Gold Compounds Inhibit the Ca2+-ATPase Activity of Brain PMCA and Human Neuroblastoma SH-SY5Y Cells and Decrease Cell Viability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gold Complexes
2.2. Preparation of Purified Synaptosomal PMCA
2.3. Ca2+-ATPase Activity
2.4. Neuroblastoma SH-SY5Y Cell Cultures, Cell Viability, and Membrane Preparation to Measure Ca2+-ATPase Activity
2.5. Statistical Analysis
3. Results
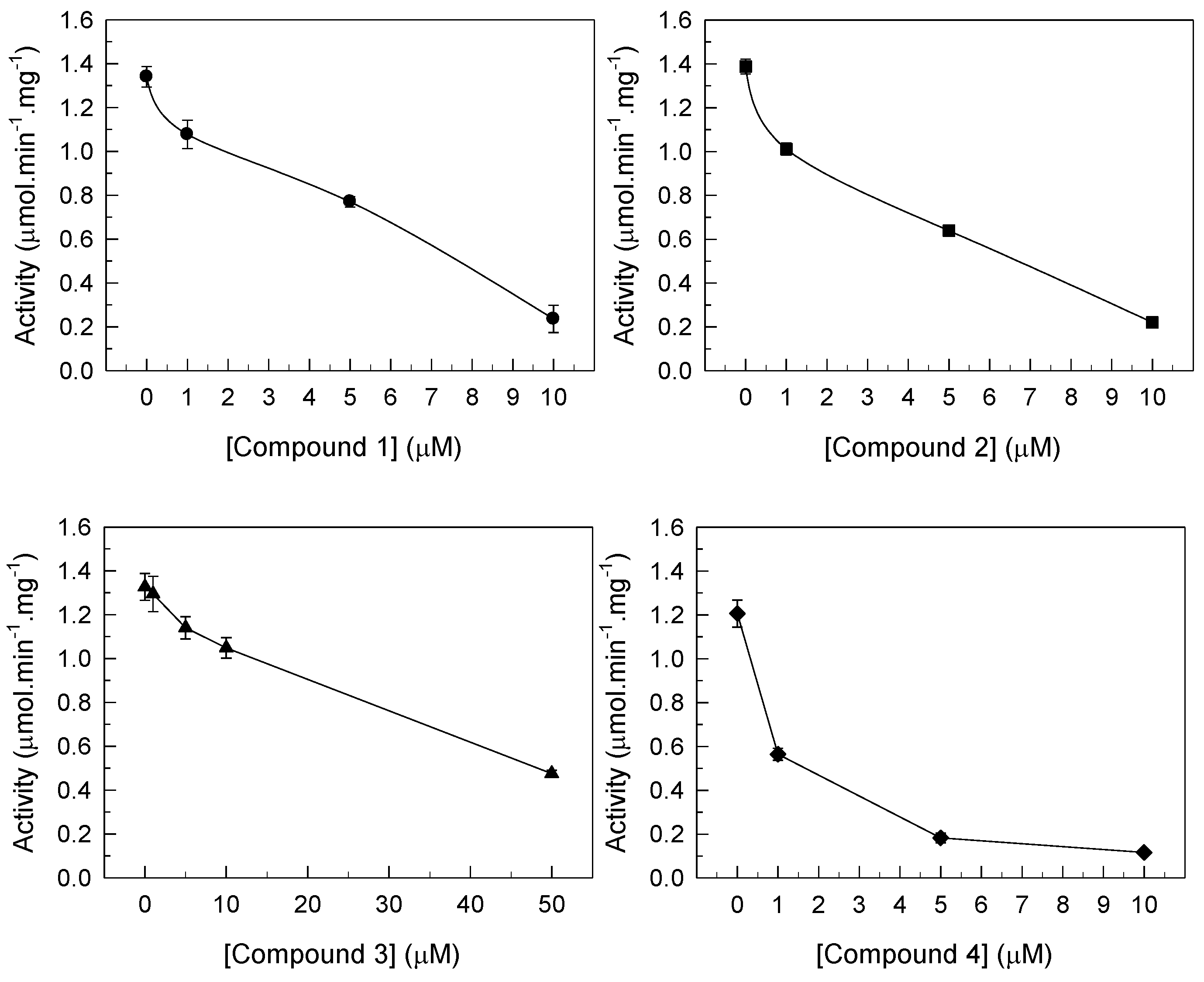
3.1. Inhibition of PMCA Activity by Gold Compounds
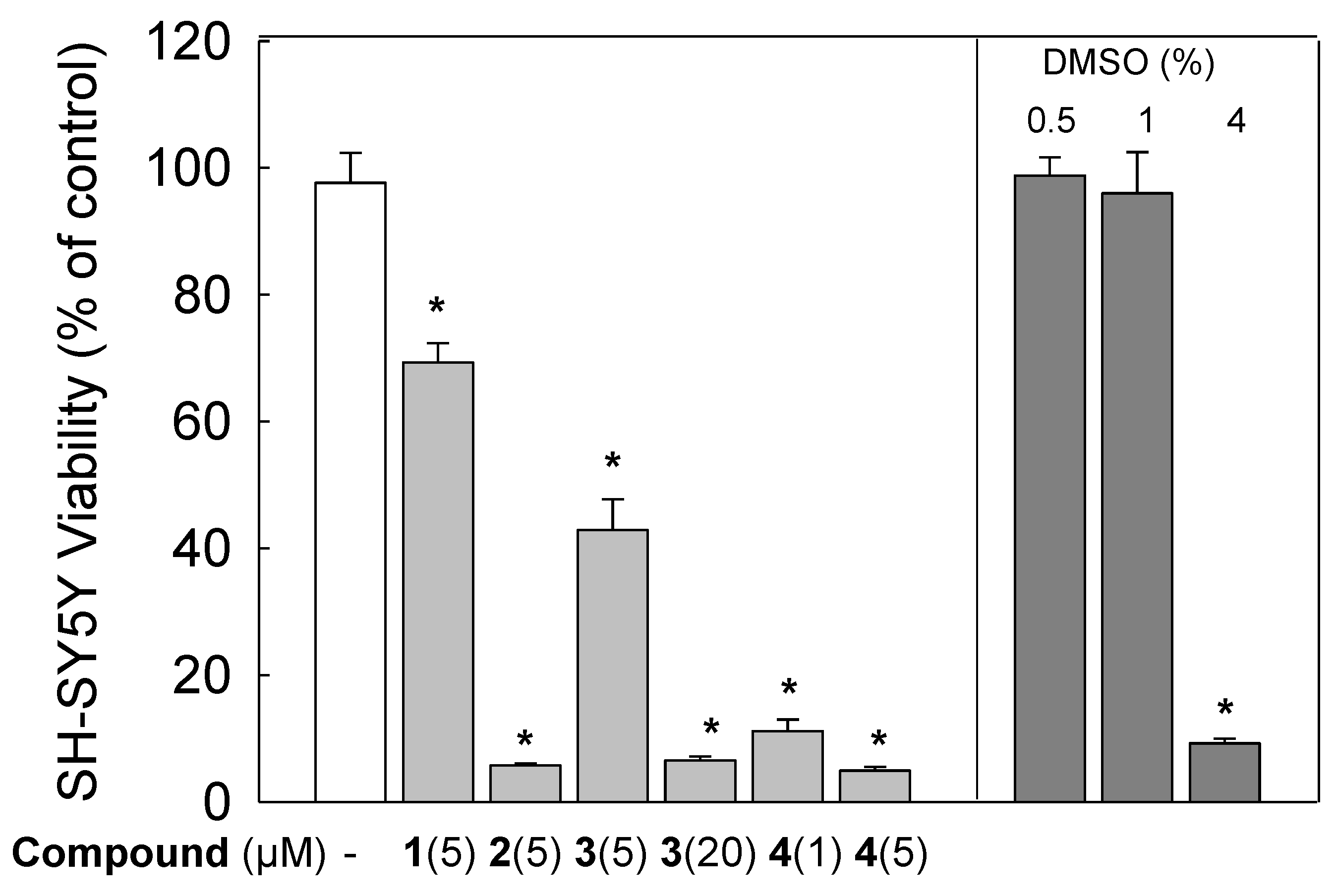
3.2. Cytotoxic Effects of Gold Compounds in SH-SY5Y Cells
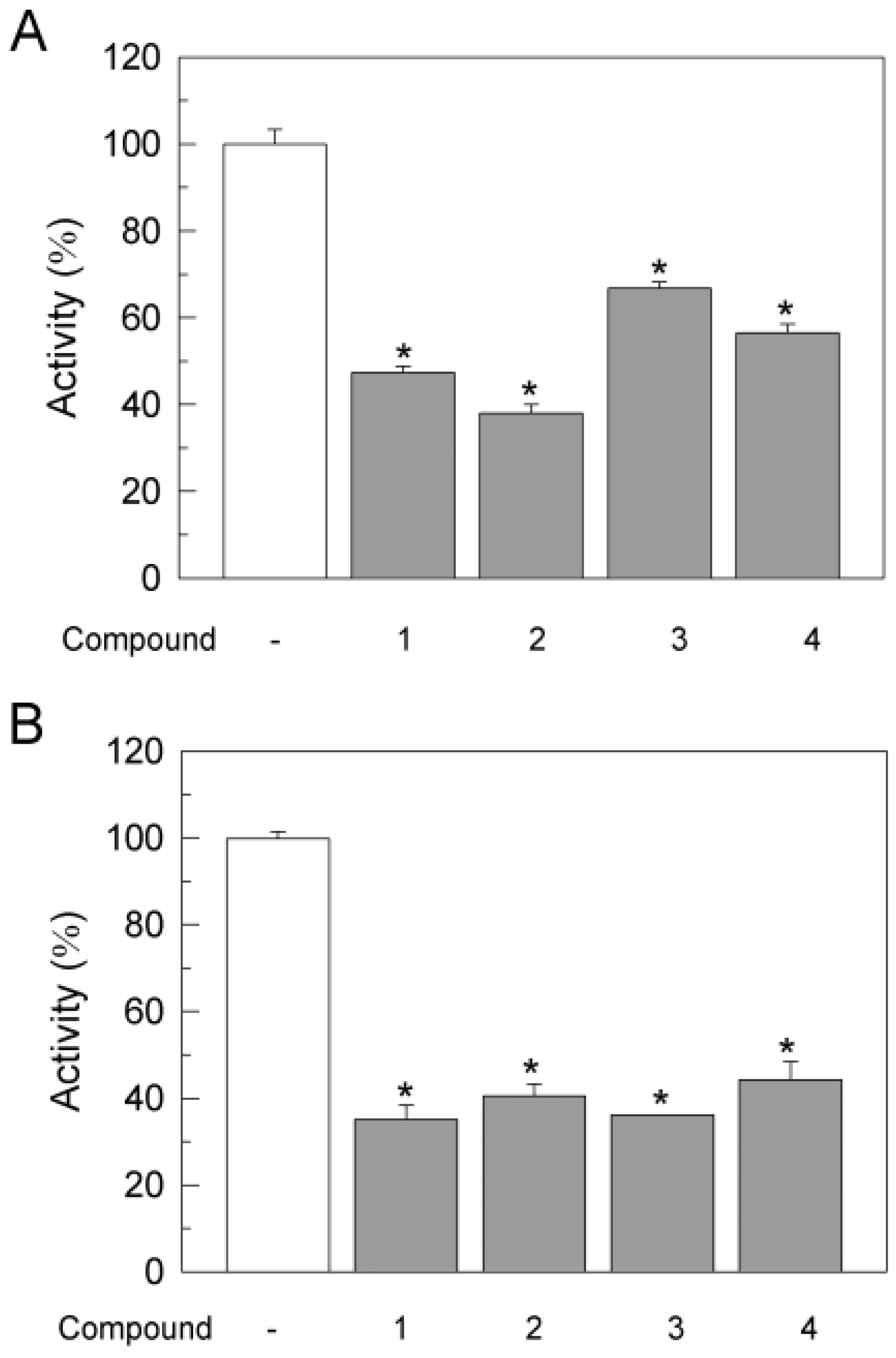
3.3. Effects of Gold Compounds on Ca2+-ATPase Activity in SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATPase | Adenosine triphosphatase |
| Auoxo6 | [(6,6′-dimethyl-2,2′-bipyridine)2Au2(μ-O)2][PF6]2 |
| BMOV | Bismaltol oxidovanadium(IV) |
| CPA | Cyclopyazonic |
| DMSO | Dimethyl sulfoxide |
| IC50 | Half maximal inhibitory concentration |
| PDC-V(V) | Pyridine-2,6-dicarboxylatodioxovanadium(V) |
| PMCA | Plasmatic membrane calcium ATPase |
| POTs | Polyoxotungstates |
| PV14O40 | Phosphotetradecavanadate |
| SERCA | Sarco(endo) plasmatic membrane calcium ATPase |
| SPCA | secretory pathway calcium ATPase |
| SR | Sarcoplasmic reticulum |
| TG | Thapsigargin |
| VO4 | Orthovanadate |
| V10O28 | Decavanadate |
References
- Carafoli, E. The Regulation of Intracellular Calcium; Springer: Boston, MA, USA, 1982; pp. 461–472. [Google Scholar]
- Domínguez, D.C.; Guragain, M.; Patrauchan, M. Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium 2015, 57, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Plattner, H.; Verkhratsky, A. The ancient roots of calcium signalling evolutionary tree. Cell Calcium 2015, 57, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Q.; Lakatta, E.G.; Cheng, H.; Zhou, Z.Q. Adaptive mechanisms of intracellular calcium homeostasis in mammalian hibernators. J. Exp. Biol. 2002, 10 Pt 19, 2957–2962. [Google Scholar] [CrossRef]
- Rappas, M.; Niwa, H.; Zhang, X. Mechanisms of ATPases-A Multi-Disciplinary Approach. Curr. Protein Pept. Sci. 2004, 5, 89–105. [Google Scholar] [CrossRef]
- Strehler, E.E.; Zacharias, D.A. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. Physiol. Rev. 2001, 81, 21–50. [Google Scholar] [CrossRef] [Green Version]
- Strehler, E.E.; Caride, A.J.; Filoteo, A.G.; Xiong, Y.; Penniston, J.T.; Enyedi, A. Plasma membrane Ca2+-ATPases as dynamic regulators of cellular calcium handling. Ann. N. Y. Acad. Sci. 2007, 1099, 226–236. [Google Scholar] [CrossRef] [Green Version]
- Wuytack, F.; Raeymaekers, L.; Missiaen, L. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium 2002, 32, 279–305. [Google Scholar] [CrossRef] [PubMed]
- Sadler, P.J.; Sue, R.E. The Chemistry of Gold Drugs. Met. Based Drugs 1994, 1, 107–144. [Google Scholar] [CrossRef] [Green Version]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- Pettenuzzo, N.; Brustolin, L.; Coltri, E.; Gambalunga, A.; Chiara, F.; Trevisan, A.; Biondi, B.; Nardon, C.; Fregona, D. Cu II and Au III Complexes with Glycoconjugated Dithiocarbamato Ligands for Potential Applications in Targeted Chemotherapy. ChemMedChem 2019, 14, 1162–1172. [Google Scholar] [CrossRef]
- Liu, W.; Gust, R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef]
- Stratton, M.; Ramachandran, A.; Camacho, E.J.M.; Patil, S.; Waris, G.; Grice, K.A. Anti-fibrotic activity of gold and platinum complexes-Au(I) compounds as a new class of anti-fibrotic agents. J. Inorg. Biochem. 2020, 206, 111023. [Google Scholar] [CrossRef]
- Azria, D.; Blanquer, S.; Verdier, J.-M.; Belamie, E. Nanoparticles as contrast agents for brain nuclear magnetic resonance imaging in Alzheimer’s disease diagnosis. J. Mater. Chem. B 2017, 5, 7216–7237. [Google Scholar] [CrossRef] [PubMed]
- Petrović, V.; Čolović, M.; Krstić, D.; Vujačić, A.; Petrović, S.; Joksić, G.; Bugarčić, Ž.; Vasić, V. In vitro effects of some gold complexes on Na+/K+ ATPase activity and cell proliferation. J. Inorg. Biochem. 2013, 124, 35–41. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Janjić, G.V.; Dramićanin, M.D.; Messori, L.; Massai, L.; Parac Vogt, T.N.; Vasić, V.M. Na/K-ATPase as a target for anticancer metal based drugs: Insights into molecular interactions with selected gold(iii) complexes. Metallomics 2017, 9, 292–300. [Google Scholar] [CrossRef]
- Fonseca, C.; Fraqueza, G.; Carabineiro, S.A.C.; Aureliano, M. The Ca2+-ATPase inhibition potential of gold(I,III) compounds. Inorganics 2020, 8, 49. [Google Scholar] [CrossRef]
- Aikman, B.; Wenzel, M.; Mósca, A.; de Almeida, A.; Klooster, W.; Coles, S.; Soveral, G.; Casini, A. Gold(III) Pyridine-Benzimidazole Complexes as Aquaglyceroporin Inhibitors and Antiproliferative Agents. Inorganics 2018, 6, 123. [Google Scholar] [CrossRef] [Green Version]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical biology of anticancer gold(iii) and gold(i) complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.L.; Carafoli, E. Ion motive ATPases. II. Energy coupling and work output. Trends Biochem. Sci. 1987, 12, 186–189. [Google Scholar] [CrossRef]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef]
- Aureliano, M.; Fraqueza, G.; Ohlin, C.A. Ion pumps as biological targets for decavanadate. Dalton Trans. 2013, 42, 11770. [Google Scholar] [CrossRef] [PubMed]
- Yatime, L.; Buch-Pedersen, M.J.; Musgaard, M.; Morth, J.P.; Winther, A.-M.L.; Pedersen, B.P.; Olesen, C.; Andersen, J.P.; Vilsen, B.; Schiøtt, B.; et al. P-type ATPases as drug targets: Tools for medicine and science. Biochim. Biophys. Acta-Bioenerg. 2009, 1787, 207–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikezić, A.V.V.; Janjić, G.V.; Bondžić, A.M.; Zarić, B.L.; Vasić-Anićijević, D.D.; Momić, T.G.; Vasić, V.M. Interaction of Au(III) and Pt(II) complexes with Na/K-ATPase: Experimental and theoretical study of reaction stoichiometry and binding sites. Metallomics 2018, 10, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, M.; Marcos, D.; Sepúlveda, M.R.; Pérez, M.; Ávila, J.; Mata, A.M. Altered Ca2+ dependence of synaptosomal plasma membrane Ca2+-ATPase in human brain affected by Alzheimer’s disease. FASEB J. 2009, 23, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, M.; Corbacho, I.; Gutierrez-Merino, C.; Mata, A.M. Methylene blue activates the PMCA activity and cross-interacts with amyloid beta-peptide, blocking Abeta-mediated PMCA inhibition. Neuropharmacology 2018, 139, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Berrocal, M.; Caballero-Bermejo, M.; Gutierrez-Merino, C.; Mata, A.M. Methylene Blue Blocks and Reverses the Inhibitory Effect of Tau on PMCA Function. Int. J. Mol. Sci. 2019, 20, 3521, PMID: 31323781; PMCID: PMC6678728. [Google Scholar] [CrossRef] [Green Version]
- Vosahlikova, M.; Roubalova, L.; Cechova, K.; Kaufman, J.; Musil, S.; Miksik, I.; Alda, M.; Svoboda, P. Na+/K+-ATPase and lipid peroxidation in forebrain cortex and hippocampus of sleep-deprived rats treated with therapeutic lithium concentration for different periods of time. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109953. [Google Scholar] [CrossRef]
- Hosseinzadeh, Z.; Hauser, S.; Singh, Y.; Pelzl, L.; Schuster, S.; Sharma, Y.; Höflinger, P.; Zacharopoulou, N.; Stournaras, C.; Rathbun, D.L.; et al. Decreased Na+/K+ ATPase Expression and Depolarized Cell Membrane in Neurons Differentiated from Chorea-Acanthocytosis Patients. Sci. Rep. 2020, 10, 8391. [Google Scholar] [CrossRef]
- Gómez-Arnaiz, S.; Tate, R.J.; Grant, M.H. Cytotoxicity of cobalt chloride in brain cell lines-A comparison between astrocytoma and neuroblastoma cells. Toxicol. Vitr. 2020, 68, 104958. [Google Scholar] [CrossRef]
- Salvador, J.M.; Mata, A.M. Purification of the synaptosomal plasma membrane (Ca2+ + Mg2+)-ATPase from pig brain. Biochem. J. 1996, 315, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sepúlveda, M.R.; Hidalgo-Sánchez, M.; Mata, A.M. A developmental profile of the levels of calcium pumps in chick cerebellum. J. Neurochem. 2005, 95, 673–683. [Google Scholar] [CrossRef]
- Sciortino, G.; Aureliano, M.; Garribba, E. Rationalizing the decavanadate(V) and oxidovanadium(IV) binding to G-actin and the competition with decaniobate(V) and ATP. Inorg. Chem. 2021, 60, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.; Sepúlveda, M.R.; Mata, A.M. Effect of spermine on the activity of synaptosomal plasma membrane Ca2+-ATPase reconstituted in neutral or acidic phospholipids. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1611, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Palacios, J.; Sepúlveda, M.R.; Lee, A.G.; Mata, A.M. Ca2+ transport by the synaptosomal plasma membrane Ca2+-ATPase and the effect of thioridazine. Biochemistry 2004, 43, 2353–2358. [Google Scholar] [CrossRef]
- Moreno, I.; Norambuena, L.; Maturana, D.; Toro, M.; Vergara, C.; Orellana, A.; Zurita-Silva, A.; Ordenes, V.R. AtHMA1 Is a Thapsigargin-sensitive Ca2+/Heavy Metal Pump. J. Biol. Chem. 2008, 283, 9633–9641. [Google Scholar] [CrossRef] [Green Version]
- Yard, N.J.; Chiesi, M.; Ball, H.A. Effect of cyclopiazonic acid, an inhibitor of sarcoplasmic reticulum Ca2+-ATPase, on the frequency-dependence of the contraction-relaxation cycle of the guinea-pig isolated atrium. Br. J. Pharmacol. 1994, 113, 1001–1007. [Google Scholar] [CrossRef] [Green Version]
- Bilmen, J.G.; Wotton, L.L.; Michelangeli, F. The inhibition of the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase by macrocyclic lactones and cyclosporin A. Biochem. J. 2002, 366, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilmen, J.G.; Khan, S.Z.; Javed, M.-H.; Michelangeli, F. Inhibition of the SERCA Ca2+ pumps by curcumin. Eur. J. Biochem. 2001, 268, 6318–6327. [Google Scholar] [CrossRef]
- Coca, R.; Soler, F.; Cortés-Castell, E.; Gil-Guillén, V.; Fernández-Belda, F. Inhibition mechanism of the intracellular transporter Ca2+-pump from sarco-endoplasmic reticulum by the antitumor agent dimethyl-celecoxib. PLoS ONE 2014, 9, e102083. [Google Scholar] [CrossRef] [Green Version]
- Gumerova, N.; Krivosudský, L.; Fraqueza, G.; Breibeck, J.; Al-Sayed, E.; Tanuhadi, E.; Bijelic, A.; Fuentes, J.; Aureliano, M.; Rompel, A. The P-type ATPase inhibiting potential of polyoxotungstates. Metallomics 2018, 10, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraqueza, G.; Fuentes, J.; Krivosudský, L.; Dutta, S.; Mal, S.S.; Roller, A.; Giester, G.; Rompel, A.; Aureliano, M. Inhibition of Na+/K+- and Ca2+-ATPase activities by phosphotetradecavanadate. J. Inorg. Biochem. 2019, 197, 110700. [Google Scholar] [CrossRef] [PubMed]
- Clausen, J.D.; Bublitz, M.; Arnou, B.; Olesen, C.; Andersen, J.P.; Møller, J.V.; Nissen, P. Crystal Structure of the Vanadate-Inhibited Ca2+-ATPase. Structure 2016, 24, 617–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraqueza, G.; Ohlin, C.A.; Casey, W.H.; Aureliano, M. Sarcoplasmic reticulum calcium ATPase interactions with decaniobate, decavanadate, vanadate, tungstate and molybdate. J. Inorg. Biochem. 2012, 107, 82–89. [Google Scholar] [CrossRef]
- Aureliano, M.; Henao, F.; Tiago, T.; Duarte, R.O.; Moura, J.J.G.; Baruah, B.; Crans, D.C. Sarcoplasmic Reticulum Calcium ATPase Is Inhibited by Organic Vanadium Coordination Compounds: Pyridine-2,6-dicarboxylatodioxovanadium(V), BMOV, and an Amavadine Analogue. Inorg. Chem. 2008, 47, 5677–5684. [Google Scholar] [CrossRef] [Green Version]
- Machado, J.F.; Correia, J.D.G.; Morais, T.S. Emerging Molecular Receptors for the Specific-Target Delivery of Ruthenium and Gold Complexes into Cancer Cells. Molecules 2021, 26, 3153. [Google Scholar] [CrossRef]
- Gamberi, T.; Pratesi, A.; Messori, L.; Massai, L. Proteomics as a tool to disclose the cellular and molecular mechanisms of selected anticancer gold compounds. Coord. Chem. Rev. 2021, 438, 213905. [Google Scholar] [CrossRef]
- Rousselle, B.; Bouyer, F.; Bayardon, J.; Laly, M.; Ghiringhelli, F.; Rousselin, Y.; Bodio, E.; Malacea-Kabbara, R. Development of a novel highly anti-proliferative family of gold complexes: Au(i)-phosphonium-phosphines. Dalton Trans. 2021, 50, 4880. [Google Scholar] [CrossRef]
- Vijaya, P.; Kaur, H.; Garg, N.; Sharma, S. Protective and therapeutic effects of garlic and tomato on cadmium-induced neuropathology in mice. J. Basic Appl. Zool. 2020, 81, 23. [Google Scholar] [CrossRef]
- Donzelli, E.; Carfi, M.; Miloso, M.; Strada, A.; Galbiati, S.; Bayssas, M.; Griffon-Etienne, G.; Cavaletti, G.; Petruccioli, M.G.; Tredici, G. Neurotoxicity of platinum compounds: Comparison of the effects of cisplatin and oxaliplatin on the human neuroblastoma cell line SH-SY5Y. J. Neurooncol. 2004, 67, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Rouco, L.; Sánchez-González, Á.; Alvariño, R.; Alfonso, A.; Vázquez-López, E.M.; García-Martínez, E.; Maneiro, M. Combined Effect of Caspase-Dependent and Caspase-Independent Apoptosis in the Anticancer Activity of Gold Complexes with Phosphine and Benzimidazole Derivatives. Pharmaceuticals 2020, 14, 10. [Google Scholar] [CrossRef] [PubMed]


| Formula | Abbreviation | Net Charge | MW (g/mol) | CAS Number |
|---|---|---|---|---|
| C6H4NAuCl2O2 | 1 | +3 | 389.97 | 88215-41-2 |
| C3H9PAuCl | 2 | +1 | 308.50 | 15278-97-4 |
| C27H36AuClN2 | 3 | +1 | 621.01 | 852445-83-1 |
| C18H15PAuCl | 4 | +1 | 494.71 | 14243-64-2 |
| Gold Compound | Km (mM) | Vmax (µmol·min−1·mg−1) | Type of Inhibition | IC50 (µM) | |
|---|---|---|---|---|---|
| 0 (µM) | 0.172 ± 0.008 | 1.92 ± 0.1 | |||
| 1 | 5 (µM) | 0.170 ± 0.008 | 0.763 ± 0.04 * | non-competitive | 4.9 ± 0.1 |
| 2 | 3 (µM) | 0.242 ± 0.012 * | 0.847 ± 0.04 * | mixed | 2.8 ± 0.2 |
| 3 | 21 (µM) | 0.262 ± 0.013 * | 0.909 ± 0.04 * | mixed | 21 ± 0.1 |
| 4 | 1 (µM) | 0.174 ± 0.008 | 0.704 ± 0.03 * | non-competitive | 0.9 ± 0.1 |
| Class of Inhibitors | Compound | IC50 (µM) | P-Type ATPase | Therapeutical Applications | References |
|---|---|---|---|---|---|
| Polycation | Spermine | 2500 | PMCA | Anticancer | [37] |
| Phenothiazine derivative | Thioridazine | 77 | PMCA | Antipsychotic drug | [38] |
| Gold(I) Compounds Gold(III) compounds | C3H9PAuCl C18H15PAuCl C27H36AuClN2 C6H4NAuCl2O2 H[AuCl4], [Au(DMSO)2Cl2]Cl [Au(bipy)Cl2]Cl | 0.8 0.9 16.3 4.5 0.7 5.5 39.8 | SERCA SERCA SERCA SERCA Na+/K+-ATPase Na+/K+-ATPase Na+/K+-ATPase | Anticancer, antifibrotic | [18] [18] [18] [18] [17] [17] [17] |
| Tungstate compounds and POTs | Se2W29 P2W18 P2W18 Sodium tungstate | 0.3 0.6 4–200 400 | SERCA | Anticancer, antibacteria, antivirus | [44] [44] [44] [47] |
| Vanadium (IV, V) compounds and POVs | PV14O40 V10O28 VO4 PDC-V(V) BMOV | 0.4 15 50 25 40 | SERCA | Insulin-mimetic properties | [45] [47] [47] [48] [48] |
| Sesquiterpene lactone | Thapsigargin | 0.001–0.029 | SERCA | Prodrugs for prostate cancer therapy | [39] |
| Indole tetraminic acid | Cyclopiazoc acid | 0.2–1.0 | SERCA | Cardioprotective action in myocardial ischemia | [40] |
| Macrocyclic lactones | Cyclosporine A Rapamycin | 62 77 | SERCA | Immunossupressant agents | [41] |
| Curcuminoides | Curcumin | 7–17 | SERCA | Antioxidants, antitumoral | [42] |
| Celecoxib analog | Dimethyl-celecoxib | 35 | SERCA | Anti-inflamatory drug | [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berrocal, M.; Cordoba-Granados, J.J.; Carabineiro, S.A.C.; Gutierrez-Merino, C.; Aureliano, M.; Mata, A.M. Gold Compounds Inhibit the Ca2+-ATPase Activity of Brain PMCA and Human Neuroblastoma SH-SY5Y Cells and Decrease Cell Viability. Metals 2021, 11, 1934. https://doi.org/10.3390/met11121934
Berrocal M, Cordoba-Granados JJ, Carabineiro SAC, Gutierrez-Merino C, Aureliano M, Mata AM. Gold Compounds Inhibit the Ca2+-ATPase Activity of Brain PMCA and Human Neuroblastoma SH-SY5Y Cells and Decrease Cell Viability. Metals. 2021; 11(12):1934. https://doi.org/10.3390/met11121934
Chicago/Turabian StyleBerrocal, Maria, Juan J. Cordoba-Granados, Sónia A. C. Carabineiro, Carlos Gutierrez-Merino, Manuel Aureliano, and Ana M. Mata. 2021. "Gold Compounds Inhibit the Ca2+-ATPase Activity of Brain PMCA and Human Neuroblastoma SH-SY5Y Cells and Decrease Cell Viability" Metals 11, no. 12: 1934. https://doi.org/10.3390/met11121934









