Verification of Possibility of Molten Steels Decopperization with ZnAl2O4
Abstract
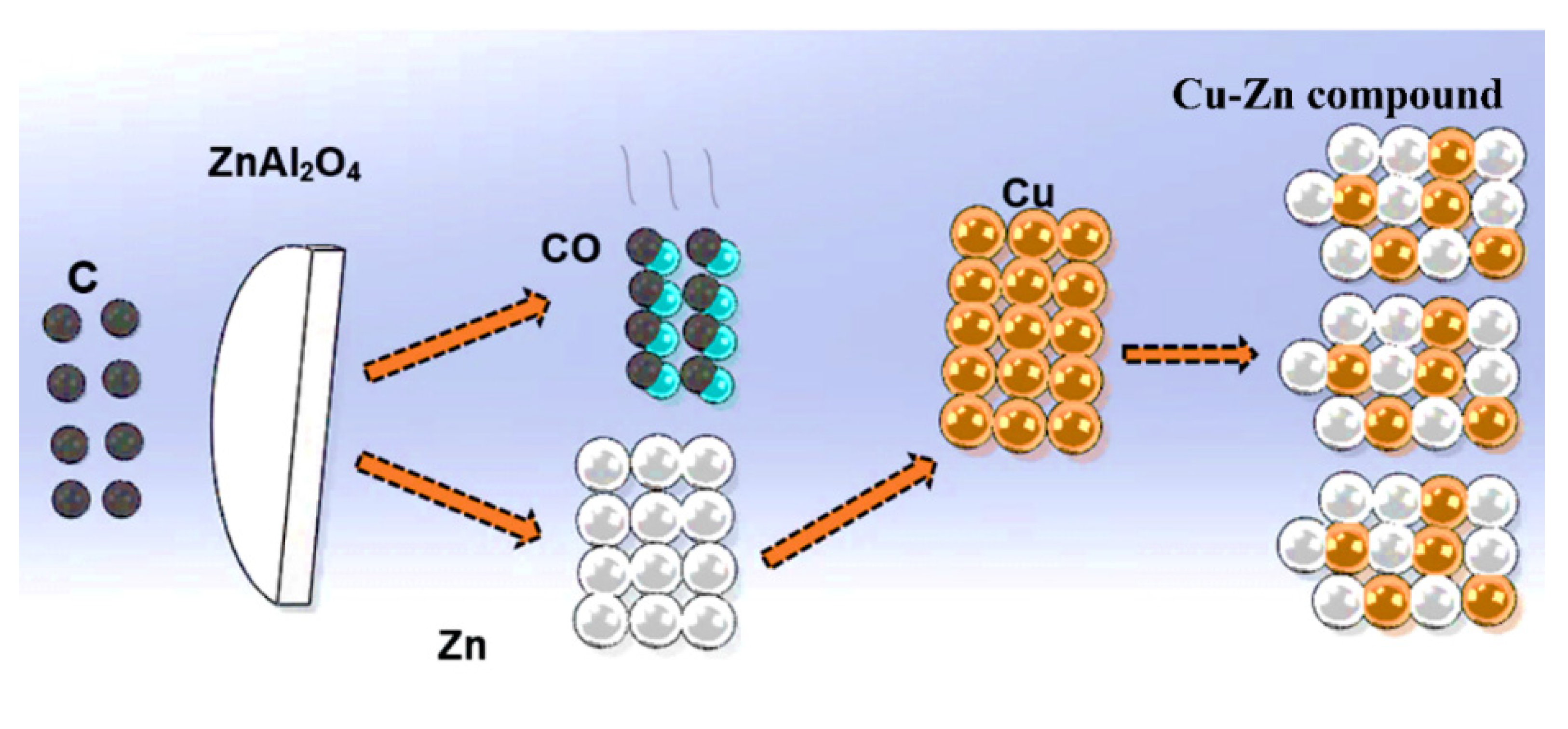
1. Introduction
2. Materials and Methods
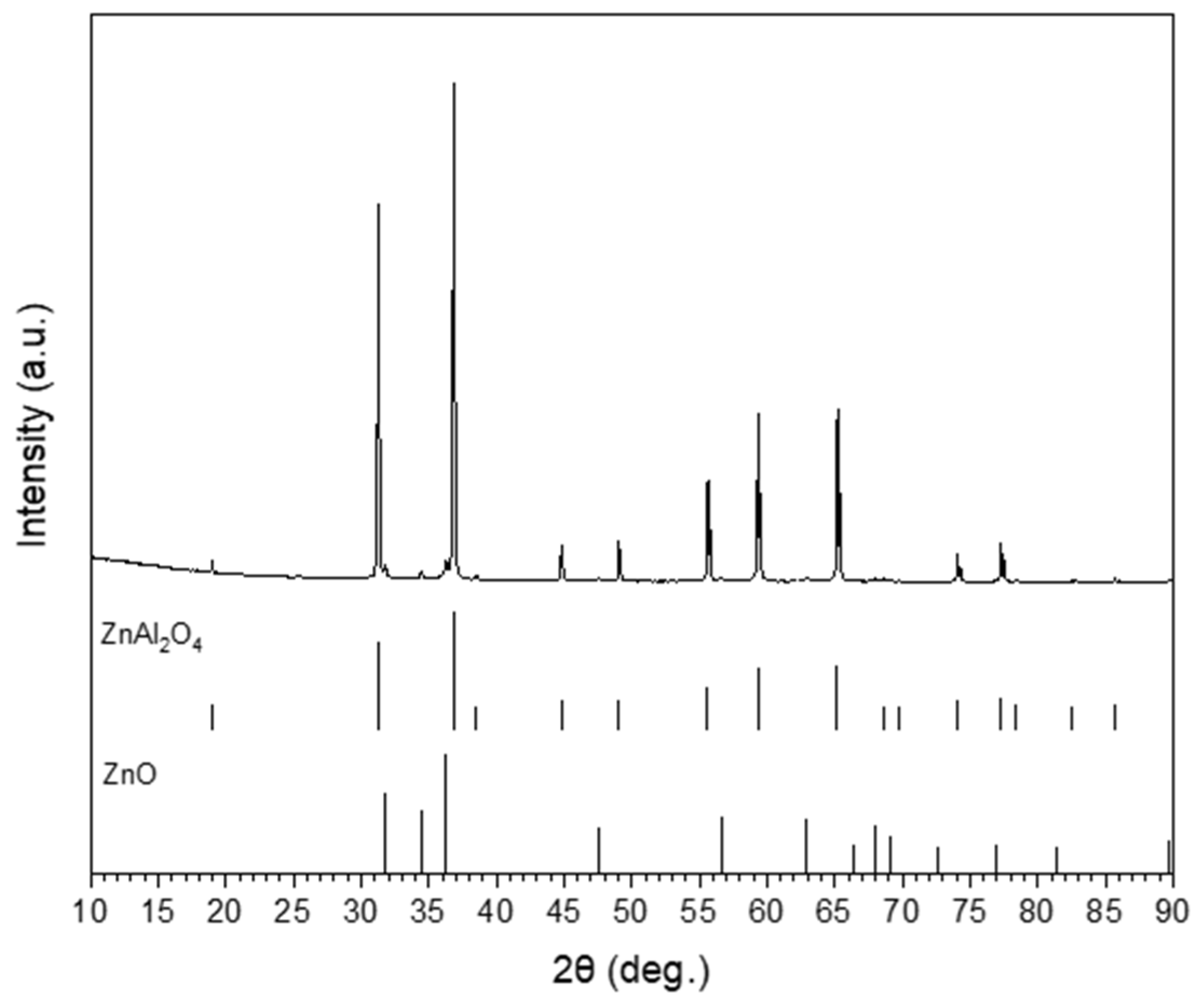
2.1. ZnAl2O4 Substrates’ Preparation
2.2. Fe-Cu Alloys’ Preparation and Experimental Setup
3. Results and Discussion
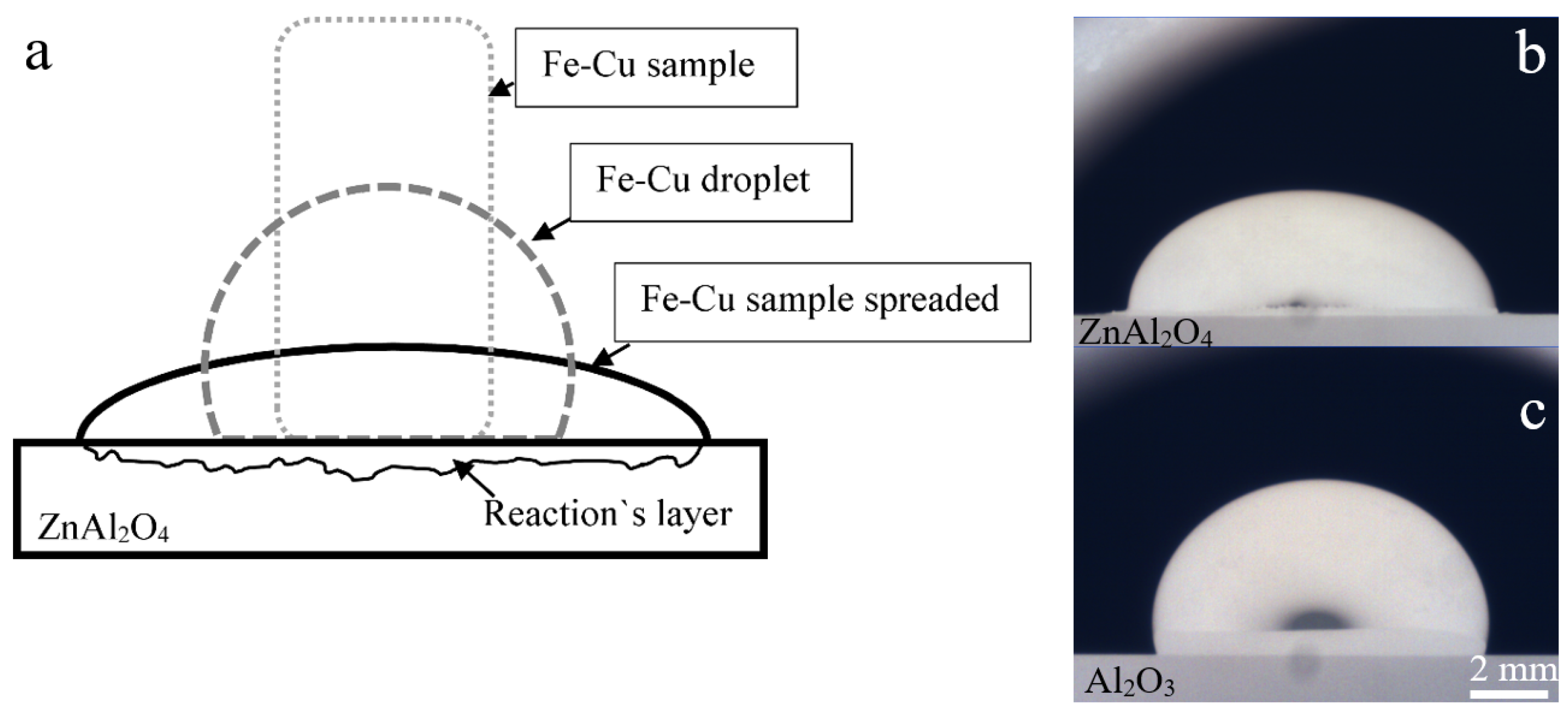
3.1. Experimental Observation
3.2. Interfacial and Cross Sectional Analysis
3.3. The Composition of Fe-Cu Alloys
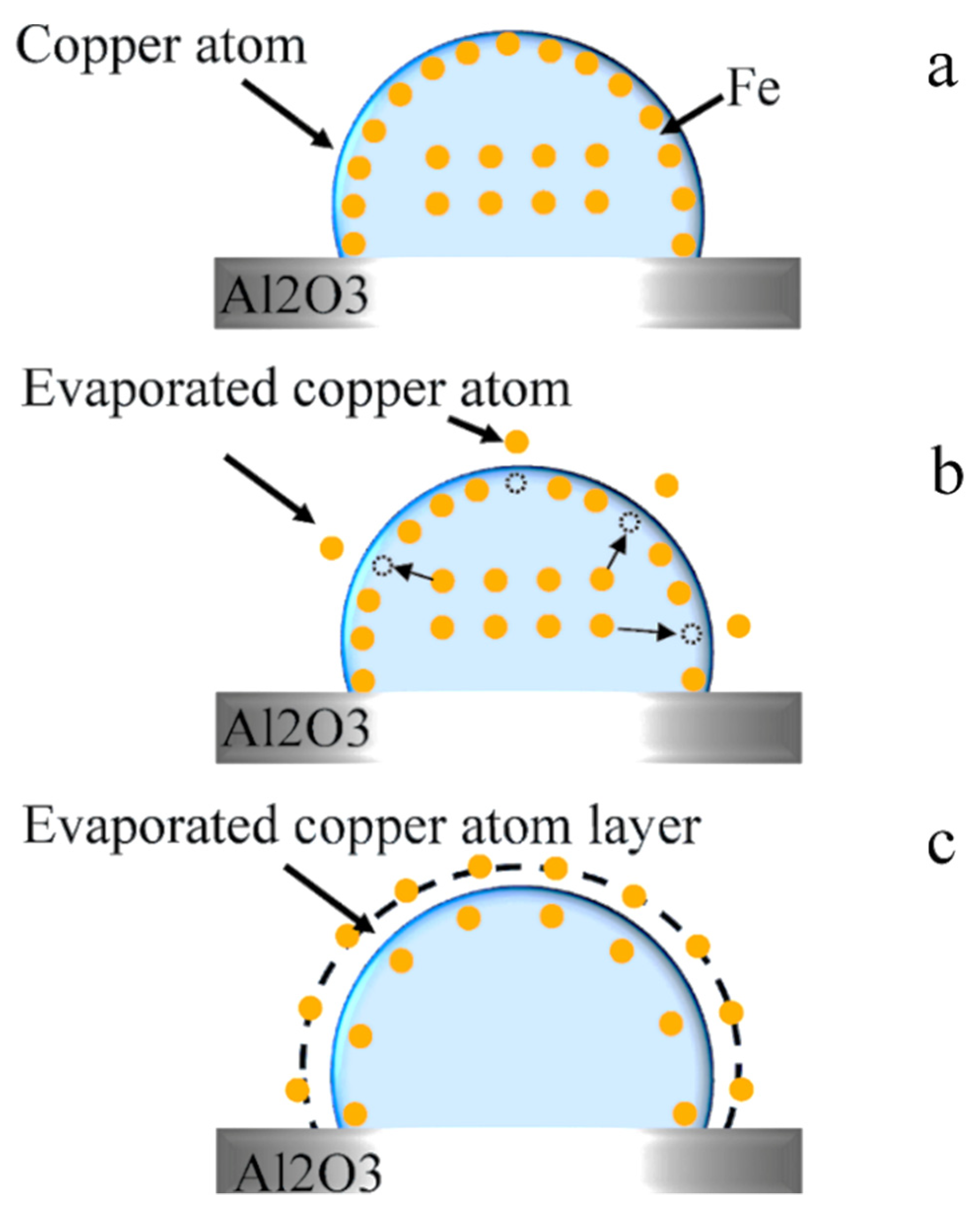
3.4. Copper Evaporation Mechanism
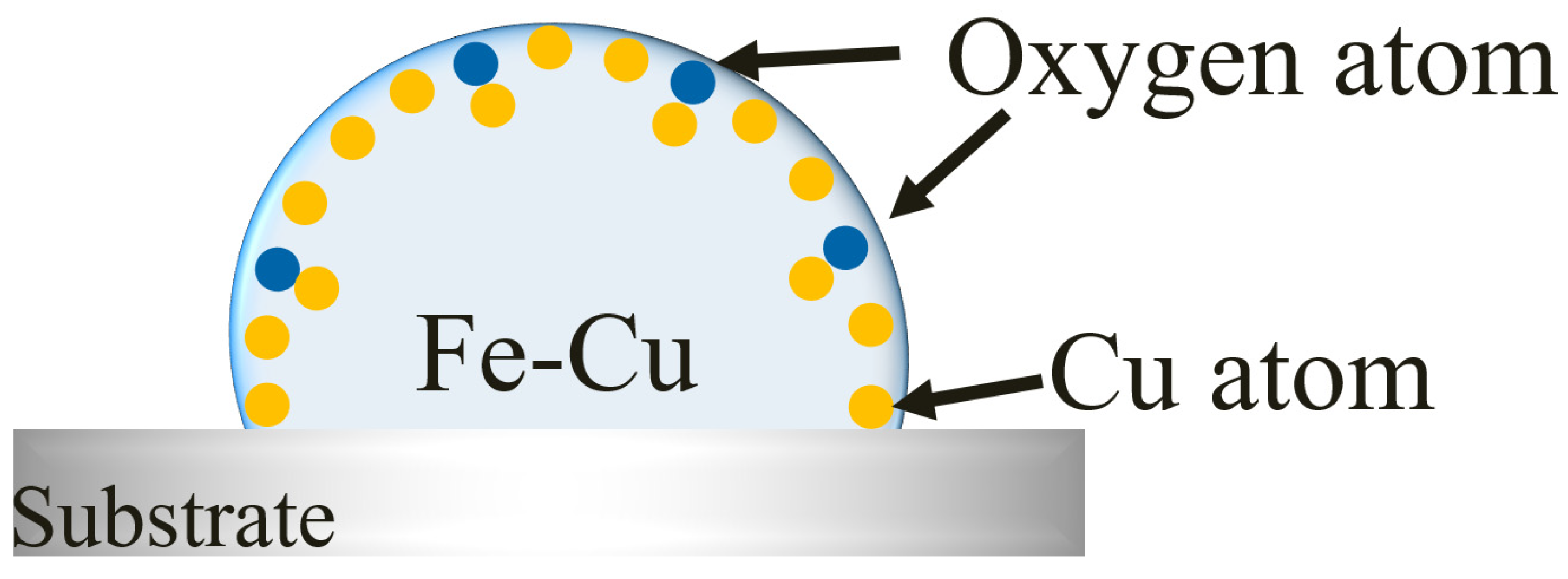
3.5. Effect of Oxygen on Copper Evaporation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rod, O.; Becker, C.; Nylén, M. Opportunities and Dangers of Using Residual Elements in Steels: A Literature Survey; Jernknotoret Report: Stockholm, Sweden, 2006. [Google Scholar]
- Savov, L.; Volkova, E. Copper and tin in steel scrap recycling. Mat. Geoenviron. 2003, 50, 627–640. [Google Scholar]
- Zhao, G.-H.; Zhang, J.; Li, J.; Li, H.-Y.; Liu, H.-T.; Ma, L.-F. Effect of copper on edge cracking behavior and microstructure of rolled austenitic stainless steel plate. J. Iron Steel Res. Int. 2021, 1–14. [Google Scholar] [CrossRef]
- Daehn, K.E.; Serrenho, A.C.; Allwood, J.M. How Will Copper Contamination Constrain Future Global Steel Recycling? Environ. Sci. Technol. 2017, 51, 6599–6606. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Guo, S.-X.; Zhou, L.; Li, Q. Slag for decopperization and sulphur control in molten steel. J. Iron Steel Res. Int. 2009, 16, 17–21. [Google Scholar] [CrossRef]
- Cohen, A.; Blander, M. Removal of copper from carbon-saturated iron with an aluminum sulfide/ferrous sulfide flux. Met. Mater. Trans. A 1998, 29, 493–495. [Google Scholar] [CrossRef]
- Shimpo, R.; Ogawa, O.; Fukaya, Y.; Ishikawa, T. Copper removal from carbon-saturated molten iron with Al2S3-FeS flux. Met. Mater. Trans. A 1997, 28, 1029–1037. [Google Scholar] [CrossRef]
- Uchida, Y.-I.; Matsui, A.; Kishimoto, Y.; Miki, Y. Fundamental Investigation on Removal of Copper from Molten Iron with Na2CO3–FeS Fluxes. ISIJ Int. 2015, 55, 1549–1557. [Google Scholar] [CrossRef]
- Savov, L.; Janke, D. Evaporation of Cu and Sn from Induction-stirred Iron-based Melts Treated at Reduced Pressure. ISIJ Int. 2000, 40, 95–104. [Google Scholar] [CrossRef]
- Zaitsev, A.I.; Zaitseva, N.E.; Shakhpazov, E.K.; Mogutnov, B.M. Evaporation of Copper from Iron Melts. ISIJ Int. 2004, 44, 639–646. [Google Scholar] [CrossRef]
- Taguchi, K.; Ono-Nakazato, H.; Usui, T. Enhancement of Evaporation Removal Rate of Copper in Molten Iron by the Silicon and/or Carbon Addition. Resour. Process. 2004, 51, 158–162. [Google Scholar] [CrossRef][Green Version]
- Savov, L.; Tu, S.; Janke, D. Methods of Increasing the Rate of Tin Evaporation from Iron-based Melts. ISIJ Int. 2000, 40, 654–663. [Google Scholar] [CrossRef][Green Version]
- Hu, X.; Yan, Z.; Jiang, P.; Zhu, L.; Chou, K.; Matsuura, H.; Tsukihashi, F. Removal of Copper from Molten Steel using FeO–SiO2–CaCl2 Flux. ISIJ Int. 2013, 53, 920–922. [Google Scholar] [CrossRef]
- Sandig, E.F.; Chebykin, D.; Prutchykova, V.V.; Fabrychnaya, O.; Volkova, O. Review: Possibilities of Steel Scrap Decopperization. Mater. Sci. Forum 2019, 959, 145–160. [Google Scholar] [CrossRef]
- Daehn, K.E.; Serrenho, A.C.; Allwood, J. Finding the Most Efficient Way to Remove Residual Copper from Steel Scrap. Met. Mater. Trans. A 2019, 50, 1225–1240. [Google Scholar] [CrossRef]
- Wieliczko, A.G.; Griszczenko, J.N.; Krucinski, M. Technological efficiency of liquid steel decopperizing. Hut. Wiad. Hut. 1998, 65, 256–259. (In Polish) [Google Scholar]
- Li, L.; Xiang, C.; Zhao, P.; Wang, C.; Tian, H.; Li, S. Decopperization in Steel Melt through Filtration. J. Iron Steel Res. Int. 1998, 10, 5–7. (In Chinese) [Google Scholar]
- Li, S.; Yu, B.; Li, S.; Li, L. Exploration on Decoppering of Molten Steel by Filtering. Engineering Chem. Metallurgy 2000, 21, 314–317. (In Chinese) [Google Scholar]
- Kim, Y.G.; Kim, I.J.; Kim, J.S.; Chung, Y.I.; Choi, D.Y. Evaluation of Surface Crack in Resistance Spot Welds of Zn-Coated Steel. Mater. Trans. 2014, 55, 171. [Google Scholar] [CrossRef]
- Shyrokykh, T.; Wei, X.; Seetharaman, S.; Volkova, O. Vaporization of Vanadium Pentoxide from CaO-SiO2-VOx Slags During Alumina Dissolution. Met. Mater. Trans. A 2021, 52, 1472–1483. [Google Scholar] [CrossRef]
- Wei, X.; Yehorov, A.; Storti, E.; Dudczig, S.; Fabrichnaya, O.; Aneziris, C.G.; Volkova, O. Phenomenon of Whiskers Formation in Al2O3-C-Refractories. Adv. Eng. Mater. 2021, 2100718. [Google Scholar] [CrossRef]
- He, J.; Zhao, J.Z.; Ratke, L. Solidification microstructure and dynamics of metastable phase transformation in undercooled liquid Cu–Fe alloys. Acta Mater. 2006, 54, 1749–1757. [Google Scholar] [CrossRef]
- Brillo, J.; Egry, I. Surface tension of nickel, copper, iron and their binary alloys. J. Mater. Sci. 2005, 40, 2213–2216. [Google Scholar] [CrossRef]
- Korobeinikov, I.I.; Chebykin, D.; Yu, X.; Seetharaman, S.; Volkova, O. Density of tin, silver and copper. Arch. Mater. Sci. Eng. 2018, 92, 28–32. [Google Scholar] [CrossRef]
- Zienert, T.; Dudczig, S.; Fabrichnaya, O.; Aneziris, C.G. Interface reactions between liquid iron and alumina–carbon refractory filter materials. Ceram. Int. 2015, 41, 2089–2098. [Google Scholar] [CrossRef]
- Labaj, J. Kinetics of Coper Evaporation from the Fe-Cu Alloys Under Reduced Pressure. Arch. Met. Mater. 2012, 57, 165–172. [Google Scholar] [CrossRef]
- Ono-Nakazato, H.; Taguchi, K.; Usui, T. Estimation of the Evaporation Rate of Copper and Tin from Molten Iron-Silicon Alloy. ISIJ Int. 2003, 43, 1105–1107. [Google Scholar] [CrossRef][Green Version]
- Ohno, R. Kinetics of Evaporation of Manganese, Copper and Sulfur from Iron Alloys in Vacuum Induction Melting. Trans. Iron Steel Inst. Jpn. 1977, 17, 732–741. [Google Scholar] [CrossRef]
- Łabaj, J.; Oleksiak, B.; Siwiec, G. Study of copper removal from liquid iron. Metalurgija 2011, 50, 265–268. [Google Scholar]
- Fischer, W.A.; Janke, D.; Stahlschmidt, K. Vergleich experimenteller Ergebnisse und theoretischer Ansätze zur Verdampfung von Begleitelementen aus Stahlschmelzen. Arch. Eisenhuttenwes. 1974, 45, 509–515. [Google Scholar] [CrossRef]
- Morohoshi, K.; Uchikoshi, M.; Isshiki, M.; Fukuyama, H. Surface Tension of Liquid Iron as Functions of Oxygen Activity and Temperature. ISIJ Int. 2011, 51, 1580–1586. [Google Scholar] [CrossRef]
- Yoshida, T.; Nagasaka, T.; Hino, M. Effect of Oxygen on the Evaporation Rate of Lead from Liquid Copper under Reduced Pressure. ISIJ Int. 2001, 41, 706–715. [Google Scholar] [CrossRef]
- Hayakawa, S.; Choh, T.; Inouye, M. Effect of Dissolved Oxygen on the Rates of Evaporation of Molten Iron and Copper. Trans. Iron Steel Inst. Jpn. 1982, 22, 637–645. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Morita, K. Evaluation of Surface Tension and Adsorption for Liquid Fe-S Alloys. ISIJ Int. 2002, 42, 588–594. [Google Scholar] [CrossRef]
- Schenck, H.; Steinmetz, E. Wirkungsparameter von Begleitelement Eisenlegierungen und Ihre Gegenseitigen Beziehungen; Verlag Stahleisen: Essen, Germany, 1968. [Google Scholar]




| Armco Iron | Fe (wt%) | C | Mn | Si | Al | O |
| 99.8 | 40 | 30 | 262 | 11 | 92 |
| Fe-Cu (wt%) | ZnAl2O4 | Al2O3 |
|---|---|---|
| 0.5% Cu, 40 ppm C | 90 min | 90 min |
| 0.5% Cu, 0.5% C | 90 min | 90 min |
| 1% Cu, 40 ppm C | 90 min | 90 min |
| 1% Cu, 0.5% C | 90 min | 90 min |
| 10% Cu, 40 ppm C | 90 min | - |
| wt% | C | Si | Mn | Al | O | Cu Wt% | Cu Loss (%) |
|---|---|---|---|---|---|---|---|
| 0.5% Cu Al2O3 | 40 | 58 | 16 | 36 | 72 | 0.382 | 23.4 |
| 0.5% Cu ZnAl2O4 | 15 | 138 | 13 | 41 | 423 | 0.411 | 17.6 |
| 1% Cu Al2O3 | 40 | 91 | 15 | 55 | 80 | 0.718 | 28.2 |
| 1% Cu ZnAl2O4 | 13 | 77 | 13 | 28 | 559 | 0.908 | 9.2 |
| 0.5% C 0.5% Cu Al2O3 | 0.516 wt% | 50 | 27 | 38 | 51 | 0.35 | 25.0 |
| 0.5% C 0.5% Cu ZnAl2O4 | 14 | 50 | 13 | 38 | 252 | 0.321 | 31.3 |
| 0.5% C 1% Cu Al2O3 | 0.457 wt% | 50 | 15 | 45 | 62 | 0.757 | 24.3 |
| 0.5% C 1% Cu ZnAl2O4 | 18 | 66 | 13 | 46 | 301 | 0.722 | 27.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Dudczig, S.; Chebykin, D.; Aneziris, C.G.; Volkova, O. Verification of Possibility of Molten Steels Decopperization with ZnAl2O4. Metals 2021, 11, 2030. https://doi.org/10.3390/met11122030
Wei X, Dudczig S, Chebykin D, Aneziris CG, Volkova O. Verification of Possibility of Molten Steels Decopperization with ZnAl2O4. Metals. 2021; 11(12):2030. https://doi.org/10.3390/met11122030
Chicago/Turabian StyleWei, Xingwen, Steffen Dudczig, Dmitry Chebykin, Christos G. Aneziris, and Olena Volkova. 2021. "Verification of Possibility of Molten Steels Decopperization with ZnAl2O4" Metals 11, no. 12: 2030. https://doi.org/10.3390/met11122030
APA StyleWei, X., Dudczig, S., Chebykin, D., Aneziris, C. G., & Volkova, O. (2021). Verification of Possibility of Molten Steels Decopperization with ZnAl2O4. Metals, 11(12), 2030. https://doi.org/10.3390/met11122030







