Modelling of the Erosive Dissolution of Metal Oxides in a Deep Eutectic Solvent—Choline Chloride/Sulfosalicylic Acid—Assisted by Ultrasonic Cavitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
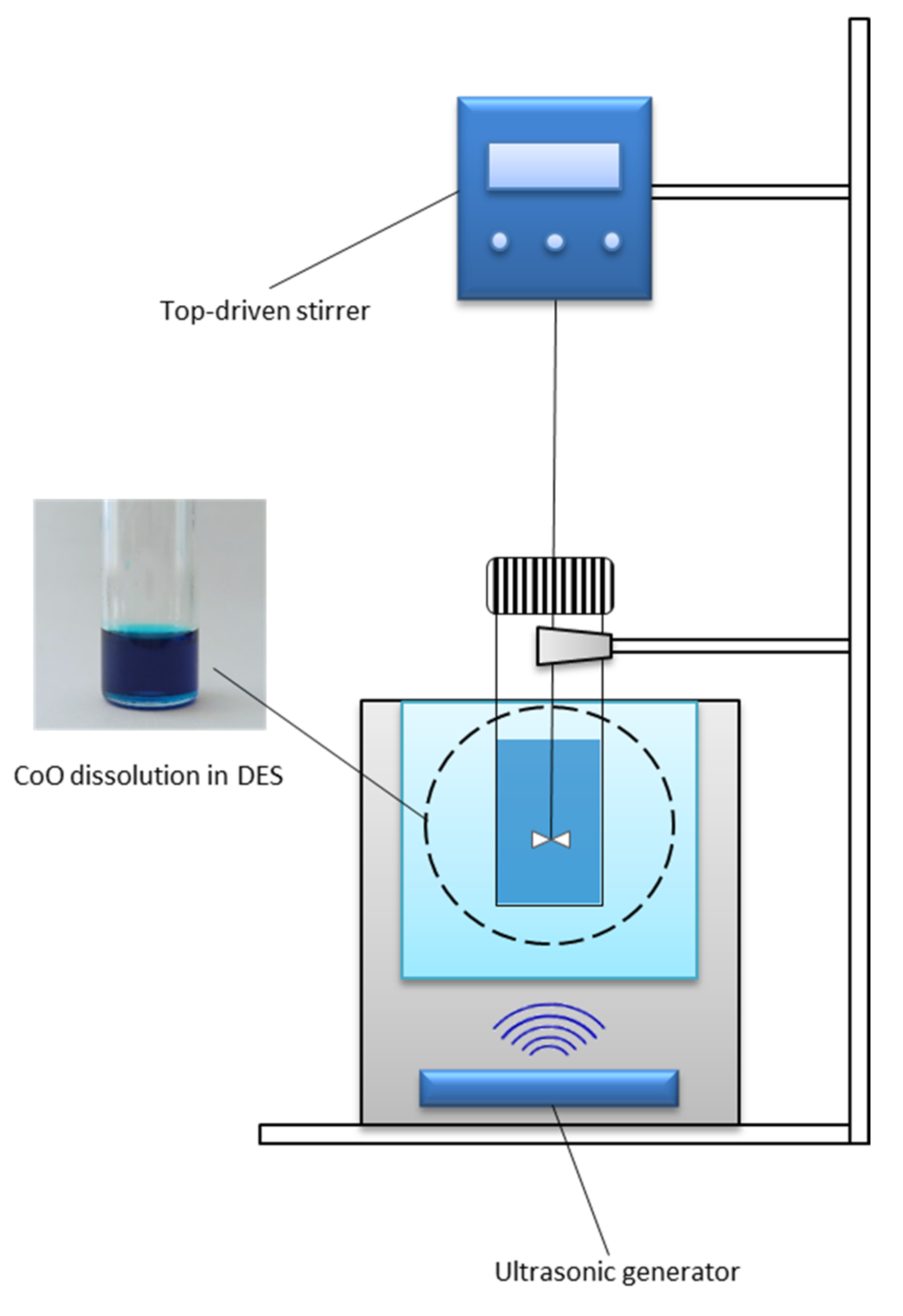
2.2. Experimental, Apparatus and Procedures
3. Theoretical Part
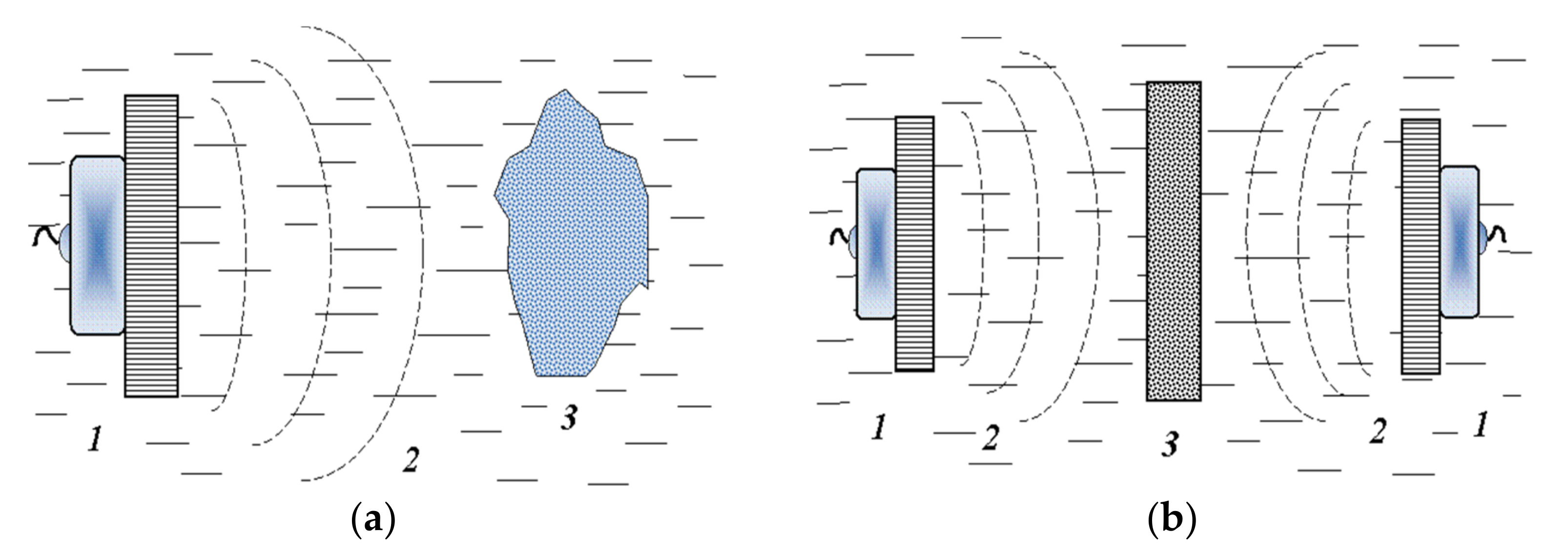
3.1. Applicable Methods and Some Basic Schemes for Solving the Problem
3.2. Force Component of the Ultrasonic Cavitation Action
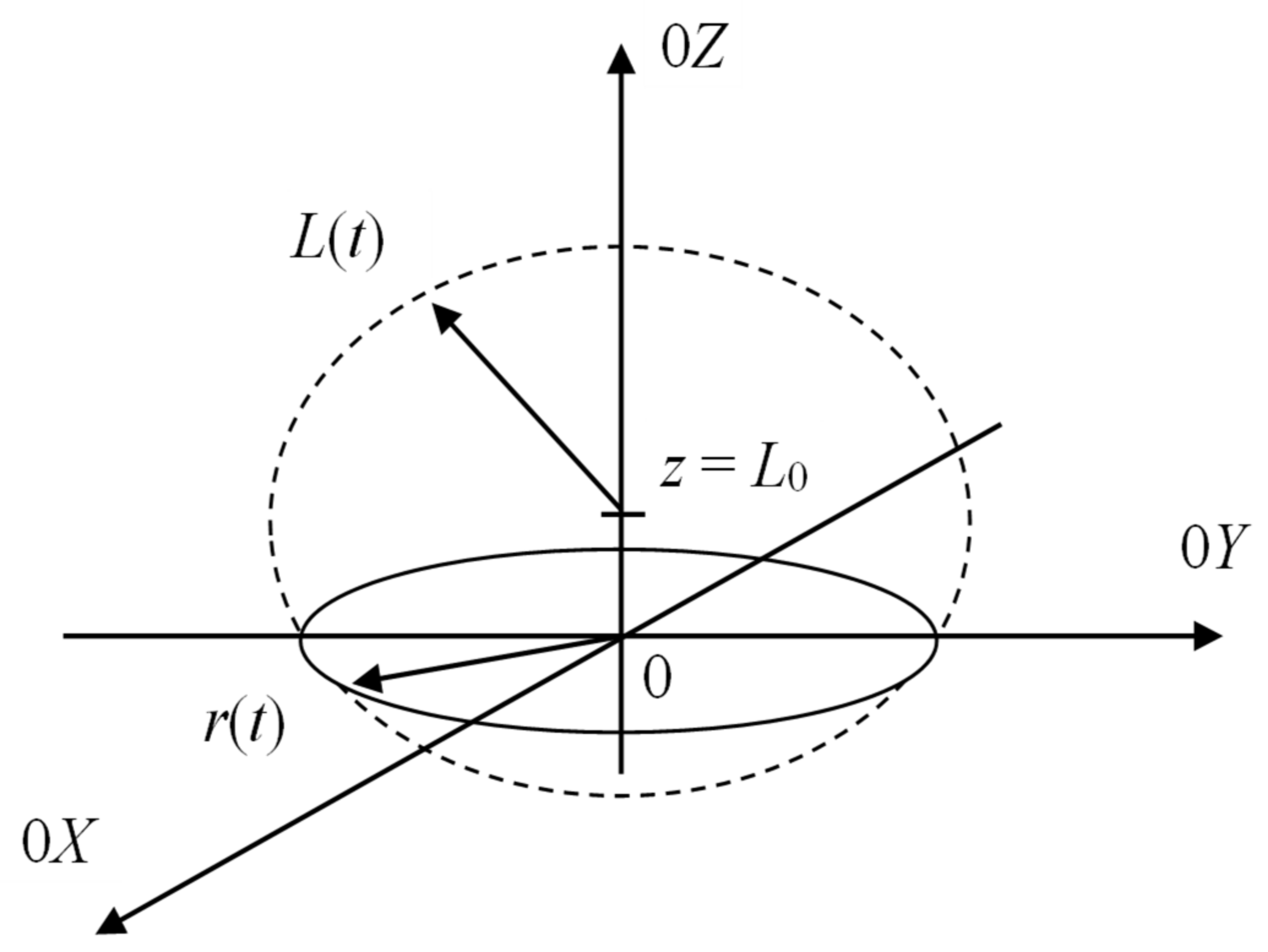
3.3. Cavitation Mechanism of Erosive Approach for Metal Oxides Dissolution in DES
4. Results and Discussion
5. Conclusions
- Efficient intensification of leaching cannot be achieved simply by acceleration of every step. Due to synchronism of some processes, it is necessary to set the operating regime in such a way that different stages do not interfere with each other and moreover provide the best conditions for the entire process realization. For example, accumulation of powder that is too fast prevents efficient dissolution due to formation of conglomerates; therefore, consistency of parameters should take this feature of the process into account;
- Ultrasound is able to boost the dissolution of metal oxide not only by its shredding (cavitation-induced) but also by creating an acoustic flow that promptly deletes the saturated solution film (interfering fast metal oxide molecules transfer) from the surface. Specific organization of such a process helps to significantly increase the efficiency of mentioned technology. However, the search for the key features and determination of optimal conditions requires additional independent study. It may result in new intriguing and practically useful recommendations and engineering solutions. In addition, results obtained during this research allow evaluating the value of flow rate in liquid. Considering the scope of mentioned schemes, including of such additional flow sources can boost the dissolution process;
- Diversity of configurations in the area of cavitation and the abundance of methods of ultrasound usage for their generation allows choosing the most efficient variant for every specific scheme. It is extremely important for maximal process intensification achievement. At the same time, one can use different types of waves, products of their interaction and features of nonlinear US modification. The latter include solitary acoustic impulses, self-focusing US beams, and nonlinear wave structures. It is possible to develop new or to modify old leaching systems with their help;
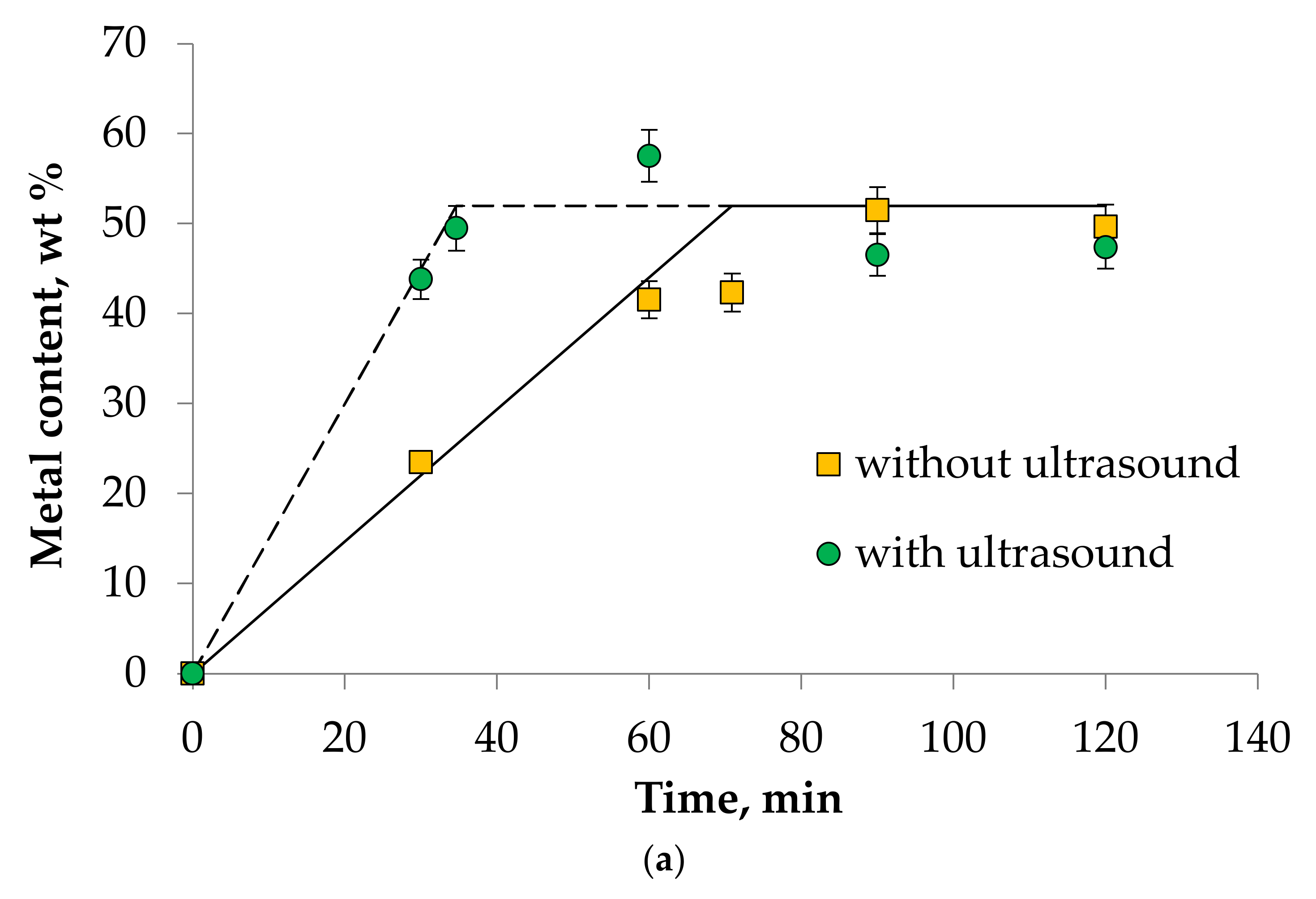
- It was found that ultrasound allows intensifying the dissolution process of Mn(III), Ni(III), and Co(II) oxides in DES choline chloride/sulfosalicylic acid (7:3). The experimental data correlate well with the theory. This fact allows us to speak about the adequacy of the proposed theoretical calculations describing kinetics of metal oxides dissolution process in DES.
Author Contributions
Funding
Conflicts of Interest
References
- Sandilya, D.K.; Kannan, A. Intensification of the Dissolution of a Sparingly Soluble Solid from a Spinning Disk in the Presence of Power Ultrasound. Ind. Eng. Chem. Res. 2011, 50, 13083–13091. [Google Scholar] [CrossRef]
- Narayana, K.L.; Swamy, K.M.; Rao, K.S.; Murty, J.S. Leaching of Metals from Ores with Ultrasound. Miner. Process. Extr. Metall. Rev. 1997, 16, 239–259. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithiumion batteries (LIBs) using organic acids as leaching reagents: A review. Resour. Conserv. Recycl. 2018, 136, 418–435. [Google Scholar] [CrossRef]
- Gui, Q.; Khan, M.I.; Wang, S.; Zhang, L. The ultrasound leaching kinetics of gold in the thiosulfate leaching process catalysed by cobalt ammonia. Hydrometallurgy 2020, 196, 105426. [Google Scholar] [CrossRef]
- Wang, X.; Srinivasakannan, C.; Duan, X.-h.; Peng, J.-h.; Yang, D.-j.; Ju, S.-h. Leaching kinetics of zinc residues augmented with ultrasound. Sep. Purif. Tech. 2013, 115, 66–72. [Google Scholar]
- Avvaru, B.; Roy, S.B.; Chowdhury, S.; Hareendran, K.N.; Pandit, A.B. Enhancement of the Leaching Rate of Uranium in the Presence of Ultrasound. Ind. Eng. Chem. Res. 2006, 45, 7639–7648. [Google Scholar] [CrossRef]
- Rao, K.S.; Narayana, K.L.; Swamy, K.M.; Murty, J.S. Influence of ultrasound in ammoniacal leaching of a copper oxide ore. Metall. Mater. Trans B 1997, 28, 721–723. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Yang, D. Determination of Antiscaling Efficiency and Dissolution Capacity for Calcium Carbonate with Ultrasonic Irradiation. Ind. Eng. Chem. Res. 2012, 51, 9266–9274. [Google Scholar] [CrossRef]
- Gradov, O.M.; Voshkin, A.A.; Zakhodyaeva, Y.A. Estimating the parameters of ultrasonically induced mass transfer and flow of liquids in the pseudomembrane method. Chem. Eng. Process. Process Intensif. 2017, 118, 54–61. [Google Scholar] [CrossRef]
- Gradov, O.M.; Voshkin, A.A.; Zakhodyaeva, Y.A. Analysis of the possible applications of the acoustic flow effect for the breakup and transfer of liquid substances in a cylindrical volume. Theor. Found. Chem. Eng. 2017, 51, 876–882. [Google Scholar] [CrossRef]
- Gradov, O.M.; Zakhodyaeva, Y.A.; Voshkin, A.A. Breakup of immiscible liquids at the interface using high-power acoustic pulses. Chem. Eng. Process. Process Intensif. 2018, 131, 125–130. [Google Scholar] [CrossRef]
- Gradov, O.M.; Zakhodyaeva, Y.A.; Zinov’eva, I.V.; Voshkin, A.A. Some Features of the Ultrasonic Liquid Extraction of Metal Ions. Molecules 2019, 24, 3549. [Google Scholar] [CrossRef]
- Gradov, O.M.; Zakhodyaeva, Y.A.; Zinov’eva, I.V.; Voshkin, A.A. Ultrasonic Intensification of Mass Transfer in Organic Acid Extraction. Processes 2021, 9, 15. [Google Scholar] [CrossRef]
- Gradov, O.M.; Zakhodyaeva, Y.A.; Voshkin, A.A. Dynamics of Mass Transfer through the Interface between Immiscible Liquids under the Resonance Effect of Ultrasound. Theor. Found. Chem. Eng. 2020, 54, 1148–1155. [Google Scholar] [CrossRef]
- Xiao, J.; Yuan, J.; Tian, Z.; Yang, K.; Yao, Z.; Yu, B.; Zhang, L. Comparison of ultrasound-assisted and traditional caustic leaching of spent cathode carbon (SCC) from aluminum electrolysis. Ultrason. Sonochem. 2018, 40, 21–29. [Google Scholar] [CrossRef]
- Karami, E.; Kuhar, L.; Bona, A.; Nikoloski, A.N. A review of electrokinetic, ultrasonic and solution pulsing methods for mass transfer enhancement in in-situ processing. Minerals Eng. 2021, 170, 107029. [Google Scholar] [CrossRef]
- Lei, C.; Aldous, I.; Hartley, J.M.; Thompson, D.L.; Scott, S.; Hanson, R.; Anderson, P.A.; Kendrick, E.; Sommerville, R.; Ryder, K.S.; et al. Lithium ion battery recycling using high-intensity ultrasonication. Green Chem. 2021, 23, 4710. [Google Scholar] [CrossRef]
- Marafi, M.; Stanislaus, A. Waste Catalyst Utilization: Extraction of Valuable Metals from Spent Hydroprocessing Catalysts by Ultrasonic-Assisted Leaching with Acids. Ind. Eng. Chem. Res. 2011, 50, 9495–9501. [Google Scholar] [CrossRef]
- Kong, J.; Xing, P.; Wei, D.; Zhuang, Y. Ultrasound-Assisted Leaching of Iron from Silicon Diamond-Wire Saw Cutting Waste. JOM 2021, 73, 791–800. [Google Scholar] [CrossRef]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Resour. Conserv. Recycl. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Sun, Z.; Cao, H.; Xiao, Y.; Sietsma, J.; Jin, W.; Agterhuis, H.; Yang, Y. Toward Sustainability for Recovery of Critical Metals from Electronic Waste: The Hydrochemistry Processes. ACS Sust. Chem. Eng. 2016, 5, 21–40. [Google Scholar] [CrossRef]
- Fedorova, M.I.; Zakhodyaeva, Y.A.; Baranchikov, A.E.; Krenev, V.A.; Voshkin, A.A. Extraction Reprocessing of Fe, Ni-Containing Parts of Ni–MH Batteries. Russ. J. Inorg. Chem. 2021, 66, 266–272. [Google Scholar] [CrossRef]
- Zakhodyaeva, Y.A.; Izyumova, K.V.; Solov’Eva, M.S.; Voshkin, A.A. Extraction separation of the components of leach liquors of batteries. Theor. Found. Chem. Eng. 2017, 51, 883–887. [Google Scholar] [CrossRef]
- Alvial-Hein, G.; Mahandra, H.; Ghahreman, A. Separation and recovery of cobalt and nickel from end of life products via solvent extraction technique: A review. J. Clean. Prod. 2021, 297, 126592. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.J.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Synthesis and Dissolution of Metal Oxides in Ionic liquids and Deep Eutectic Solvents. Molecules 2019, 25, 78. [Google Scholar] [CrossRef] [Green Version]
- Zürner, P.; Frisch, G. Leaching and selective extraction of indium and tin from zinc flue dust using an oxalic acid based deep eutectic solvent. ACS Sust. Chem. Eng. 2019, 7, 5300–5308. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; McKenzie, K.J.; Obi, S.U. Solubility of Metal Oxides in Deep Eutectic Solvents Based on Choline Chloride. J. Chem. Eng. Data. 2006, 51, 1280. [Google Scholar] [CrossRef]
- Pateli, I.M.; Thompson, D.; Alabdullah, S.; Abbott, A.P.; Jenkin, G.; Hartley, J. The effect of pH and hydrogen bond donor on the dissolution of metal oxides in deep eutectic solvents. Green Chem. 2019, 22, 5476. [Google Scholar] [CrossRef]
- Macchioni, V.; Carbone, K.; Cataldo, A.; Fraschini, R.; Bellucci, S. Lactic acid-based deep eutectic solvents for the extraction of bioactive metabolites of Humulus lupulus L.: Supramolecular organization, phytochemical profiling and biological activity. Sep. Purif. Technol. 2021, 264, 118039. [Google Scholar] [CrossRef]
- Lakka, A.; Karageorgou, I.; Kaltsa, O.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D. Polyphenol Extraction from Humulus lupulus (Hop) Using a Neoteric Glycerol/L-Alanine Deep Eutectic Solvent: Optimisation, Kinetics and the Effect of Ultrasound-Assisted Pretreatment. AgriEngineering 2019, 1, 30. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wu, Y.; Li, J.; Wang, A.; Li, G.; Ren, X.; Yin, W. Ultrasound-assisted deep eutectic solvent extraction of echinacoside and oleuropein from Syringa pubescensTurcz. Ind. Crops Prod. 2020, 151, 112442. [Google Scholar] [CrossRef]
- Schaeffer, N.; Passos, H.; Gras, M.; Rodriguez Vargas, S.J.; Neves, M.C.; Svecova, L.; Papaiconomou, N.; Coutinho, J.A.P. Selective separation of manganese, cobalt and nickel in a fully aqueous system. ACS Sustain. Chem. Eng. 2020, 8, 12260–12269. [Google Scholar] [CrossRef]
- Zinov’eva, I.V.; Fedorova, A.Y.; Milevskii, N.A.; Zakhodyaeva, Y.A.; Voshkin, A.A. A Deep Eutectic Solvent Based on Choline Chloride and Sulfosalicylic Acid: Properties and Applications. Theor. Found. Chem. Eng. 2021, 55, 371–379. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Figurovskaya, V.N.; Ivanov, V.M. Molecular absorption spectroscopy of 4-(2-pyridilazo)resorcinolcomplexes as alternative for the atomic absorption spectroscopy. Mosc. Univ. Chem. Bull. 1992, 33, 570–574. [Google Scholar]
- Flynn, H.G. Physics of acoustic cavitation in liquids. In Physical Acoustics; Mason, W.P., Ed.; John Wiley & Sons: New York, NY, USA, 1964; Volume 16, pp. 57–172. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradov, O.M.; Zinov’eva, I.V.; Zakhodyaeva, Y.A.; Voshkin, A.A. Modelling of the Erosive Dissolution of Metal Oxides in a Deep Eutectic Solvent—Choline Chloride/Sulfosalicylic Acid—Assisted by Ultrasonic Cavitation. Metals 2021, 11, 1964. https://doi.org/10.3390/met11121964
Gradov OM, Zinov’eva IV, Zakhodyaeva YA, Voshkin AA. Modelling of the Erosive Dissolution of Metal Oxides in a Deep Eutectic Solvent—Choline Chloride/Sulfosalicylic Acid—Assisted by Ultrasonic Cavitation. Metals. 2021; 11(12):1964. https://doi.org/10.3390/met11121964
Chicago/Turabian StyleGradov, Oleg M., Inna V. Zinov’eva, Yulia A. Zakhodyaeva, and Andrey A. Voshkin. 2021. "Modelling of the Erosive Dissolution of Metal Oxides in a Deep Eutectic Solvent—Choline Chloride/Sulfosalicylic Acid—Assisted by Ultrasonic Cavitation" Metals 11, no. 12: 1964. https://doi.org/10.3390/met11121964
APA StyleGradov, O. M., Zinov’eva, I. V., Zakhodyaeva, Y. A., & Voshkin, A. A. (2021). Modelling of the Erosive Dissolution of Metal Oxides in a Deep Eutectic Solvent—Choline Chloride/Sulfosalicylic Acid—Assisted by Ultrasonic Cavitation. Metals, 11(12), 1964. https://doi.org/10.3390/met11121964








