Investigation on Vanadium Chemistry in Basic-Oxygen-Furnace (BOF) Slags—A First Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Bulk Chemical Analysis
2.2.2. X-ray Powder Diffraction (PXRD)
2.2.3. Electron Probe Microanalysis (EPMA)
2.2.4. Modeling
3. Results
3.1. Bulk Chemical Analysis
3.2. X-ray Powder Diffraction
3.3. EPMA—Analysis and Calculations
- − A: Calcium-orthosilicate-like compound (COS, X2YO4) with Ca, Si, and less V and P).
- − B/C: Wüstite-like primitive oxides (WLO, XO with Ca, Mg, and Fe).
- − D: Dicalcium ferrite solid solution (DFS, X2Y2O5 with Ca, Fe, and less Al, Mg, V, Cr, and Mn).
3.3.1. Modeling of the Composition of the Different Grain Types—Characterization of COS
3.3.2. Modeling of the Composition of the Different Grain Types—Characterization of DFS
4. Discussion
4.1. Vanadium Host Compounds—COS and DFS
4.2. Modeling
4.3. Critical Evaluation of the Virtual Component Modeling Results
4.3.1. Presence of V3+, V4+, and V5+
4.3.2. Ferrous Iron Next to Pentavalent Vanadium
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Meas. A.1 | Ca2SiO4 | Ca2VO4 | Ca3(VO4)2 | Ca3(PO4)2 | Fe2SiO4 | Opt. | CaO | |||
| F | 0.813379 | 0.027384 | 0.020103 | 0.028910 | 0.017558 | 0.063008 | ||||
| Ca | 45.3 | 37.9 | 1.1 | 0.7 | 1.1 | 40.8 | 4.5 | |||
| Fe2+ | 0.96 | 0.96 | 0.96 | |||||||
| Fe3+ | ||||||||||
| V4+ | 1.3 | 0.7 | 0.7 | |||||||
| V5+ | 0.6 | 0.6 | ||||||||
| Si | 13.5 | 13.3 | 0.2 | 13.5 | ||||||
| P5+ | 0.58 | 0.58 | 0.58 | |||||||
| O | 35.4 | 30.2 | 0.9 | 0.7 | 1.2 | 0.6 | 33.7 | 1.8 | ||
| Meas. A.3 | Ca2SiO4 | Ca2VO4 | Ca3(VO4)2 | Ca3(PO4)2 | Fe2SiO4 | Ca2CrO4 | Opt. | CaO | ||
| F | 0.296813 | 0.591268 | 0.039931 | 0.035279 | 0.010947 | 0.019858 | 0.000573 | |||
| Ca | 41.7 | 13.8 | 24.3 | 1.4 | 1.4 | 0.8 | 41.7 | 0.04 | ||
| Fe2+ | 0.6 | 0.6 | 0.6 | |||||||
| Fe3+ | ||||||||||
| V4+ | 16.6 | 15.4 | 15.4 | |||||||
| V5+ | 1.2 | 1.2 | ||||||||
| Si | 4.89 | 4.8 | 0.2 | 4.99 | ||||||
| P5+ | 0.70 | 0.70 | 0.70 | |||||||
| Cr4+ | 0.526 | 0.526 | 0.526 | |||||||
| O | 32.8 | 11.0 | 19.4 | 1.5 | 1.5 | 0.4 | 0.7 | 34.4 | 0.02 | |
| Meas. D.2 | Ca2Fe2O5 | Ca2FeAlO5 | Ca2FeTiO5 | Ca2AlVO5 | Ca2CrFeO5 | Ca2AlMnO5 | Opt. | CaO | ||
| F | 0.390686 | 0.040088 | 0.220377 | 0.140206 | 0.113393 | 0.024584 | 0.058106 | |||
| Ca | 31.3 | 11.5 | 1.3 | 6.7 | 4.7 | 3.4 | 0.8 | 28.5 | 4.2 | |
| Fe2+ | 24.0 | 16.1 | 0.9 | 4.7 | 2.4 | 24.0 | ||||
| V3+ | 3.0 | 3.0 | 3.0 | |||||||
| Al3+ | 2.3 | 0.4 | 1.6 | 0.3 | 2.3 | |||||
| Cr3+ | 2.2 | 2.2 | 2.2 | |||||||
| Ti3+ | 4.0 | 4.0 | 4.0 | |||||||
| Mn3+ | 0.56 | 0.56 | 0.56 | |||||||
| O | 27.4 | 11.5 | 1.3 | 6.7 | 4.7 | 3.4 | 0.8 | 28.4 | 1.7 | |
| Meas. D.3 | Ca2Fe2O5 | Ca2FeAlO5 | Ca2FeTiO5 | Ca2AlVO5 | Ca2CrFeO5 | Ca2AlMnO5 | Opt. | CaO | ||
| F | 0.516164 | 0.051918 | 0.165283 | 0.136467 | 0.056696 | 0.022828 | 0.020915 | |||
| Ca | 30.5 | 15.2 | 1.7 | 5.0 | 4.6 | 1.67 | 0.8 | 29.00 | 1.50 | |
| Fe2+ | 27.08 | 21.2 | 1.2 | 3.50 | 1.18 | 27.08 | ||||
| V3+ | 2.92 | 2.92 | 2.92 | |||||||
| Al3+ | 2.37 | 0.57 | 1.55 | 0.25 | 2.37 | |||||
| Cr3+ | 1.1 | 1.1 | 1.1 | |||||||
| Ti3+ | 3.0 | 3.0 | 3.0 | |||||||
| Mn3+ | 0.52 | 0.52 | 0.52 | |||||||
| O | 26.86 | 15.2 | 1.71 | 5.01 | 4.59 | 1.69 | 0.76 | 28.94 | 0.60 | |
References
- Worldsteel Association. 2020 World Steel in Figures; Worldsteel Association: Brussels, Belgium, 2020; pp. 11–12. [Google Scholar]
- Preßlinger, H.; Mayr, M.; Apfolterer, R. Quantitative phase evaluation of converter slags. Steel Res. 1999, 70, 209–214. [Google Scholar] [CrossRef]
- Aarabi-Karasgani, M.; Rashchi, F.; Mostoufi, N.; Vahidi, E. Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy 2010, 102, 14–21. [Google Scholar] [CrossRef]
- Kelley, K.D.; Scott, C.T.; Polyak, D.E.; Kimball, B.E. Vanadium; Schulz, K.J., DeYoung, J.H., Jr., Seal, R.R., II, Bradley, D.C., Eds.; U.S. Geological Survey Professional Paper 1802; Critical Mineral Resources of the United States-Economic and Environmental Geology and Prospects for Future Supply: Reston, VA, USA, 2017; pp. U1–U36.
- Pourret, O.; Dia, A. Encyclopedia of Geochemistry; White, B., Casey, W., Goldstein, S., Harnett, H., Marty, B., Yurimoto, H., Eds.; Chapter Vanadium; Springer: Deutschland, Germany, 2018; pp. 1474–1476. [Google Scholar] [CrossRef] [Green Version]
- Holleman, A.F.; Wiberg, N.; Wiberg, E. Lehrbuch der Anorganischen Chemie. Walter de Gruyter: Berlin, Germany, 2007. [Google Scholar] [CrossRef]
- Toplis, M.J.; Corgne, A. An experimental study of element partitioning between magnetite, clinopyroxene and iron-bearing silicate liquids with particular emphasis on vanadium. Contrib. Miner. Petrol. 2002, 144, 22–37. [Google Scholar] [CrossRef]
- Righter, K.; Leeman, W.P.; Hervig, R.L. Partitioning of Ni, Co and V between spinel-structured oxides and silicate melts: Importance of spinel composition. Chem. Geol. 2006, 227, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, H. An electrochemical series of redox couples in silicate melts: A review and applications to geochemistry. J. Geophys. Res. 1987, 92, 9225. [Google Scholar] [CrossRef]
- Inoue, R.; Suito, H. Distribution of Vanadium between Liquid Iron and MgO-saturated Slags of the System CaO-MgO-FeOx&SiO2. Tetsu-to-Hagane 1982, 68, 1532–1540. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Li, J.; Yu, Y.; Zhu, H. Effect of Cooling Rate on Crystallization Behavior of CaO-SiO2-MgO-Cr2O3 Based Slag. High Temp. Mater. Process. 2020, 39, 74–80. [Google Scholar] [CrossRef]
- Hobson, A.J.; Stewart, D.I.; Bray, A.W.; Mortimer, R.J.G.; Mayes, W.M.; Rogerson, M. Mechanism of Vanadium Leaching during Surface Weathering of Basic Oxygen Furnace Steel Slag Blocks: A Microfocus X-ray Absorption Spectroscopy and Electron Microscopy Study. Environ. Sci. Technol. 2017, 51, 7823–7830. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Qin, T.; Li, X.; Liu, Y.; Huang, J.-H.; Wu, Z. First-principles investigation of vanadium isotope fractionation in solution and during adsorption. Earth Planet. Sci. Lett. 2015, 426, 216–224. [Google Scholar] [CrossRef]
- Preßlinger, H.; Klepp, K.O. Vanadium in converter slags. Steel Res. 2016, 73, 522–525. [Google Scholar] [CrossRef]
- Hobson, A.J.; Stewart, D.I.; Bray, A.W.; Mortimer, R.J.G.; Mayes, W.M.; Riley, A.L. Behaviour and fate of vanadium during the aerobic neutralisation of hyperalkaline slag leachate. Sci. Total Environ. 2018, 643, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Dill, H.G. The “chessboard” classification scheme of mineral deposits: Mineralogy and geology from aluminum to zirconium. Earth-Sci. Rev. 2010, 100, 1–420. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B: Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Merlet, C. Quantitative Electron Probe Microanalysis: New Accurate Φ (ρz) Description. In Electron Microbeam Analysis; Springer: Vienna, Austria, 1992; pp. 107–115. [Google Scholar] [CrossRef]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Saalfeld, H. Kristallchemische Untersuchungen im System Ca2SiO4–Ca3(PO4)2. Beitrag zur Frage der Diadochie des Siliciums und Phosphors. Z. Krist.-Cryst. Mater. 1971, 197, 133. [Google Scholar] [CrossRef]
- McConnell, D.; Verhoek, F. Crystals, minerals and chemistry. J. Chem. Educ. 1963, 40, 512. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Dong, Y. Enrichment and crystallization of vanadium in factory steel slag. Metallurgist 2011, 55, 401–409. [Google Scholar] [CrossRef]
- Wachsmuth, F.; Geiseler, J.; Fix, W.; Koch, K.; Schwerdtfeger, K. Contribution to the Structure of BOF-Slags and its Influence on Their Volume Stability. Can. Metall. Q. 1981, 20, 279–284. [Google Scholar] [CrossRef]
- Mohan, K.; Glasser, F.P. The thermal decomposition of Ca3SiO5 at temperatures below 1250 °C I. Pure C3S and the influence of excess CaO or Ca2SiO4. Cem. Concr. Res. 1977, 7, 1–7. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. The principles of distribution of chemical elements in minerals and rocks. The seventh Hugo Müller Lecture, delivered before the Chemical Society on March 17th. J. Chem. Soc. 1937, 655–673. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Mittelstadt, R.; Schwerdtfeger, K. The dependence of the oxidation state of vanadium on the oxygen pressure in melts of VOx, Na2O-VOx, and CaO-SiO2-VOx. Metall. Trans. B 1990, 21, 111–120. [Google Scholar] [CrossRef]
- Sakkas, K.; Kapelar, S.; Panias, D.; Nomikos, P.; Sofianos, A. Slag Atlas, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany; Deutschland, Germany, 1995; pp. 10–19. [Google Scholar]
- Farah, H. Oxidation-reduction equilibria of vanadium in CaO-SiO2, CaO-Al2O3-SiO2 and CaO-MgO-SiO2 melts. J. Mater. Sci. 2003, 38, 1885–1894. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Seetharaman, S. Determination of Vanadium Oxidation States in CaO-MgO-Al2O3-SiO2-VOx System by K Edge XANES Method. Steel Res. Int. 2016, 87, 199–209. [Google Scholar] [CrossRef]
- Park, J.M. Iron redox equilibria in BOF slag equilibrated with ambient air. Steel Res. 2002, 73, 39–43. [Google Scholar] [CrossRef]
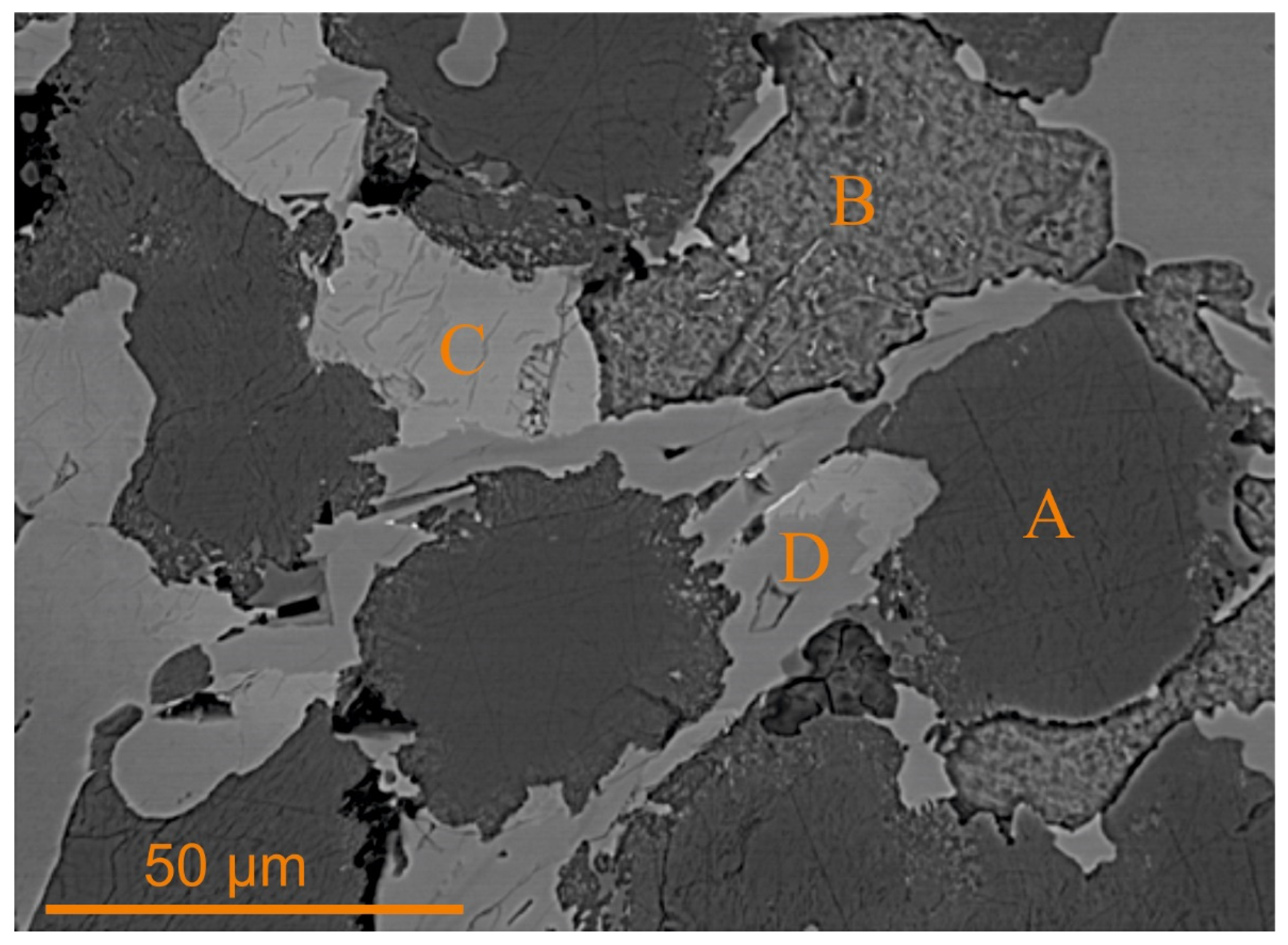
| Ca | Fe | Si | Mg | Al | Cr | P | Mn | V | Ti |
|---|---|---|---|---|---|---|---|---|---|
| 29.7 (0.3) | 15.8 (0.3) | 5.26 (0.15) | 5.18 (0.16) | 0.60 (0.06) | 0.48 (0.05) | 0.221 (0.012) | 2.23 (0.02) | 1.90 (0.04) | 0.76 (0.02) |
| Ca2SiO4 | Ca2Fe2O5 | (Fe,Mg)O | CaO | Ca(OH)2 | CaCO3 | Spinel |
|---|---|---|---|---|---|---|
| 45 | 14 | 19 | 3 | 12 | 6 | 1 |
| A-Type | B-Type | C-Type | D-Type | |
|---|---|---|---|---|
| Ca | 42 (4) | 54.4 (1.4) | 1.6 (0.6) | 30.7 (0.5) |
| Fe | 0.8 (0.4) | 11.1 (0.5) | 47 (5) | 26 (3) |
| Si | 9 (4) | 0.09 (0.03) | 0.022 (0.009) | 0.46 (0.11) |
| V | 8 (8) | 0.033 (0.009) | 0.09 (0.02) | 2.9 (0.2) |
| Cr | 0.3 (0.2) | 0.10 (0.05) | 1.3 (0.6) | 1.4 (0.9) |
| P | 0.7 (0.1) | 0.020 (0.005) | - | - |
| O | 33 (3) | 27.1 (0.2) | 28.5 (1.5) | 27.0 (0.5) |
| Al | 0.10 (0.05) | 0.006 (0.003) | 0.013 (0.007) | 2.4 (0.2) |
| Mg | - | 1.0 (0.3) | 18 (4) | 0.43 (0.11) |
| Mn | 0.05 (0.02) | 4.7 (0.3) | 6.5 (0.9) | 0.54 (0.07) |
| Ti | 0.24 (0.09) | - | - | 3.3 (1.0) |
| COS-Si | COS-V | ||||
|---|---|---|---|---|---|
| Grain | A.1 | A.2 | A.3 | A.4 | Pure Ca2SiO4 |
| Ca | 45.3 (0.4) | 45.5 (0.3) | 41.7 (0.6) | 41.9 (0.3) | 46.54 |
| Fe | 0.96 (0.07) | 1.0 (0.3) | 0.6 (0.2) | 0.8 (0.5) | - |
| Si | 13.5 (0.4) | 13.7 (0.3) | 4.89 (0.13) | 4.8 (0.2) | 16.31 |
| V | 1.3 (0.4) | 1.1 (0.2) | 16.6 (0.4) | 16.2 (0.3) | - |
| Cr | 0.082 (0.011) | 0.092 (0.013) | 0.526 (0.010) | 0.50 (0.03) | - |
| P | 0.58 (0.02) | 0.59 (0.02) | 0.70 (0.07) | 0.72 (0.11) | - |
| O | 35.4 (0.3) | 35.7 (0.3) | 32.8 (0.3) | 32.8 (0.3) | 30.08 |
| Meas. A.2 | Ca2SiO4 | Ca2VO4 | Ca3(VO4)2 | Ca3(PO4)2 | Fe2SiO4 | Opt. | CaO | ||
| F | 0.824853 | 0.023171 | 0.017010 | 0.029576 | 0.018244 | 0.061952 | |||
| Ca | 45.5 | 38.4 | 1.0 | 0.6 | 1.1 | 40.5 | 4.7 | ||
| Fe2+ | 1.0 | 1.0 | 1.0 | ||||||
| Fe3+ | |||||||||
| V4+ | 1.1 | 0.6 | 0.6 | ||||||
| V5+ | 0.5 | 0.5 | |||||||
| Si | 13.7 | 13.5 | 0.3 | 13.7 | |||||
| P5+ | 0.59 | 0.59 | 0.59 | ||||||
| O | 35.7 | 30.6 | 0.8 | 0.6 | 1.2 | 0.6 | 35.0 | 1.8 | |
| Meas. A.4 | Ca2SiO4 | Ca2VO4 | Ca3(VO4)2 | Ca3(PO4)2 | Fe2SiO4 | Ca2CrO4 | Opt. | CaO | |
| F | 0.282071 | 0.577020 | 0.038969 | 0.035905 | 0.014595 | 0.018957 | 0.021793 | ||
| Ca | 41.9 | 13.1 | 23.7 | 1.3 | 1.4 | 0.8 | 40.3 | 1.6 | |
| Fe2+ | 0.8 | 0.8 | 0.8 | ||||||
| Fe3+ | |||||||||
| V4+ | 16.2 | 15.1 | 15.1 | ||||||
| V5+ | 1.1 | 1.1 | |||||||
| Si | 4.8 | 4.6 | 0.2 | 4.8 | |||||
| P5+ | 0.72 | 0.72 | 0.72 | ||||||
| Cr4+ | 0.50 | 0.50 | 0.50 | ||||||
| O | 32.8 | 10.5 | 18.9 | 1.4 | 1.5 | 0.5 | 0.6 | 33.4 | 0.6 |
| Grain | D.1 | D.2 | D.3 | Ideal Ca2Fe2O5 |
|---|---|---|---|---|
| Ca | 30.5 (0.3) | 31.3 (0.8) | 30.50 (0.03) | 29.49 |
| Fe | 28 (2) | 24 (3) | 27.08 (0.05) | 41.09 |
| Ti | 3.0 (0.8) | 4.0 (1.1) | 3.0 (0.3) | - |
| V | 2. 8 (0.2) | 3.0 (0.205) | 2.92 (0.06) | - |
| Cr | 1.1 (0.8) | 2.2 (1.0) | 1.1 (0.2) | - |
| Al | 2.4 (0.2) | 2.3 (0.4) | 2.37 (0.04) | - |
| Mn | 0.55 (0.04) | 0.56 (0.06) | 0.52 (0.02) | - |
| O | 27.0 (0.7) | 27.4 (0.4) | 26.86 (0.11) | 29.43 |
| Meas. D.1 | Ca2Fe2O5 | Ca2FeAlO5 | Ca2FeTiO5 | Ca2AlVO5 | Ca2CrFeO5 | Ca2AlMnO5 | Opt. | CaO | |
|---|---|---|---|---|---|---|---|---|---|
| F | 0.534560 | 0.059061 | 0.165283 | 0.130859 | 0.056696 | 0.024145 | 0.012059 | ||
| Ca | 30.5 | 15.8 | 1.9 | 5.0 | 4.4 | 1.7 | 0.8 | 29.6 | 0.9 |
| Fe3+ | 28.0 | 22.0 | 1.34 | 3.5 | 1.2 | 28.0 | |||
| V3+ | 2.8 | 2.8 | 2.8 | ||||||
| Al3+ | 2.4 | 0.7 | 1.4 | 0.3 | 2.4 | ||||
| Cr3+ | 1.1 | 1.1 | 1.1 | ||||||
| Ti3+ | 3.0 | 23.0 | 3.0 | ||||||
| Mn3+ | 0.55 | 0.55 | 0.55 | ||||||
| O | 27.00 | 15.7 | 1.9 | 5.0 | 4.4 | 1.7 | 0.8 | 29.6 | 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunderlich, S.; Schirmer, T.; Fittschen, U.E.A. Investigation on Vanadium Chemistry in Basic-Oxygen-Furnace (BOF) Slags—A First Approach. Metals 2021, 11, 1869. https://doi.org/10.3390/met11111869
Wunderlich S, Schirmer T, Fittschen UEA. Investigation on Vanadium Chemistry in Basic-Oxygen-Furnace (BOF) Slags—A First Approach. Metals. 2021; 11(11):1869. https://doi.org/10.3390/met11111869
Chicago/Turabian StyleWunderlich, Sophie, Thomas Schirmer, and Ursula E. A. Fittschen. 2021. "Investigation on Vanadium Chemistry in Basic-Oxygen-Furnace (BOF) Slags—A First Approach" Metals 11, no. 11: 1869. https://doi.org/10.3390/met11111869
APA StyleWunderlich, S., Schirmer, T., & Fittschen, U. E. A. (2021). Investigation on Vanadium Chemistry in Basic-Oxygen-Furnace (BOF) Slags—A First Approach. Metals, 11(11), 1869. https://doi.org/10.3390/met11111869






