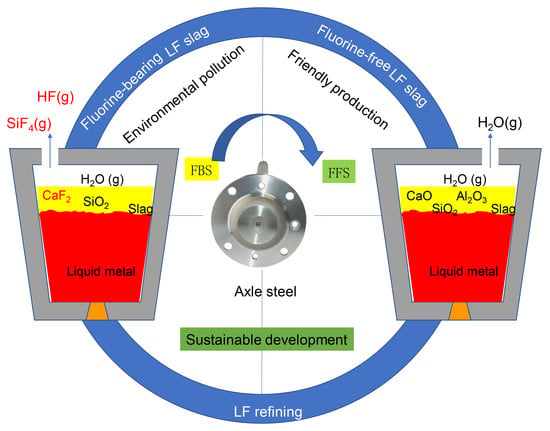
Fundamental Research on Fluorine-Free Ladle Furnace Slag for Axle Steel of Electric Multiple Unit Vehicles
Abstract
1. Introduction
2. Research Methods
2.1. Theoretical Analysis
2.2. Laboratory Tests
2.3. Industrial Scale Tests
3. Results and Discussion
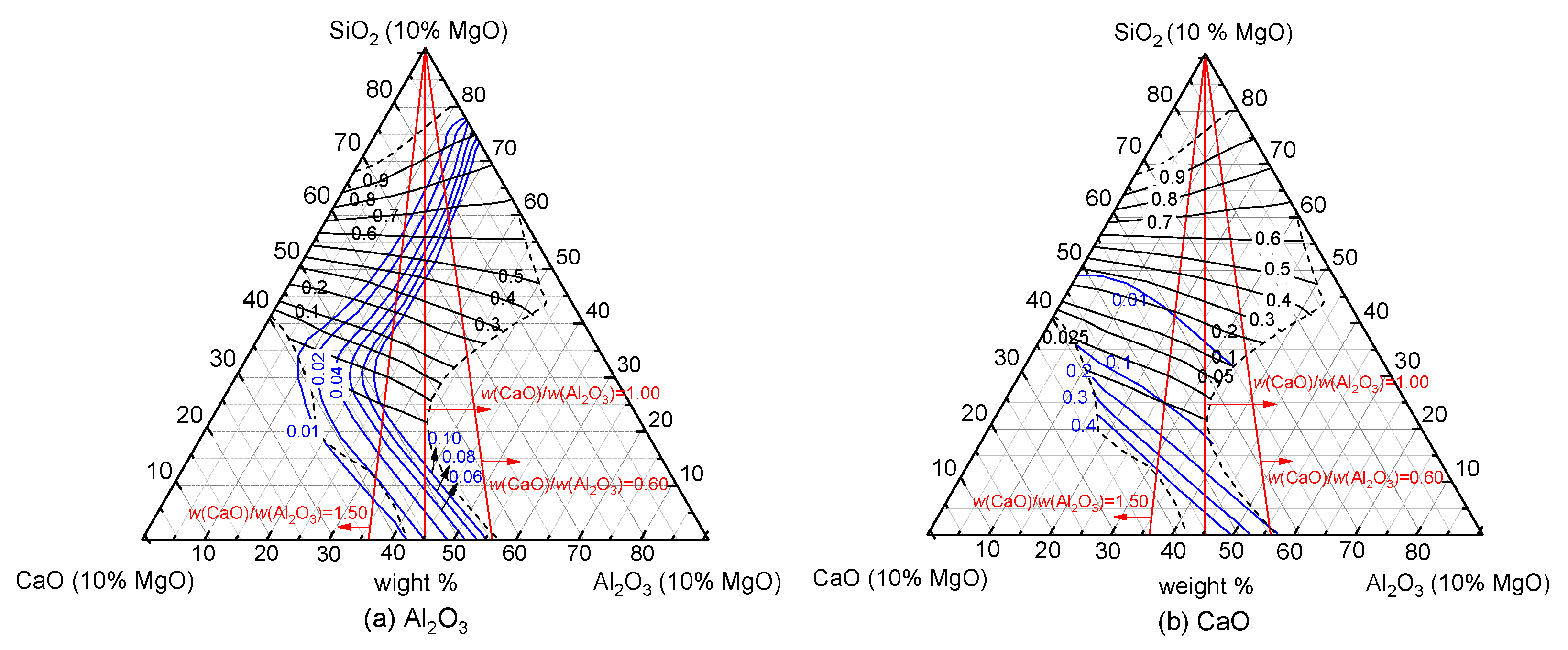
3.1. Theoretical Analysis
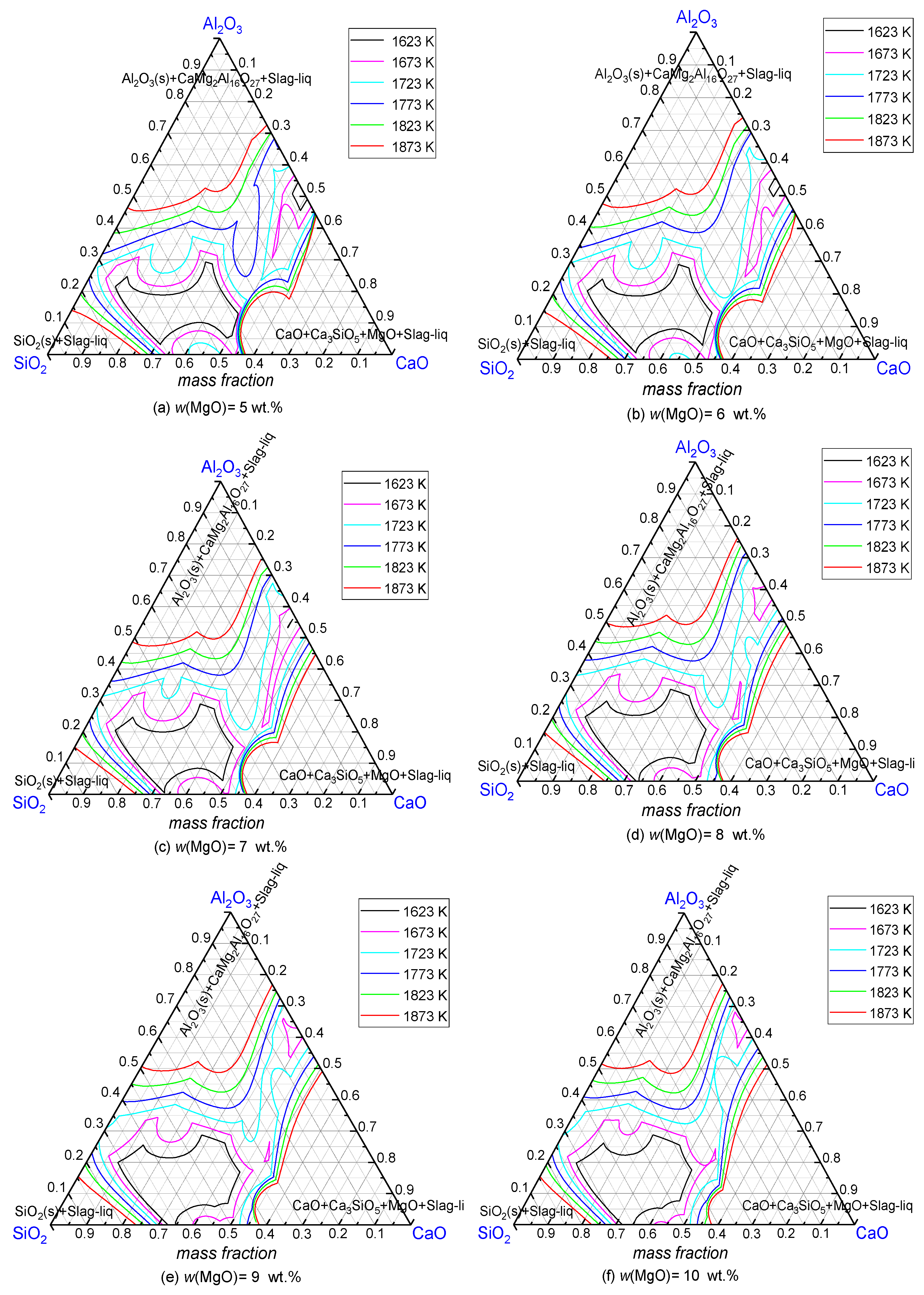
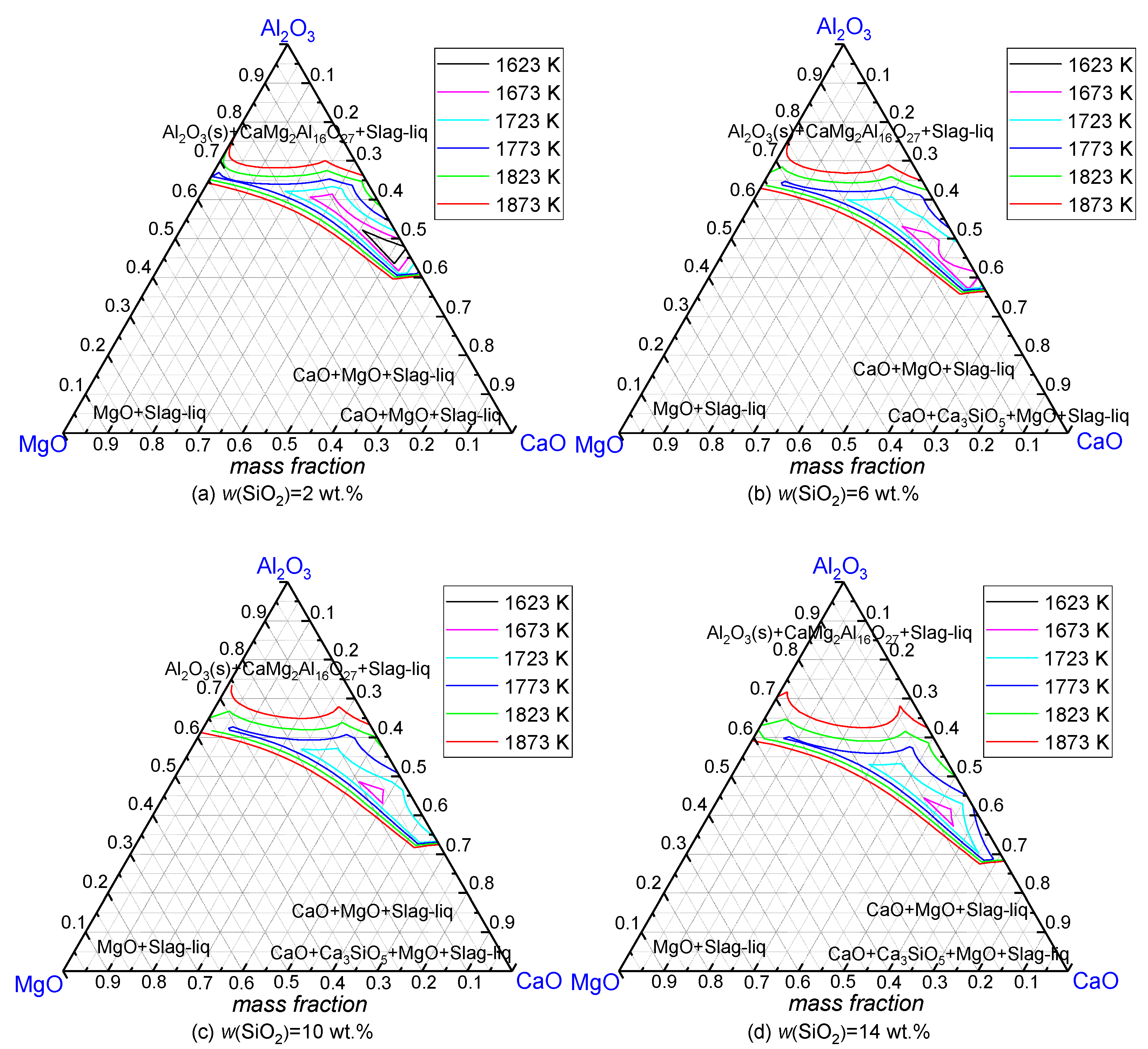
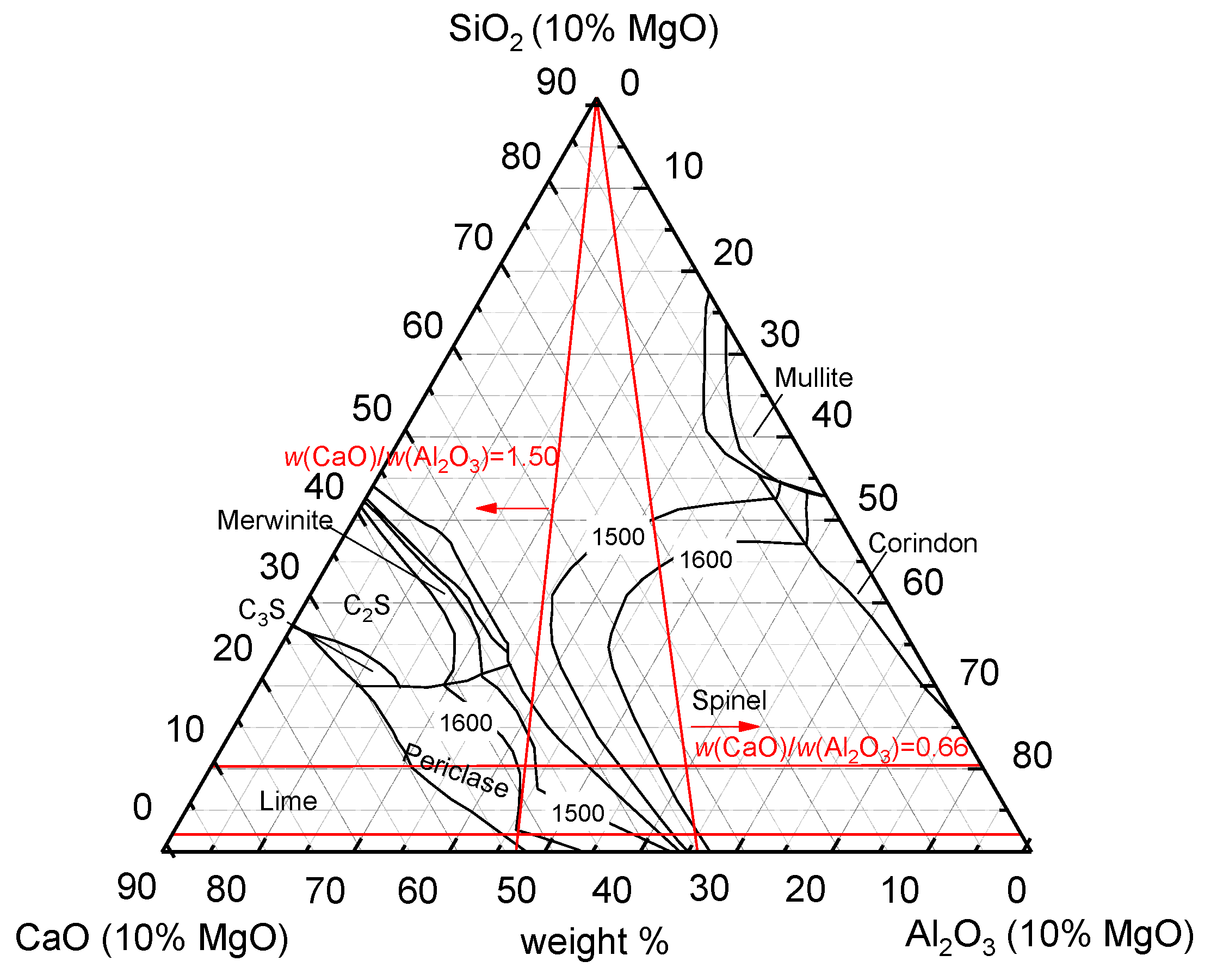
3.1.1. MgO
3.1.2. SiO2
3.1.3. Mass Ratio of w(CaO)/w(Al2O3)
3.2. Laboratory Tests
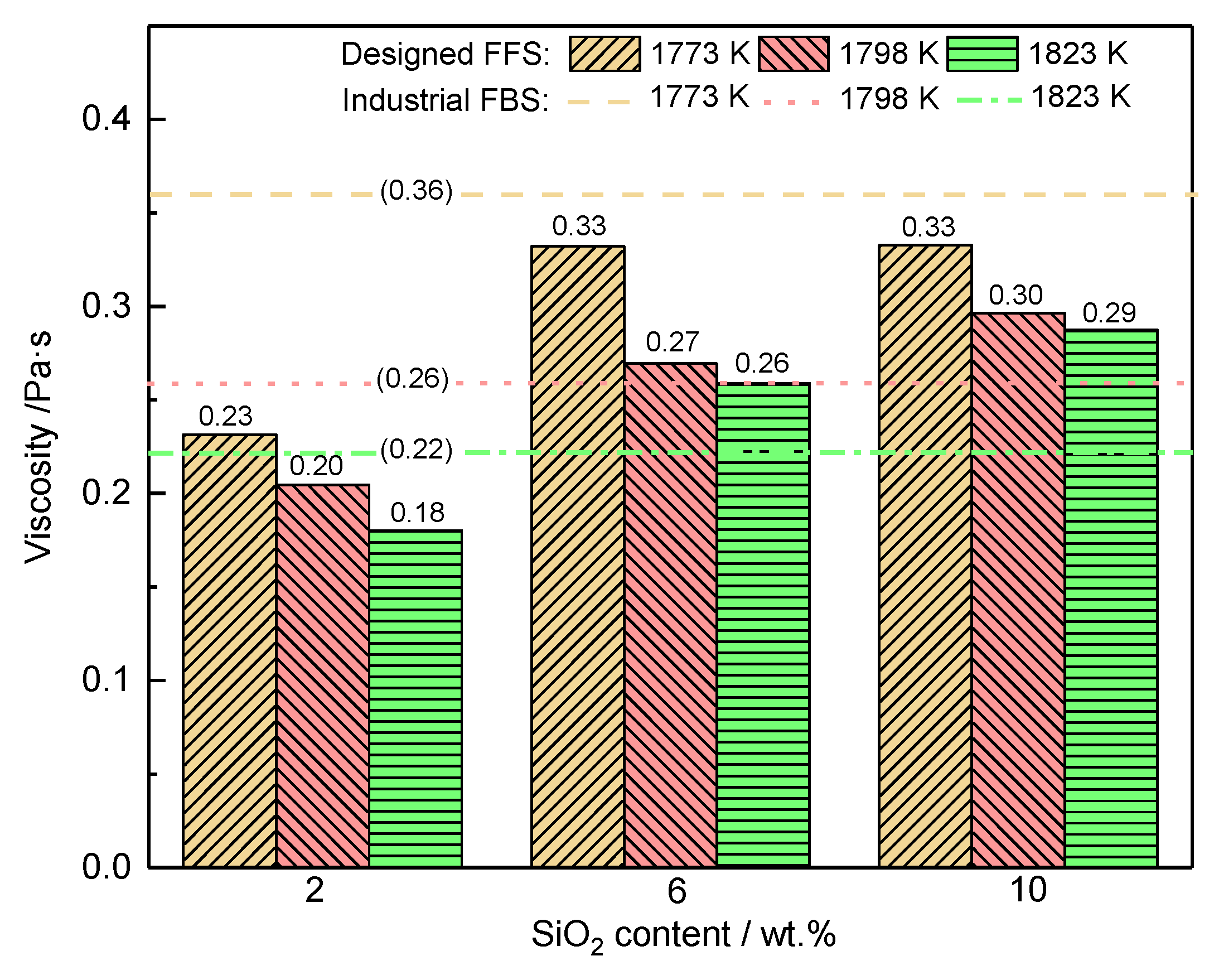
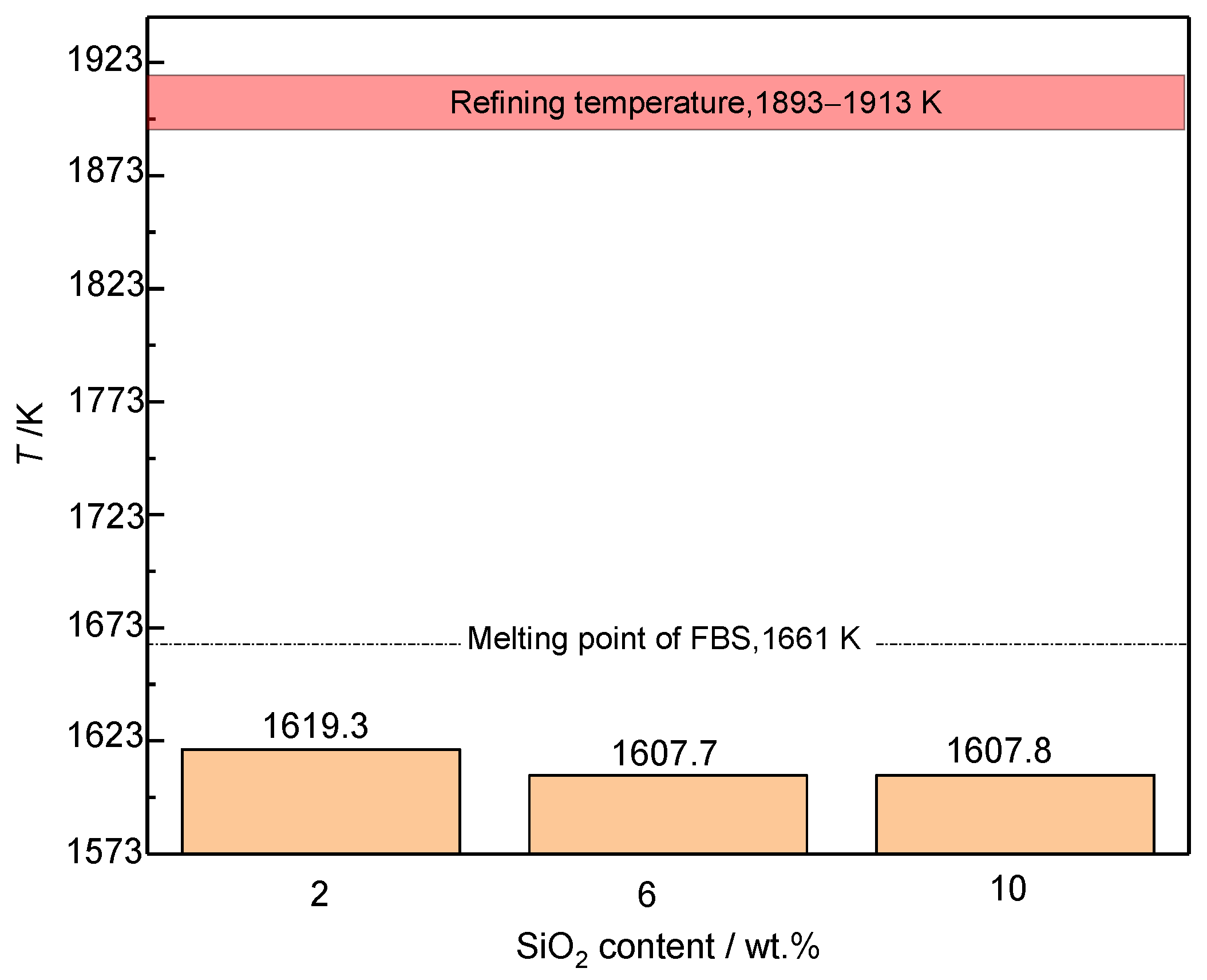
3.2.1. SiO2
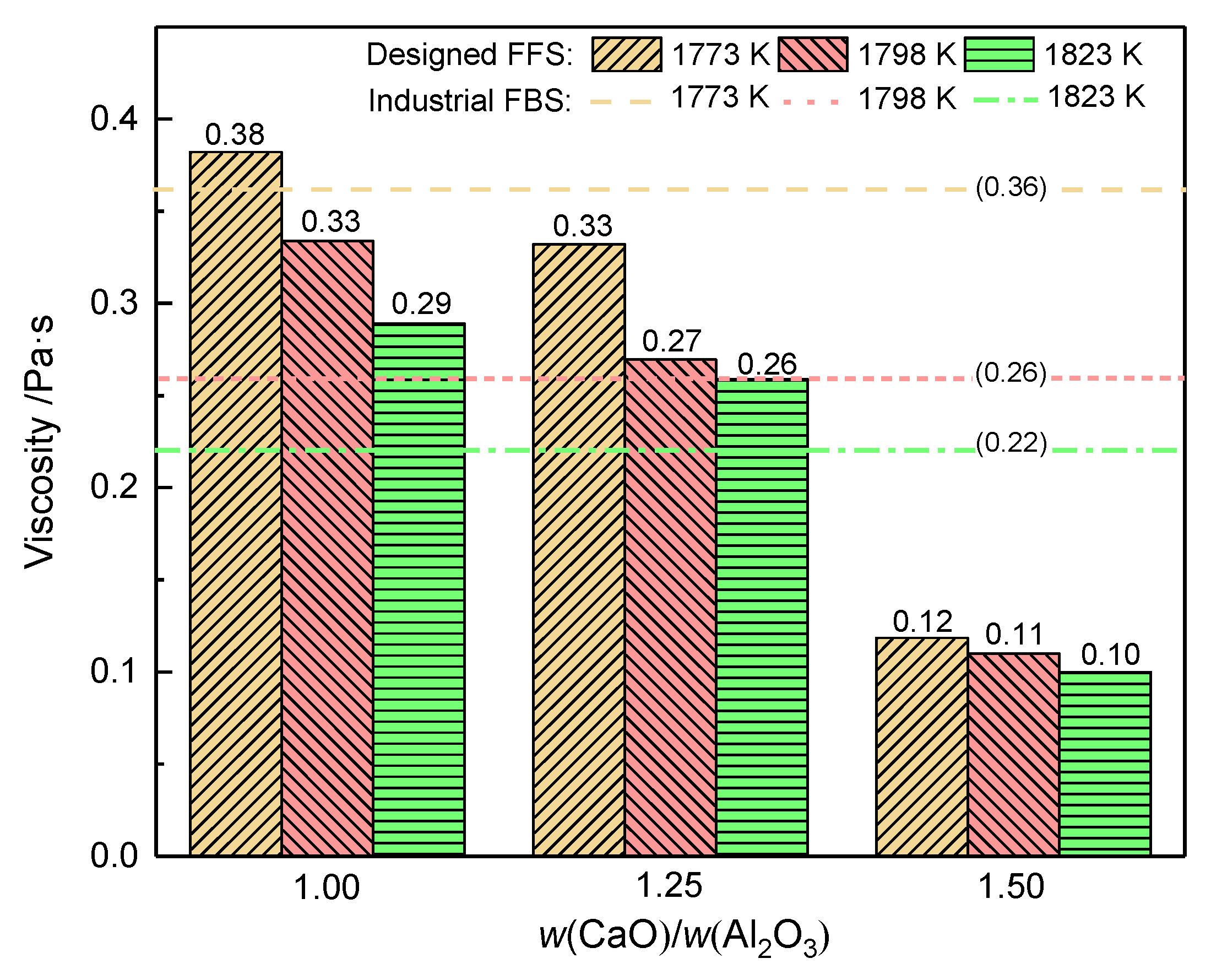
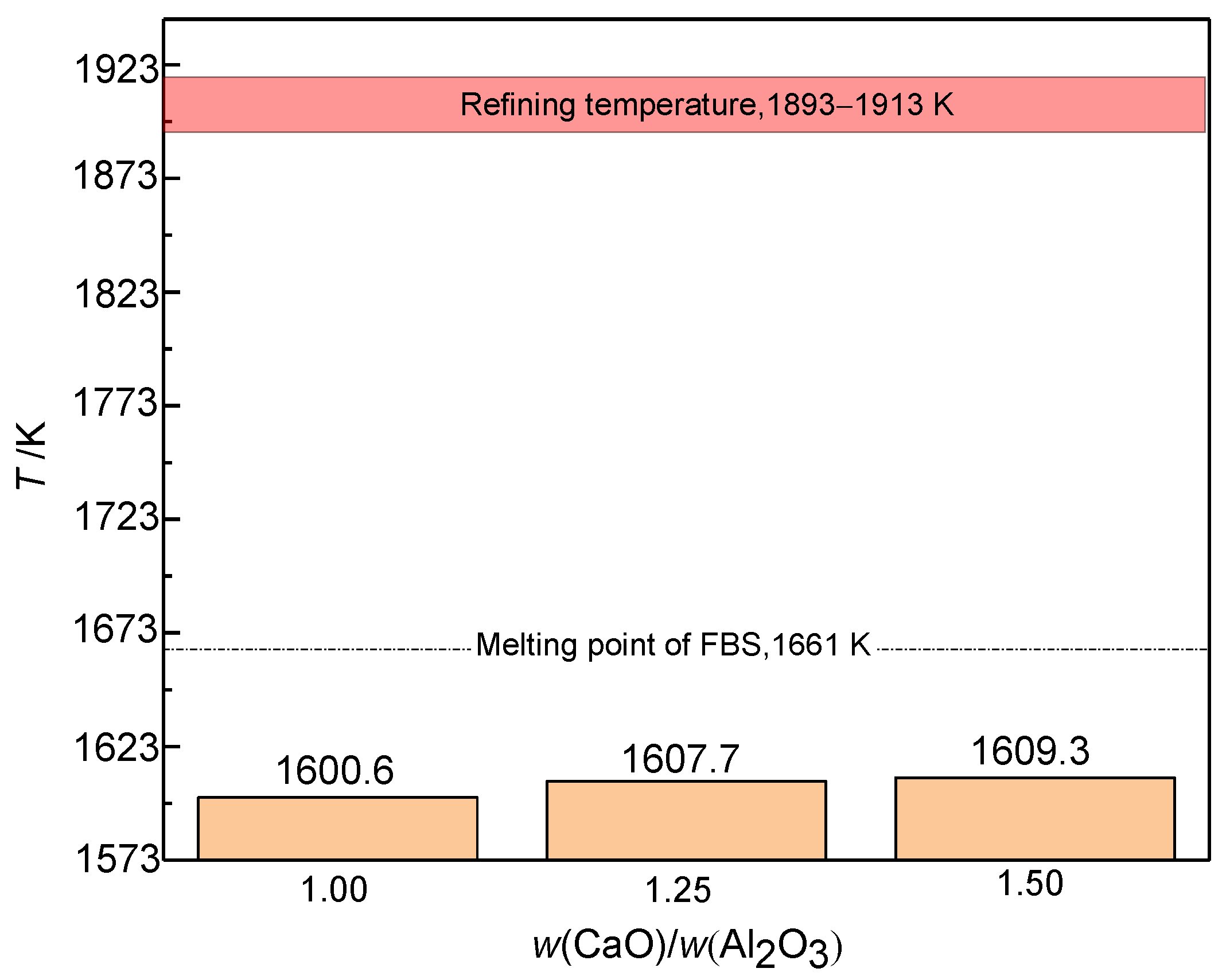
3.2.2. Mass Ratio w(CaO)/w(Al2O3)
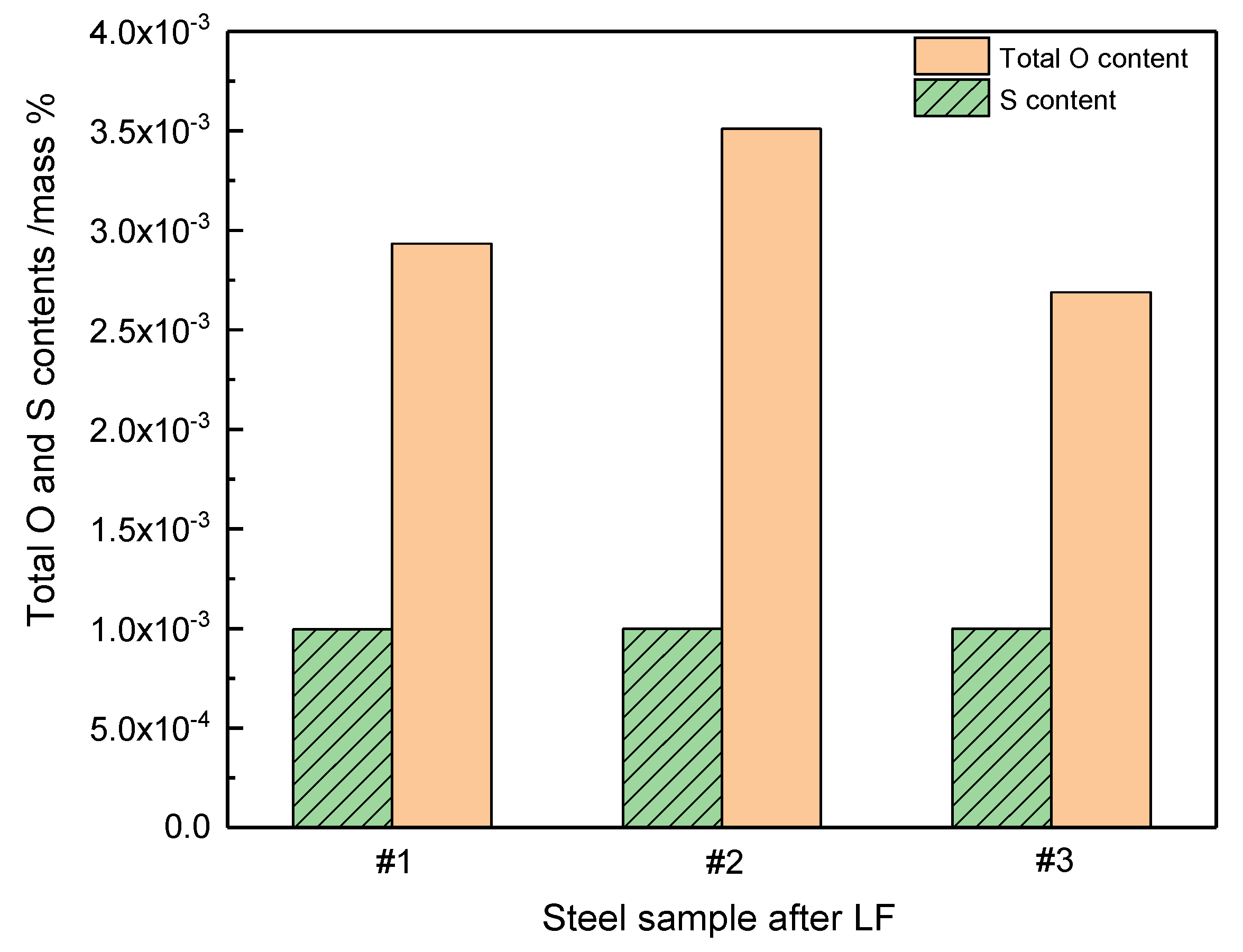
3.3. Industrial Scale Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FBS | Fluorine-bearing refining slag |
| FFS | Fluorine-free ladle furnace slag |
| EMU | Electric multiple unit |
| EAF | Electric arc furnace |
| LF | Ladle furnace |
| VD | Vacuum degassing |
| RH | Ruhrstahl-Heraeus process |
| CC | Continuous casting |
| SEM | Scanning electron microscope |
| EDS | Energy disperse spectroscopy |
References
- Li, S.X. Effects of inclusions on very high cycle fatigue properties of high strength steels. Int. Mater. Rev. 2012, 57, 92–114. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, X.H.; Chen, B.; Wang, W.J. Laboratory study on evolution mechanisms of non-metallic inclusions in high strength alloyed steel refined by high basicity slag. ISIJ Int. 2010, 50, 95–104. [Google Scholar] [CrossRef]
- Reis, B.H.; Bielefeldt, W.V.; Vilela, A.C.F. Efficiency of inclusion absorption by slags during secondary refining of steel. ISIJ Int. 2014, 54, 1584–1591. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Effect of CaO/Al2O3 ratio of ladle slag on formation behavior of inclusions in Mn and V alloyed steel. ISIJ Int. 2018, 54, 1584–1591. [Google Scholar]
- Cheng, P.C.; Huang, S.G.; Pandelaers, L.; Dyck, J.V.; Guo, M.X. Effect of the CaO-Al2O3-Based top slag on the cleanliness of stainless steel during secondary metallurgy. Metall. Mater. Trans. B 2013, 44, 1105–1119. [Google Scholar]
- Wu, L.S.; Gran, J.; Sichen, D. The Effect of calcium fluoride on slag viscosity. Metall. Mater. Trans. B 2011, 42, 928–931. [Google Scholar] [CrossRef]
- Jie, Q.; Liu, C.J.; Jiang, M.F. Properties investigation of CaO-Al2O3-SiO2-Li2O-B2O3-Ce2O3 mould flux with different w(CaO)/w(Al2O3) for heat-resistant steel continuous casting. Can. Metall. Q. 2017, 56, 212–220. [Google Scholar]
- Seetharaman, S.; Mukai, K.; Sichen, D. Viscosities of slags—An overview. Steel Res. Int. 2005, 76, 267–278. [Google Scholar] [CrossRef]
- Ohta, M.; Kubo, T.; Morita, K. Effects of CaF2, MgO and SiO2 addition on sulfide capacities of the CaO-Al2O3 slag. Tetsu-to-Hagane 2013, 89, 742–749. [Google Scholar] [CrossRef][Green Version]
- Guo, B.Q.; Bao, Y.P.; Wang, M.; Lin, L. Optimization of CaO- SiO2-Al2O3-MgO-CaF2 refining slag in low-carbon and aluminum-containing 20Mn2 steel. Chin. J. Eng. 2015, 37, 1415–1421. [Google Scholar]
- Park, J.H.; Min, D.J.; Song, H.S. The Effect of CaF2 on the viscosities and structures of CaO-SiO2(-MgO)-CaF2 slags. Metall. Mater. Trans. B 2002, 33, 723–729. [Google Scholar] [CrossRef]
- Peng, K.W.; Zhang, P.J.; Xie, G.H.; Ma, L. Study on properties of Al2O3 –CaO-Na2CO3 slag system. Appl. Mech. Mater. 2011, 269, 117–119. [Google Scholar]
- Liu, C.J.; Zhang, R.; Zhao, X.B.; Jia, J.X.; Min, Y. Quantification of phosphorus structures in CaO-SiO2-P2O5 glasses via raman spectroscopy. J. Non-Cryst. Solids 2020, 557, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.B.; Liu, C.J.; Jiang, M.F. Effect of fluorine on melt structure for CaO-SiO2-CaF2 and CaO-Al2O3-CaF2 by molecular dynamics simulations. ISIJ Int. 2020, 60, 2176–2182. [Google Scholar] [CrossRef]
- Persson, M.; Seetharaman, S.; Seetharaman, S. Kinetic Studies of Fluoride Evaporation from Slags. ISIJ Int. 2007, 12, 1711–1717. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Tan, Z.; Qu, B.; Cui, Y.R. The volatilization behaviour of typical fluorine-containing slag in steelmaking. Soc. Open Sci. 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Mao, H.X.; Hu, H.T.; Ma, G.J. Contamination of fluorine in CC mould powder to environment and countermeasures. Steelmaking 1999, 15, 41–45. [Google Scholar]
- Wang, W.L.; Cai, D.; Zhang, L. A review of fluorine-free mold flux development. ISIJ Int. 2018, 58, 1957–1964. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, Z.T.; Liu, L.L.; Wang, X.D. Investigation of the viscosity and structural properties of CaO-SiO2-TiO2 slags. Metall. Mater. Trans. B 2014, 45, 1389–1397. [Google Scholar] [CrossRef]
- Gao, J.X.; Wen, G.H.; Sun, Q.H.; Tang, P.; Liu, Q. The Influence of Na2O on the solidification and crystallization behavior of CaO-SiO2-Al2O3-based mold flux. Metall. Mater. Trans. B 2015, 46, 1850–1859. [Google Scholar] [CrossRef]
- Tang, Z.F.; Qiao, J.L.; Jiang, X.Y. Kinetics of Na2O evaporation from CaO-Al2O3-SiO2-MgO-TiO2-Na2O slags. Ironmak. Steelmak. 2017, 44, 237–245. [Google Scholar] [CrossRef]
- Wu, C.C.; Cheng, G.G.; Long, H. Effect of Ce2O3 and CaO/Al2O3 on the Phase, melting temperature and viscosity of CaO-Al2O3-10 mass% SiO2 based slags. High Temp. Mater. Processes. High Temp. Mater. Process. 2013, 33, 77–84. [Google Scholar] [CrossRef]
- Keshari, B.K.K.; Kumar, S.; Roy, S.; Pratihar, T.K. Optimising slag composition & gas purging in a LF to reduce noise and improve refining. Steel Times Int. 2012, 36, 17–19. [Google Scholar]
- Xu, J.F.; Zhang, J.Y.; Jie, C.; Ruan, F.; Chou, K.C. Experimental measurements and modelling of viscosity in CaO-Al2O3-MgO slag system. Ironmak. Steelmak. 2011, 38, 329–337. [Google Scholar] [CrossRef]
- Hai, F.; Wang, C.X.; Zhang, J.M.; Qie, J.C. Development trends of environmental protection technologies for Chinese steel industry. J. Iron Steel Ress. Int. 2017, 24, 235–242. [Google Scholar]
- Wang, Y.; Wen, Z.; Cao, X. Environmental efficiency evaluation of China’s iron and steel industry: A process -level data envelopment analysis. Sci. Total Environ. 2020, 707, 1–52. [Google Scholar] [CrossRef]
- Ökvist, L.S. The melting properties of tuyere slags with and without flux injection into the blast furnace. ISIJ Int. 2001, 41, 1429–1436. [Google Scholar] [CrossRef]
- Mills, K.C. Slag Atlas, VDEh, 2nd ed.; Verlag Stahleisen GmbH: Düsseldorf, Germany, 1995. [Google Scholar]
- Almeida, R.A.M.; Vieira, D.; Bielefeldt, W.V.; Vilela, A.C.F. MgO saturation analisys of CaO-SiO2-FeO-MgO-Al2O3 slag system. Mater. Res. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, X.; Liu, C.J.; Jiang, M.F.; Saxén, H.; Zevenhoven, R. Corrosion behavior of MgO-C ladle refractory by molten slag. Steel Res. Int. 2020, 92, 2000497. [Google Scholar] [CrossRef]
- Dong, Y.; Jiang, Z.; Cao, Y.; Zhang, H.; Shen, H. Effect of MgO and SiO2 on surface tension of fluoride containing slag. J. Cent. South. Univ. 2014, 21, 4104–4108. [Google Scholar] [CrossRef]
- Chang, Y.E.; Lin, C.M.; Shen, J.M. Effect of MgO content on the viscosity, foaming life, and bonding in liquid and liquid/Solid CaO-SiO2-MgO-5Al2O3-30FeO slags. Met.—Open Access Metall. J. 2021, 11, 249. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.H.; Sohn, I.; Min, D.J. The effect of MgO on the viscosity of the CaO-SiO2-20 wt%Al2O3-MgO slag system. Steel Res. Int. 2010, 81, 261–264. [Google Scholar] [CrossRef]
- Vázquez, A.B.; Caballero, A.; Pena, P. Quaternary System Al2O3-CaO-MgO-SiO2: II Study of the Crystallization Volume of MgAl2O4. J. Am. Ceram. Soc. 2005, 88, 1949–1957. [Google Scholar] [CrossRef]
- Song, M.; Shu, Q.; Sichen, D. Viscosities of the quaternary Al2O3-CaO-MgO-SiO2 slags. Steel Res. Int. 2010, 82, 260–268. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Li, B.; Bai, C. Relationship between structure and viscosity of CaO-SiO2-MgO-30.00 wt.%Al2O3 slag by molecular dynamics simulation with FT-IR and Raman spectroscopy. Ironmak. Steelmak. 2017, 45, 1–11. [Google Scholar] [CrossRef]
- Liao, J.L.; Zhang, Y.Y.; Sridhar, S. Effect of Al2O3/SiO2 ratio on the viscosity and structure of slags. ISIJ Int. 2012, 52, 753–758. [Google Scholar] [CrossRef]
- Zhang, R.; Min, Y.; Wang, Y.; Zhao, X.; Jia, X.J.; Liu, C. Viscosity estimation of multicomponent slags of CaOSiO2-Al2O3-FexO system based on microstructure analysis. Energy Fuel. 2020, 34, 1–40. [Google Scholar]
- Liang, X. Effect of additives on melting point and viscosity of rh refining slag. Open Mater. Sci. J. 2011, 5, 9–14. [Google Scholar] [CrossRef][Green Version]
- Yang, G.G.; Wang, X.H. Inclusion evolution after calcium addition in low carbon Al-Killed steel with ultra low sulfur content. ISIJ Int. 2015, 55, 126–133. [Google Scholar] [CrossRef]
- Liu, C.; Gao, X.; Ueda, S. Change in Composition of inclusions through the reaction between Al-killed steel and the slag of CaO and MgO Saturation. ISIJ Int. 2019, 59, 268–276. [Google Scholar] [CrossRef]
- Gu, C.; Wang, M.; BaO, Y.P.; Wang, F.M.; Lian, J.H. Quantitative Analysis of Inclusion Engineering on the Fatigue Property Improvement of Bearing Steel. Metal 2019, 9, 476. [Google Scholar] [CrossRef]




| No. | CaO | Al2O3 | SiO2 | MgO | CaF2 | w(CaO)/w(Al2O3) |
|---|---|---|---|---|---|---|
| 1 | 46.11 | 36.89 | 10.00 | 7.00 | - | 1.25 |
| 2 | 48.33 | 38.67 | 6.00 | 7.00 | - | 1.25 |
| 3 | 50.56 | 40.44 | 2.00 | 7.00 | - | 1.25 |
| 4 | 43.50 | 43.50 | 6.00 | 7.00 | - | 1.00 |
| 5 | 52.20 | 34.80 | 6.00 | 7.00 | - | 1.50 |
| 6 | 55.07 | 30.91 | 1.84 | 7.18 | 5.00 | 1.78 |
| No. | CaO | Al2O3 | SiO2 | MgO | CaF2 | w(CaO)/w(Al2O3) |
|---|---|---|---|---|---|---|
| #1 | 53.54 | 37.31 | 3.72 | 5.43 | - | 1.44 |
| #2 | 57.50 | 31.18 | 7.10 | 4.22 | - | 1.84 |
| #3 | 55.15 | 30.01 | 1.84 | 7.18 | 5.82 | 1.84 |
| C | Si | Mn | P | S | Cr | Mo | Ni | V | Al | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.24–0.32 | 0.20–0.40 | 0.60–0.90 | ≤0.01 | ≤0.01 | 0.90–1.20 | 0.20–0.30 | 0.50–1.50 | ≤0.06 | 0.01–0.04 | ≤0.20 |
| Point | Al | O | Mg | Ca |
|---|---|---|---|---|
| +1 | 27.96 | 72.04 | - | - |
| +2 | 25.95 | 64.03 | 10.02 | - |
| +3 | 8.29 | 46.12 | 44.45 | 1.15 |
| +4 | 3.86 | 45.86 | 50.30 | - |
| +5 | 25.27 | 55.85 | 4.40 | 14.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Mei, X.; Gao, L.; Zhang, J.; Wang, Z.; Sun, L.; Zevenhoven, R.; Saxén, H. Fundamental Research on Fluorine-Free Ladle Furnace Slag for Axle Steel of Electric Multiple Unit Vehicles. Metals 2021, 11, 1973. https://doi.org/10.3390/met11121973
Zhao Q, Mei X, Gao L, Zhang J, Wang Z, Sun L, Zevenhoven R, Saxén H. Fundamental Research on Fluorine-Free Ladle Furnace Slag for Axle Steel of Electric Multiple Unit Vehicles. Metals. 2021; 11(12):1973. https://doi.org/10.3390/met11121973
Chicago/Turabian StyleZhao, Qing, Xiaohui Mei, Lei Gao, Jinwen Zhang, Zhixiang Wang, Lifeng Sun, Ron Zevenhoven, and Henrik Saxén. 2021. "Fundamental Research on Fluorine-Free Ladle Furnace Slag for Axle Steel of Electric Multiple Unit Vehicles" Metals 11, no. 12: 1973. https://doi.org/10.3390/met11121973
APA StyleZhao, Q., Mei, X., Gao, L., Zhang, J., Wang, Z., Sun, L., Zevenhoven, R., & Saxén, H. (2021). Fundamental Research on Fluorine-Free Ladle Furnace Slag for Axle Steel of Electric Multiple Unit Vehicles. Metals, 11(12), 1973. https://doi.org/10.3390/met11121973