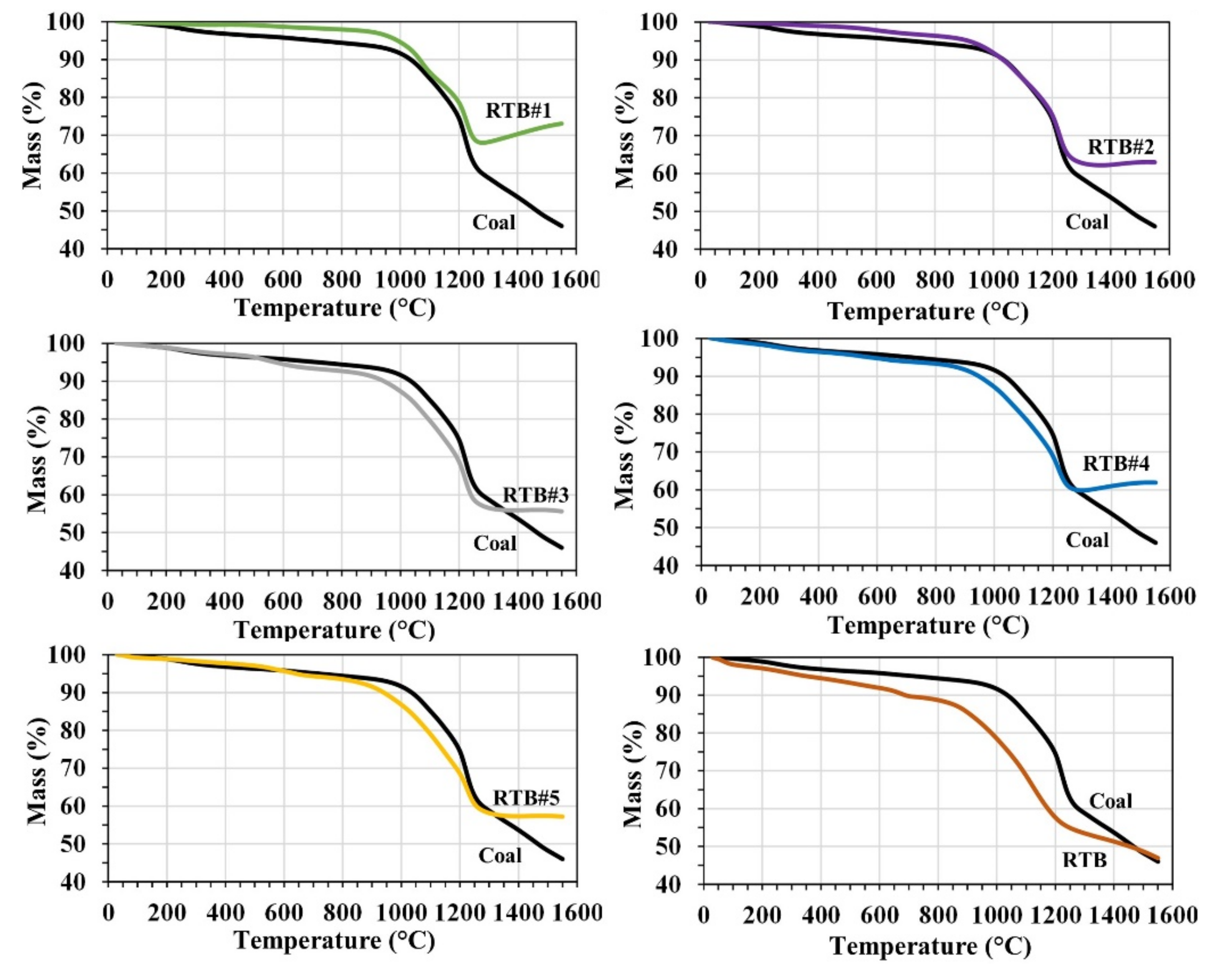
3.1. TGA Analysis of Carbon Composite Pellet (CCP)
3.2. High-Temperature Interaction of the Carbon-Scale Composite Pellet (CCP)
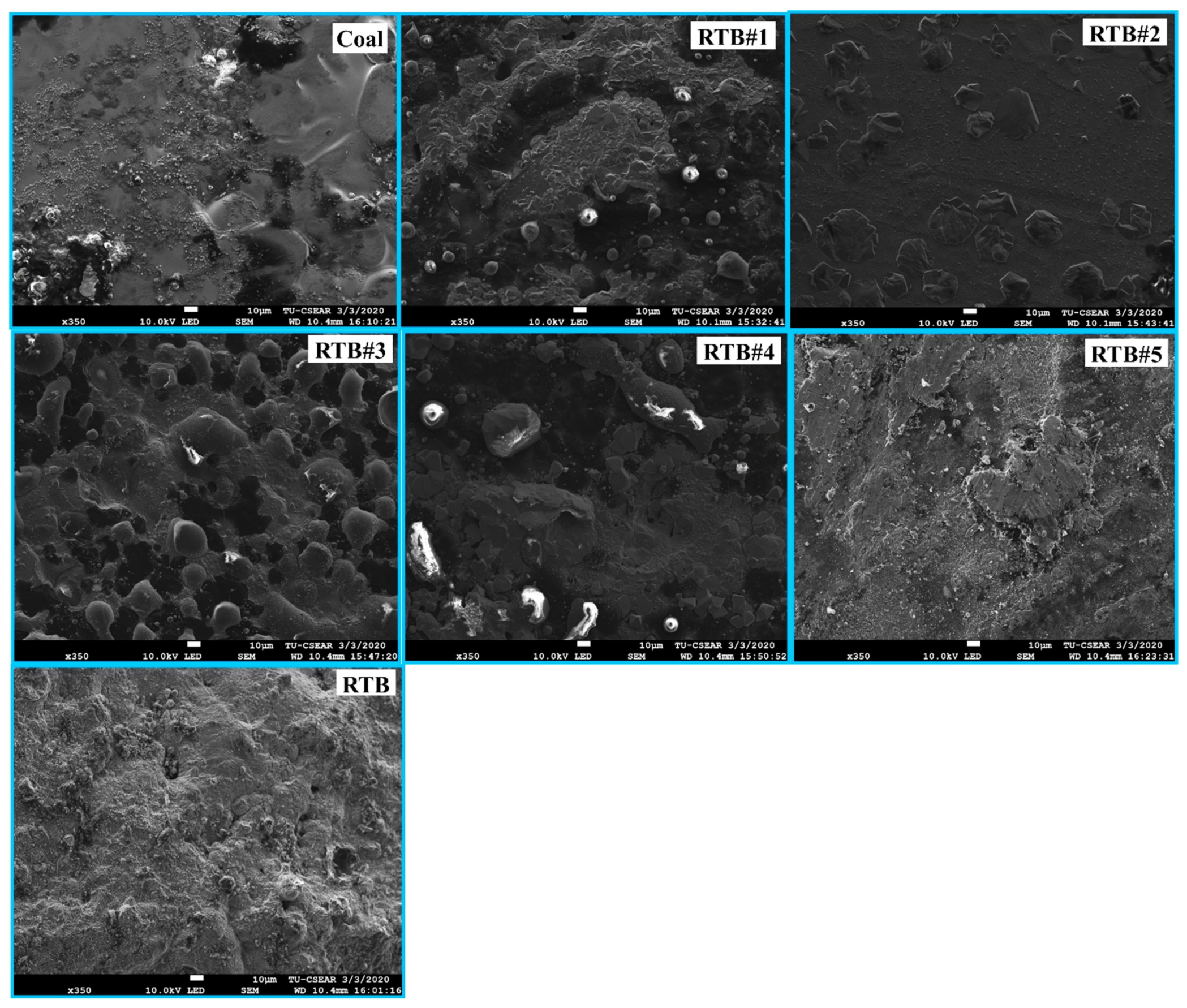
3.3. Effect of RTB on the Formation of Metallic Iron Droplets
3.3.1. Carbon Structure
3.3.2. Volatiles
3.4. Preliminary Cost Analysis for Using RTB as a Carbon Source in Steelmaking Process
4. Conclusions
- From the TGA results, the reduction of mill-scale in the CCP occurs at about 1100–1200 °C for all cases. The weight of CCP in the cases of coal and RTB keeps decreasing until 1550 °C with the weight loss of 53.98 and 53.08 wt.%, respectively. This indicates a slow reduction behavior and incomplete reduction. For blends RTB#1–RTB#5, the CCP weight stabilizes until 1550 °C due to the complete reduction, with the weight loss ranging between 26.92–44.36 wt.%.
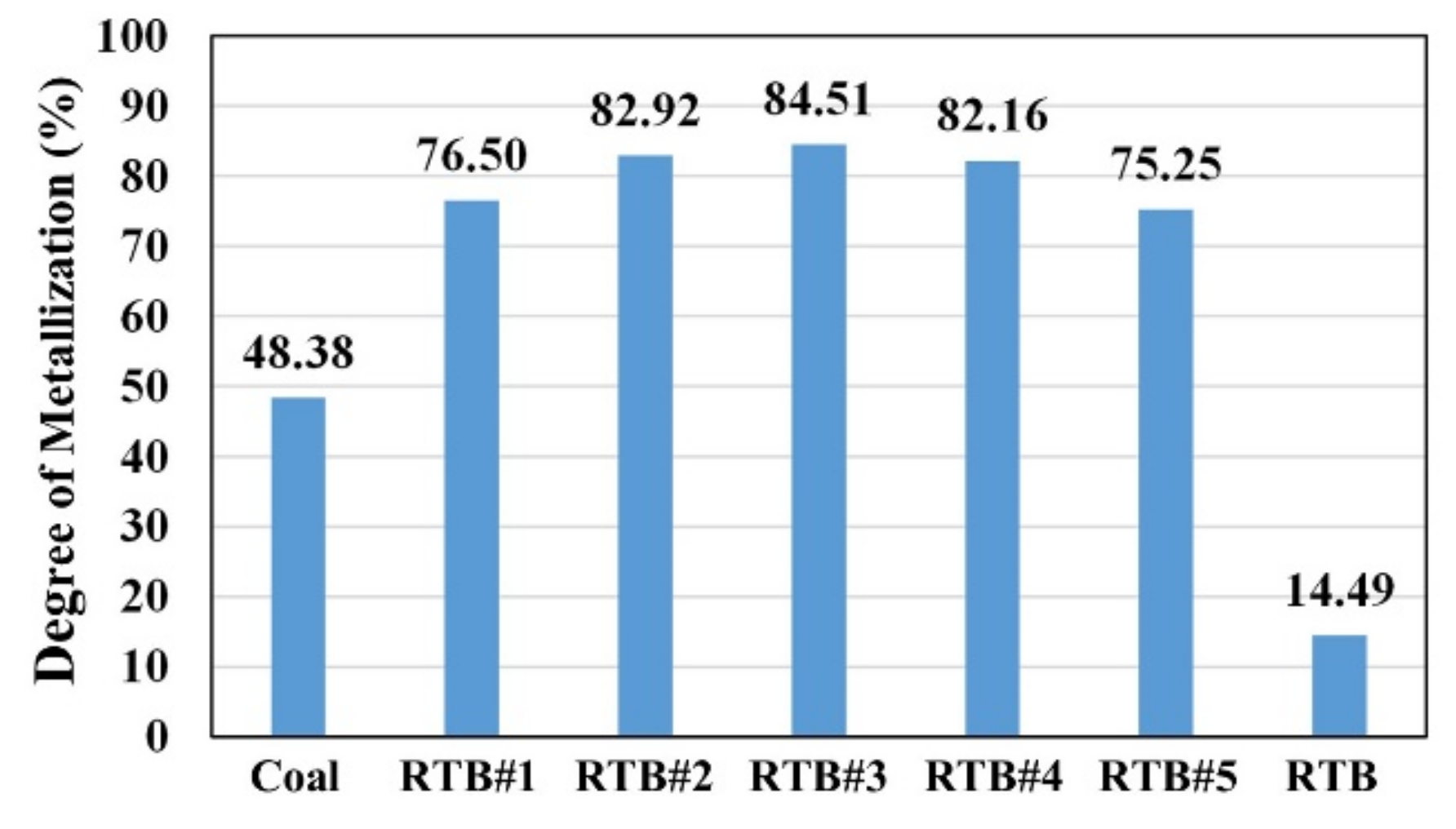
- The reduction of mill scale in the CCP at 1550 °C occurs after the first min for all cases, except for RTB. The reduced Fe droplets were found to separate clearly from the black residues in the crucible. The degree of metallization (DOM) of the CCP produced from coal and RTB was 48.38% and 14.49%, respectively, and the metallic iron obtained was 23.05 wt.% for coal, while it was only 6.09 wt.% for RTB.
- On the other hand, the CCP produced from blends RTB#1–RTB#5 shows higher extent of iron oxides reduction with the metallic iron of 35.28–39.82 wt.%. The DOM ranges between 75.25–84.51%, which is about two and six times higher than that in the cases of parent coal and RTB, respectively.
- Among the blends, RTB#3 showed the maximum DOM of 84.51%. The increase of RTB content in the blends up to 50 wt.% (RTB#5), caused the decrease in DOM of the CCP.
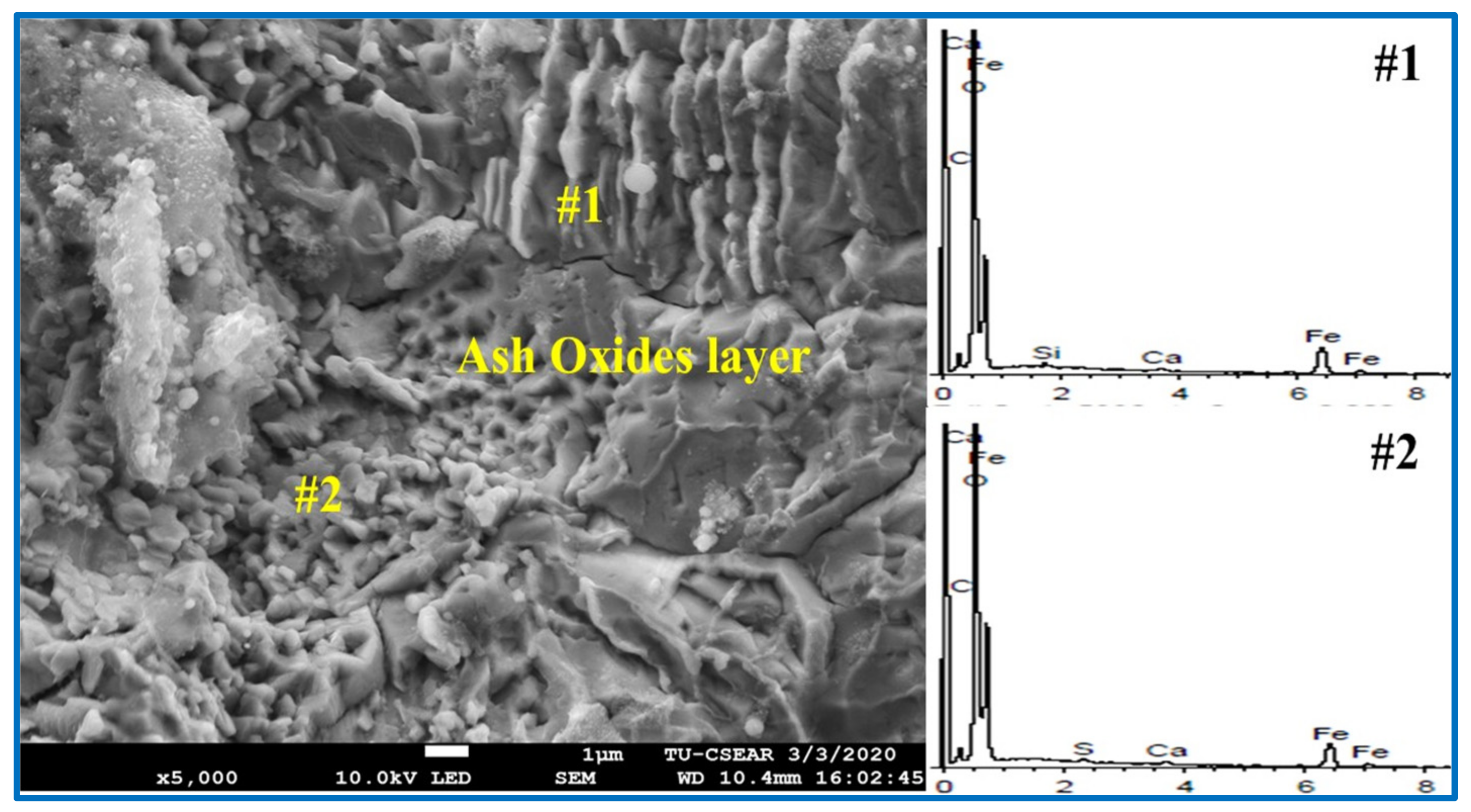
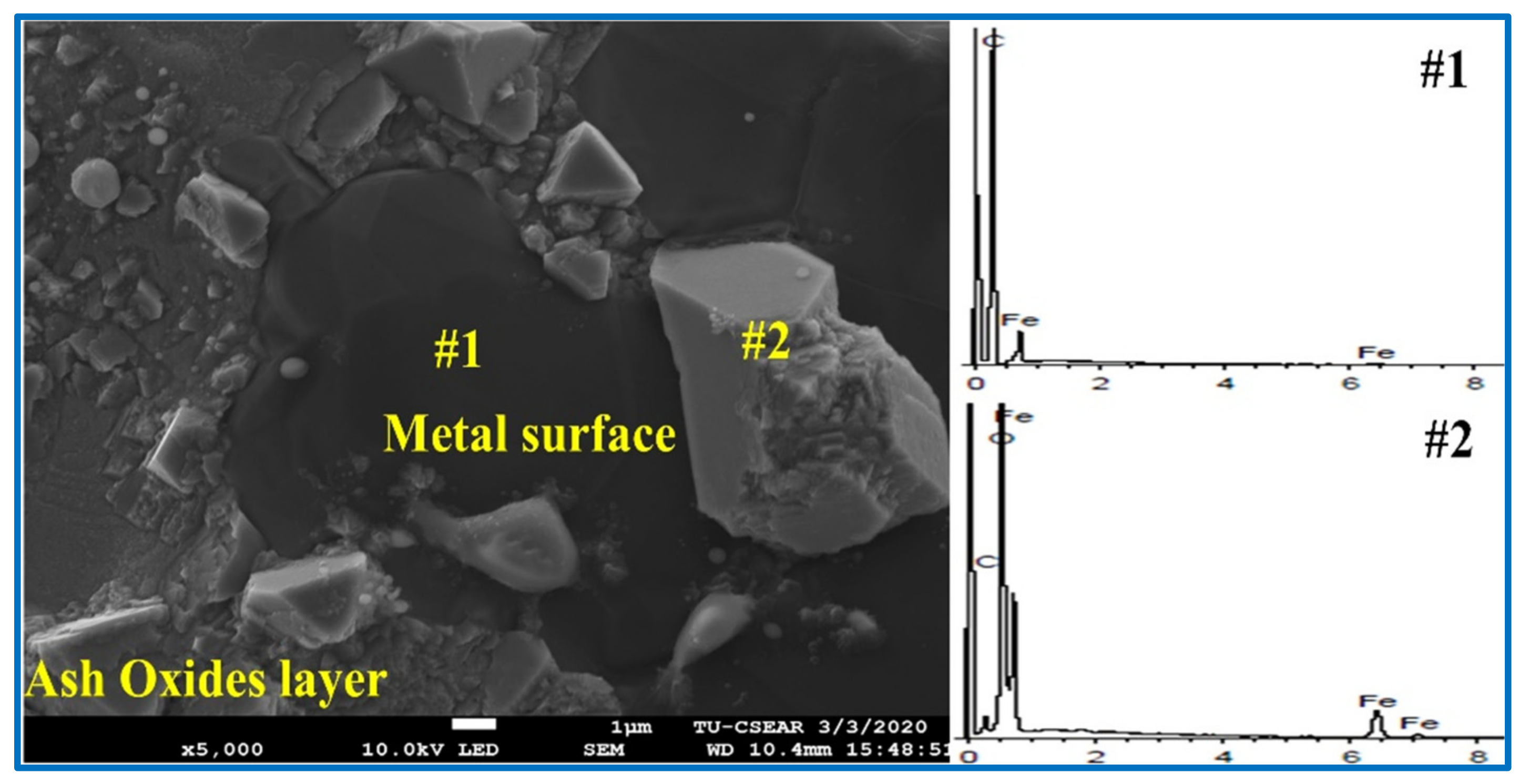
- The addition of RTB into coal was not found to significantly change the composition and carbon structure of chars produced compared to the parent coal, but rather for the ash chemistry. CaO in rubber tree bark played a role in forming ash layer as the interfacial product on the metal surface, and thus the separation between metallic Fe and slag phases. The excess of CaO content in the system hinders the reduction of mill scale, thus the optimum CaO content is essential.
- Rubber tree bark, which is a local-based resource, can be used as a reductant for mill scale at 1550 °C by optimally blending with coal up to 30 wt.%. This can reduce the consumption of fossil fuel by 30 wt.% and reduce the production cost in steelmaking industries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sahajwalla, V.; Rahman, M.; Khanna, R.; Saha-Chaudhury, N.; O’Kane, P.; Skidmore, C.; Knights, D. Recycling waste plastics in EAF steelmaking: Carbon/slag interactions of HDPE-Coke blends. Steel Res. Int. 2009, 80, 535–543. [Google Scholar]
- Zaharia, M.; Sahajwalla, V.; Khanna, R.; Koshy, P.; O’Kane, P. Carbon/slag interactions between coke/rubber blends and EAF slag at 1550 °C. ISIJ Int. 2009, 49, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Dankwah, J.R.; Koshy, P.; Saha-Chaudhury, N.; O’Kane, P.; Skidmore, C.; Knights, D.; Sahajwalla, V. Reduction of FeO in EAF steelmaking slag by metallurgical coke and waste plastics blends. ISIJ Int. 2011, 51, 498–507. [Google Scholar] [CrossRef] [Green Version]
- Dankwah, J.R.; Koshy, P.; O’Kane, P.; Sahajwalla, V. Reduction of FeO in EAF steelmaking slag by blends of metallurgical coke and end-of-life tyre. Steel Res. Int. 2012, 83, 766–774. [Google Scholar] [CrossRef]
- Kongkarat, S.; Khanna, R.; Koshy, P.; O’KANE, P.; Sahajwalla, V. Recycling waste Polymers in EAF steelmaking: Influence of polymer composition on carbon/slag interactions. ISIJ Int. 2012, 52, 385–393. [Google Scholar] [CrossRef] [Green Version]
- Kongkarat, S.; Cherdhirunkorn, B.; Thongreang, R. Utilization of waste HDPE for Sustainable EAF steelmaking: Carbon dissolution into liquid steel. Steel Res. Int. 2017, 88, 1600168. [Google Scholar] [CrossRef]
- Ueki, Y.; Nunome, Y.; Yoshiie, R.; Naruse, I.; Nishibata, Y.; Aizawa, S. Effect of woody biomass addition on coke properties. ISIJ Int. 2014, 11, 2454–2460. [Google Scholar] [CrossRef] [Green Version]
- Yunos, N.; Zaharia, M.; Asriidris, M.; Nath, D.; Khanna, R.; Sahajwalla, V. Recycling agricultural waste from palm shells during electric arc furnace steelmaking. Energy Fuels 2012, 26, 278. [Google Scholar] [CrossRef]
- Baganiti, M.C.; Kan, T.; Evans, T.J.; Strezov, V. Iron ore reduction by biomass volatiles. J. Sustain. Metall. 2021, 7, 215–226. [Google Scholar]
- Eissa, M.; Ahmed, A.; El-Fawkhry, M. Conversion of mill scale waste into valuable Products via carbothermic reduction. J. Metall. 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.I.; Lopez, F.A.; Torralba, J.M. Recycling of steel plant mill scale via iron ore pelletisation process. Ironmak. Steelmak. 2009, 36, 409–415. [Google Scholar]
- El-Hussiny, N.A.; Shalabi, M.E.H. A self-reduced intermediate product from iron and steel plants waste materials using a briquetting process. Powder Technol. 2011, 205, 217–223. [Google Scholar] [CrossRef]
- Bagatini, M.C.; Zymla, V.; Osorio, E.; Vilela, A.C.F. Characterization and reduction Behavior of mill scale. ISIJ Int. 2011, 51, 1072. [Google Scholar] [CrossRef] [Green Version]
- Jagannath, P.; Satadal, G.; Manik Chandra, G.; Swatantra, P.; Thirumalachari, V. Development of Pellet-Sinter Composite Agglomerate for Blast Furnace. ISIJ Int. 2014, 54, 620–627. [Google Scholar]
- Martin, M.I.; Lopez, F.A.; Torralba, J.M. Production of sponge iron powder by reduction of rolling mill scale. Ironmak. Steelmak. 2012, 39, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Sen, R.; Dehiya, S.; Pandel, U.; Banerjee, M.K. Utilization of low grade coal for direct reduction of mill scale to obtain sponge iron: Effect of reduction time and particle size. Procedia Earth Planet. Sci. 2015, 11, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wang, D.R.; He, Y.D.; Qi, H.B.; Wei, G. Reduction of oxide scale on hot-rolled Strip steels by carbon monoxide. Mater. Lett. 2008, 62, 3500–3502. [Google Scholar]
- Benchiheub, O.; Mechachti, S.; Serrai, S.; Khalifa, M.G. Elaboration of iron powder from mill scale. J. Mater Environ. Sci. 2010, 1, 267–276. [Google Scholar]
- Mechachti, S.; Benchiheub, O.; Serrai, S.; Shalabi, M.E.H. Preparation of iron Powders by Reduction of Rolling Mill Scale. Int. J. Sci. Eng. Res. 2013, 4, 1467–1472. [Google Scholar]
- Guan, C.; Li, J.; Tan, N.; He, Y.; Zhang, S. Reduction of oxide scale on hot-rolled steel by hydrogen at low temperature. Int. J. Hydrog. Energy 2014, 39, 15116–15124. [Google Scholar] [CrossRef]
- Joshi, C.; Dhokey, N.B. Study of Kinetics of Mill Scale Reduction: For PM Applications. Trans. Indian Inst. Met. 2015, 68, 31–35. [Google Scholar] [CrossRef]
- Sista, K.S.; Dwarapudi, S.; Nerune, V.P. Direct reduction recycling of mill scale through iron powder synthesis. ISIJ Int. 2019, 5, 787–794. [Google Scholar] [CrossRef] [Green Version]
- Ye, Q.; Zhu, H.; Peng, J.; Khannan, C.S.; Chen, J.; Dai, L.; Liu, P. Preparation of Reduced Iron Powders from Mill Scale with Microwave Heating: Optimization Using Response Surface Methodology. Metall. Mater. Trans. B 2013, 44B, 1478–1485. [Google Scholar] [CrossRef]
- Ye, Q.; Zhu, H.; Zhang, L.; Ma, J.; Zhou, L.; Liu, P.; Chen, J.; Chen, G.; Peng, J. Preparation of reduced iron powder using combined distribution of wood-charcoal by microwave heating. J. Alloy. Compd. 2014, 613, 102–106. [Google Scholar] [CrossRef]
- Khaerudini, D.S.; Chanif, I.; Insiyanda, D.R.; Destyorini, F.; Alva, S.; Premono, A. Preparation and characterization of mill scale industrial waste reduced by biomass-based carbon. J. Sustain. Metall. 2019, 5, 510–518. [Google Scholar] [CrossRef]
- Available online: http://www.basel.int/ (accessed on 6 September 2021).
- Strezov, V.; Ziolkowski, A.; Evans, T.J.; Nelson, P.F. Assessment of evolution of loss Ignition matter during heating of iron ores. J. Therm. Anal. Calorim. 2010, 100, 901–907. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Holee, D.; Zheng, C. Characteristics of hemicellulose, Cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Barrett, D. Partial Oxidation of Methane Using Iron Oxide as Donor. Ind. Eng. Chem. Process Des. Develop. 1972, 11, 415–420. [Google Scholar] [CrossRef]
- Ovesen, C.V.; Stoltze, P.; Norskov, J.K.; Campbell, C.T. A kinetic model of the water gas Shift reaction. J. Catal. 1992, 134, 445–468. [Google Scholar] [CrossRef]
- Biswas, A.K. Principles of Blast Furnace Ironmaking: Theory and Practice, 2nd ed.; Cootha Publishing House: Brisbane, QLD, Australia, 2010. [Google Scholar]
- McCarthy, F.; Khanna, R.; Sahajwalla, V.; Simento, N. Interfacial phenomena occurring during iron/char interactions in a blast furnace. ISIJ Int. 2005, 45, 1261–1268. [Google Scholar] [CrossRef] [Green Version]
- Jouhari, K.; Datta, P. Effect of calcium carbonate on degree of reduction of mill scale by coal. In Proceedings of the Seminar on Experimental Approaches in Pyrometallurgy Research, Bhubaneswar, India, 20–21 April 2000; Allied Publishers: New Delhi, India, 2000; p. 137. [Google Scholar]
- Chengyi, D.; Xuewei, L.V.; Yun, C.; Chenguang, B. Crystallization kinetics of 2CaO·Fe2O3 and CaO·Fe2O3 in the CaO–Fe2O3 system. ISIJ Int. 2016, 56, 1157–1163. [Google Scholar]
- LI, H.; Guo, X. Determination of Gibbs Free Energy of Formation from Elements for Ca4Fe9O17 by Solid-state Galvanic Cell. Metall. Mater. Trans. B 2015, 46B, 278–285. [Google Scholar] [CrossRef]





| Experimental Technique | Experimental Parameter | Reducing Agent | Product | Literatures |
|---|---|---|---|---|
| Direct Reduction | 1050–1150 °C 3–12 h | Coke | Fe powder | Martin et al. [15] |
| Direct Reduction | 900 °C 30–90 min | Low grade coal | Fe powder | Sen et al. [16] |
| Direct Reduction | 750–1050 °C 20–60 min | Carbon monoxide | Fe powder | Benchiheub et al. [18] |
| Direct Reduction | 600–1300 °C 1–4 h | Hydrogen | Fe powder | Sista et al. [22] |
| Direct reduction | 1050–1150 °C 20–60 min | Wood char coal | Fe powder | Ye et al. [23] |
| Direct reduction | 900–1000 °C 1 and 2 h | Coconut fiber | Fe powder | Khaerudini et al. [25] |
| Oxides (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Fe(T) | Fe2O3 | Al2O3 | SiO2 | CaO | SO3 | TiO2 | K2O | P2O5 |
| 69.70 | 93.66 | 0.82 | 1.42 | 0.17 | 0.08 | 0.04 | 0.02 | 0.04 |
| Reductants Name | Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| Fixed C * | Volatile * | Ash * | S ** | H | N | |
| Coal | 84.74 | 9.96 | 5.30 | 0.56 | 1.86 | 1.13 |
| RTB#1 | 85.27 | 6.46 | 8.27 | 0.51 | 0.97 | 0.99 |
| RTB#2 | 82.79 | 8.19 | 9.01 | 0.50 | 0.81 | 0.94 |
| RTB#3 | 81.39 | 8.03 | 10.58 | 0.45 | 0.88 | 0.88 |
| RTB#4 | 79.68 | 10.25 | 10.07 | 0.46 | 0.92 | 0.85 |
| RTB#5 | 80.03 | 9.52 | 10.45 | 0.45 | 0.91 | 0.77 |
| RTB | 56.76 | 24.48 | 18.76 | 0.02 | 0.7 | 0.75 |
| Reductants | SiO2 | Al2O3 | Fe2O3 | CaO | SO3 | TiO2 | K2O | P2O5 |
|---|---|---|---|---|---|---|---|---|
| Coal | 53.55 | 34.12 | 7.15 | 1.10 | 1.26 | 1.42 | 1.01 | 0.39 |
| RTB | 3.19 | 3.98 | 0.22 | 75.94 | 2.10 | 0.06 | 13.8 | 0.70 |
| CCP Name | Composition | Total Weight (g) | |
|---|---|---|---|
| Carbon (g) | Scale (g) | ||
| Coal | 3.74 | 10 | 13.74 |
| RTB#1 | 3.72 | 10 | 13.72 |
| RTB#2 | 3.83 | 10 | 13.83 |
| RTB#3 | 3.89 | 10 | 13.89 |
| RTB#4 | 3.98 | 10 | 13.98 |
| RTB#5 | 3.96 | 10 | 13.96 |
| RTB | 5.58 | 10 | 15.58 |
| Samples | Input (mg) | Inflection Point, I.P. (°C) | % Output at I.P. | % Wt. Loss at I.P | % Output at 1550 °C | % Wt. Loss at 1550 °C |
|---|---|---|---|---|---|---|
| Coal | 9.736 | 1223.33 | 56.54 | 43.46 | 46.02 | 53.98 |
| RTB#1 | 12.362 | 1225.00 | 68.02 | 31.98 | 73.08 | 26.92 |
| RTB#2 | 11.898 | 1223.00 | 62.46 | 37.54 | 62.97 | 37.03 |
| RTB#3 | 11.392 | 1220.00 | 56.04 | 43.96 | 55.64 | 44.36 |
| RTB#4 | 9.282 | 1217.67 | 60.30 | 39.70 | 61.91 | 38.09 |
| RTB#5 | 13.145 | 1224.33 | 57.35 | 42.65 | 57.25 | 42.75 |
| RTB | 10.651 | 1120.33 | 53.48 | 46.52 | 46.92 | 53.08 |
| CCP Name | Before | % Fe(Met) | ||
|---|---|---|---|---|
| % Fixed Carbon | % Fe2O3 | % Fe(T) | After 30 min | |
| Coal | 23.07 | 68.17 | 47.64 | 23.05 |
| RTB#1 | 23.12 | 68.27 | 47.70 | 36.49 |
| RTB#2 | 22.93 | 67.72 | 47.33 | 39.24 |
| RTB#3 | 22.79 | 67.43 | 47.12 | 39.82 |
| RTB#4 | 22.68 | 67.00 | 46.82 | 38.47 |
| RTB#5 | 22.70 | 67.09 | 46.88 | 35.28 |
| RTB | 20.33 | 60.12 | 42.01 | 6.09 |
| CCP Name | LC (nm) |
|---|---|
| Coal | 1.1893 |
| RTB#1 | 1.1453 |
| RTB#2 | 1.1432 |
| RTB#3 | 1.0310 |
| RTB#4 | 0.9960 |
| RTB#5 | 1.0078 |
| RTB | - |
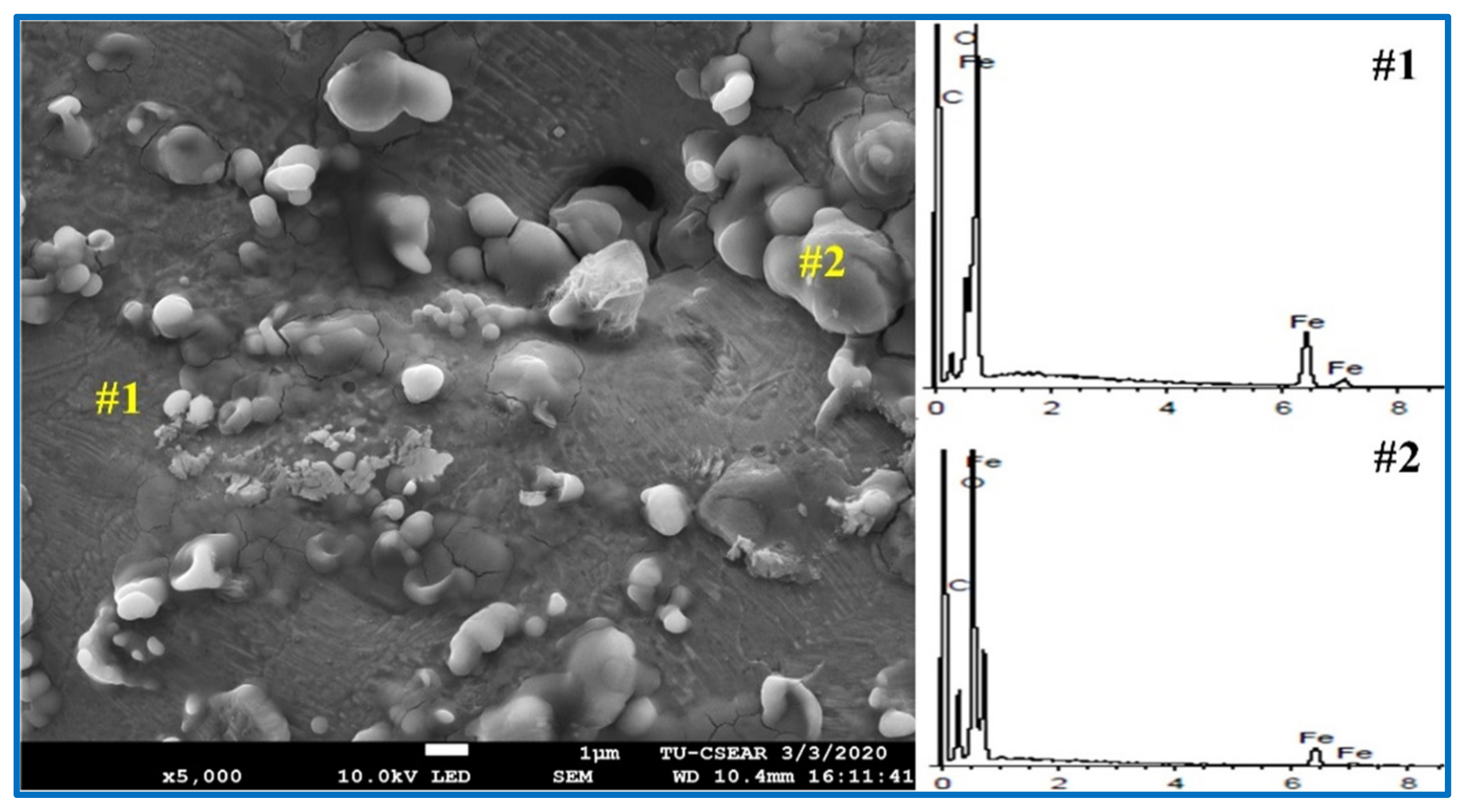
| Samples | Point | Element (Atomic %) | ||||
|---|---|---|---|---|---|---|
| C | Fe | O | Ca | S | ||
| Coal | 1 | 16.74 | 68.55 | 14.70 | - | - |
| 2 | 27.92 | 26.20 | 45.88 | - | - | |
| RTB#3 | 1 | 92.27 | 7.73 | - | - | - |
| 2 | 8.52 | 38.25 | 53.23 | - | - | |
| RTB | 1 | 7.69 | 37.52 | 54.01 | 0.46 | 0.32 |
| 2 | 9.35 | 33.14 | 56.65 | 0.47 | 0.39 | |
| Size (Nm) | Price (Thai Baht/Ton) | Quantity (Kg/Ton of Steel) | Location |
|---|---|---|---|
| 1–3 | 6000 | 24 | EAF |
| 3–6 | 7500 | 14 | EAF |
| 10–15 | 9000 | 3 | Ladle furnace |
| Samples | Coal | RTB | Price |
|---|---|---|---|
| (wt.%) | (wt.%) | (Thai Bath/Ton) | |
| Coal | 100 | 0 | 6700 |
| RTB#1 | 90 | 10 | 6230 |
| RTB#2 | 80 | 20 | 5760 |
| RTB#3 | 70 | 30 | 5290 |
| RTB#4 | 60 | 40 | 4820 |
| RTB#5 | 50 | 50 | 4350 |
| RTB | 0 | 100 | 2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kongkarat, S.; Khumpa, J. Utilization of Rubber Tree Bark for Reduction of Mill Scale at 1550 °C: Implication for Sustainable Wastes Recycling in Steelmaking Process. Metals 2021, 11, 1738. https://doi.org/10.3390/met11111738
Kongkarat S, Khumpa J. Utilization of Rubber Tree Bark for Reduction of Mill Scale at 1550 °C: Implication for Sustainable Wastes Recycling in Steelmaking Process. Metals. 2021; 11(11):1738. https://doi.org/10.3390/met11111738
Chicago/Turabian StyleKongkarat, Somyote, and Jintana Khumpa. 2021. "Utilization of Rubber Tree Bark for Reduction of Mill Scale at 1550 °C: Implication for Sustainable Wastes Recycling in Steelmaking Process" Metals 11, no. 11: 1738. https://doi.org/10.3390/met11111738
APA StyleKongkarat, S., & Khumpa, J. (2021). Utilization of Rubber Tree Bark for Reduction of Mill Scale at 1550 °C: Implication for Sustainable Wastes Recycling in Steelmaking Process. Metals, 11(11), 1738. https://doi.org/10.3390/met11111738






