Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation
Abstract
1. Introduction
1.1. State-of-the-Art in Recycling Li-ion Batteries
1.1.1. Thermal Preconditioning
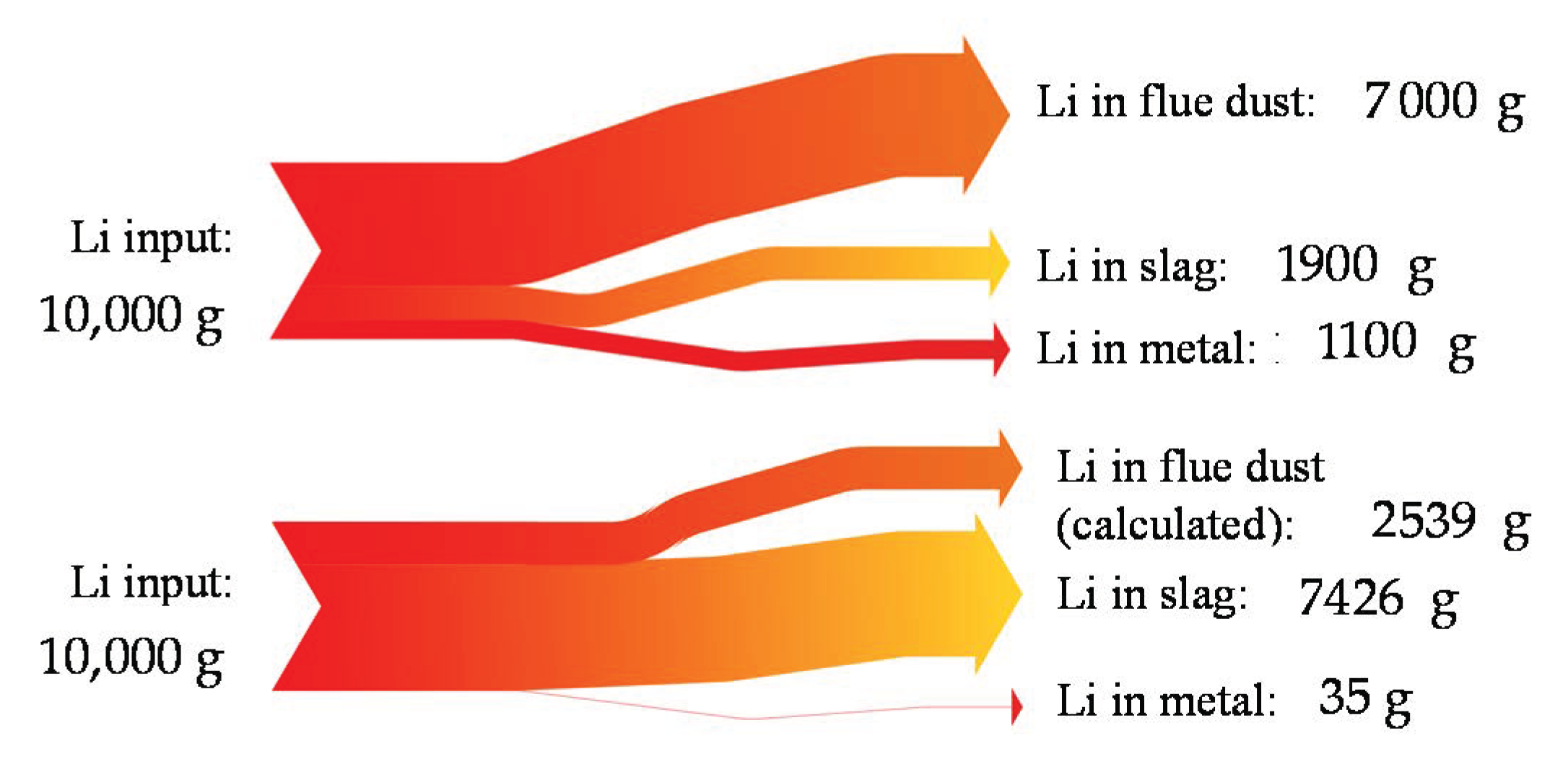
1.1.2. Lithium Behavior in Pyro- and Hydrometallurgical Recycling Steps and Need for Early-Stage Li-Separation
1.1.3. Liquid–Gas Carbonation (Indirect Carbonation)
1.1.4. Solid–Gas Carbonation (Direct Thermal Carbonation)
1.1.5. The Role of Supercritical CO2 (SCCO2)
2. Materials and Methods
2.1. Recycling Concept with Integrated Early-Stage Li-Recovery
- A filter cake with mainly carbon, nickel, cobalt, manganese, aluminum and copper fragments and a share of lithium (→ C-filter cake);
- A lithium-bearing filtrate (solution)
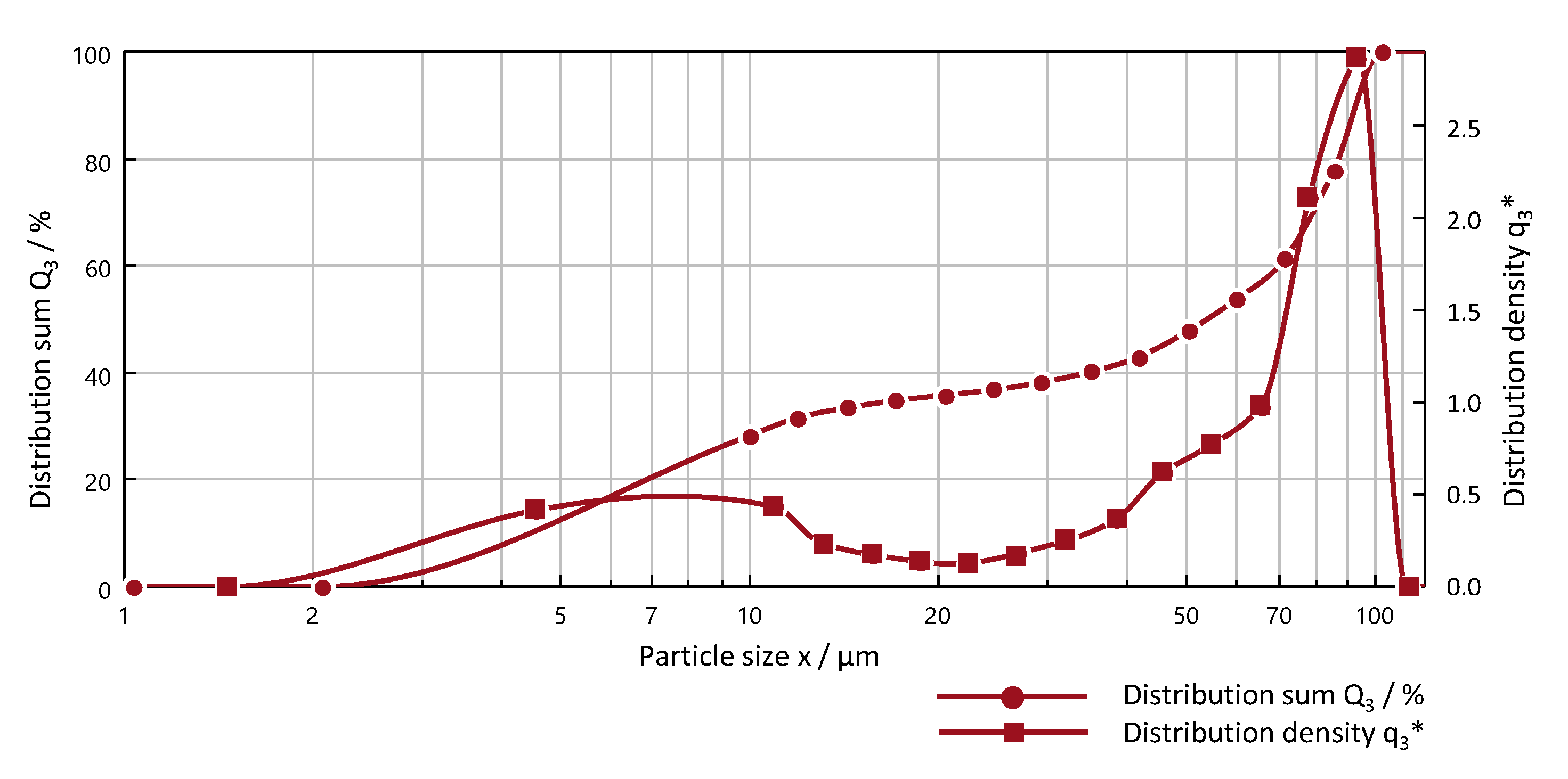
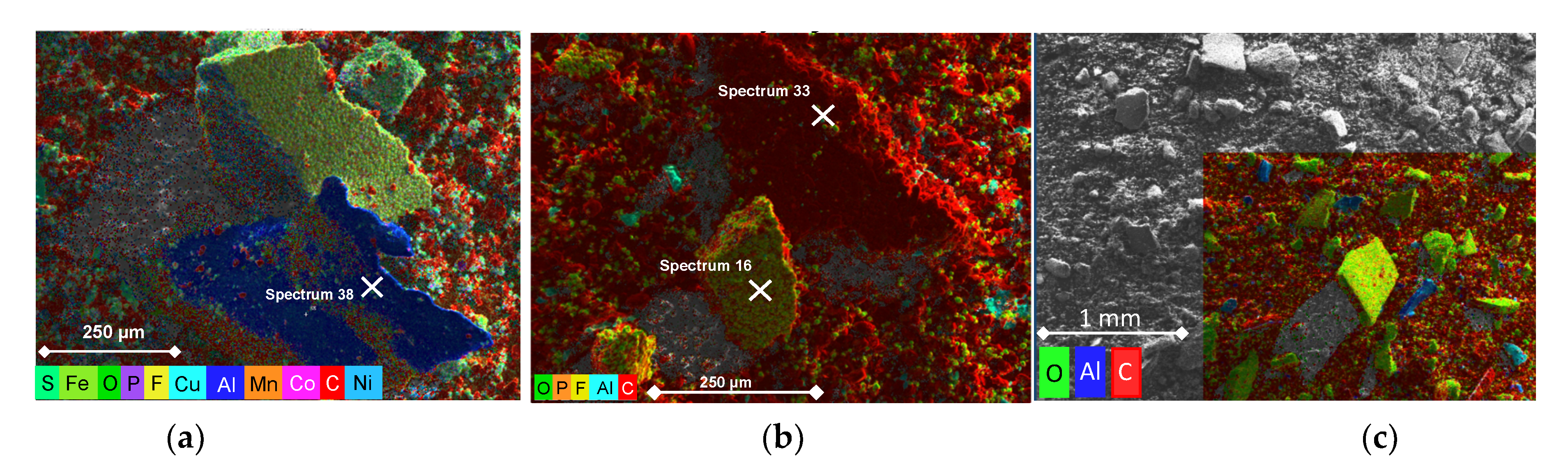
2.2. Material Characterization
2.3. Neutral Leaching Reference Tests in Deionized H2O
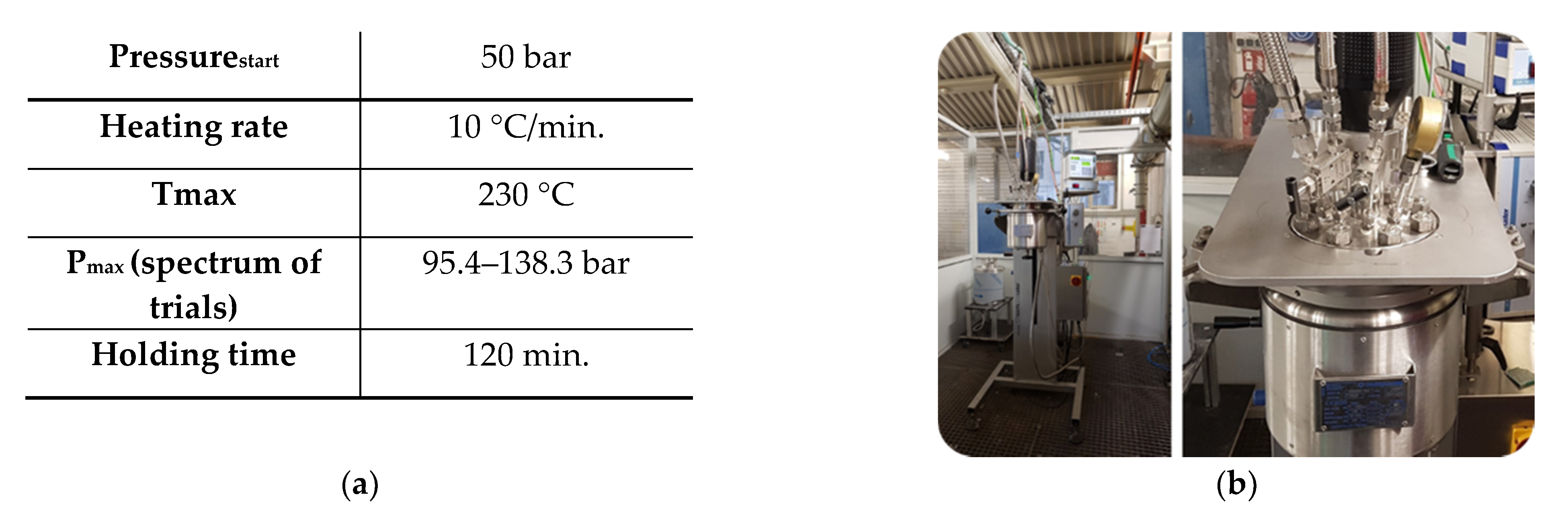
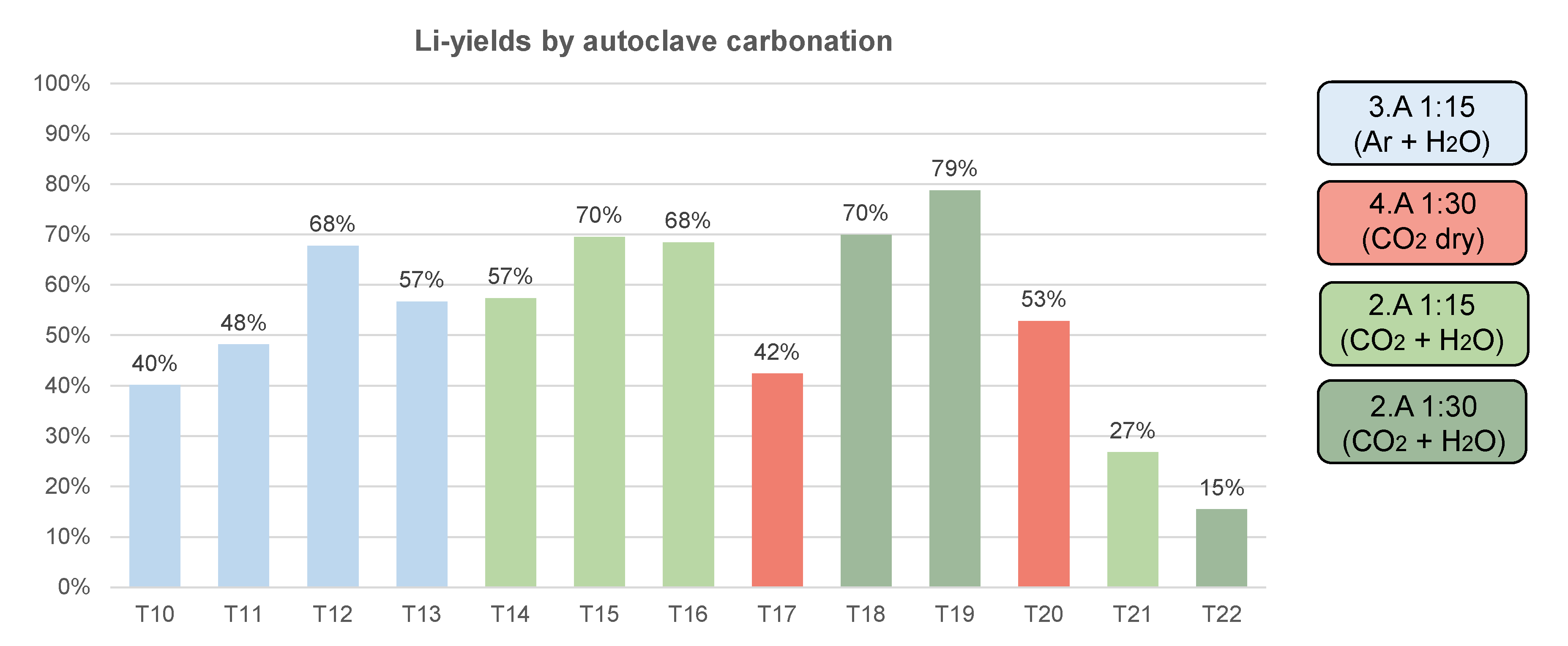
2.4. Carbonation by Supercritical CO2
3. Results
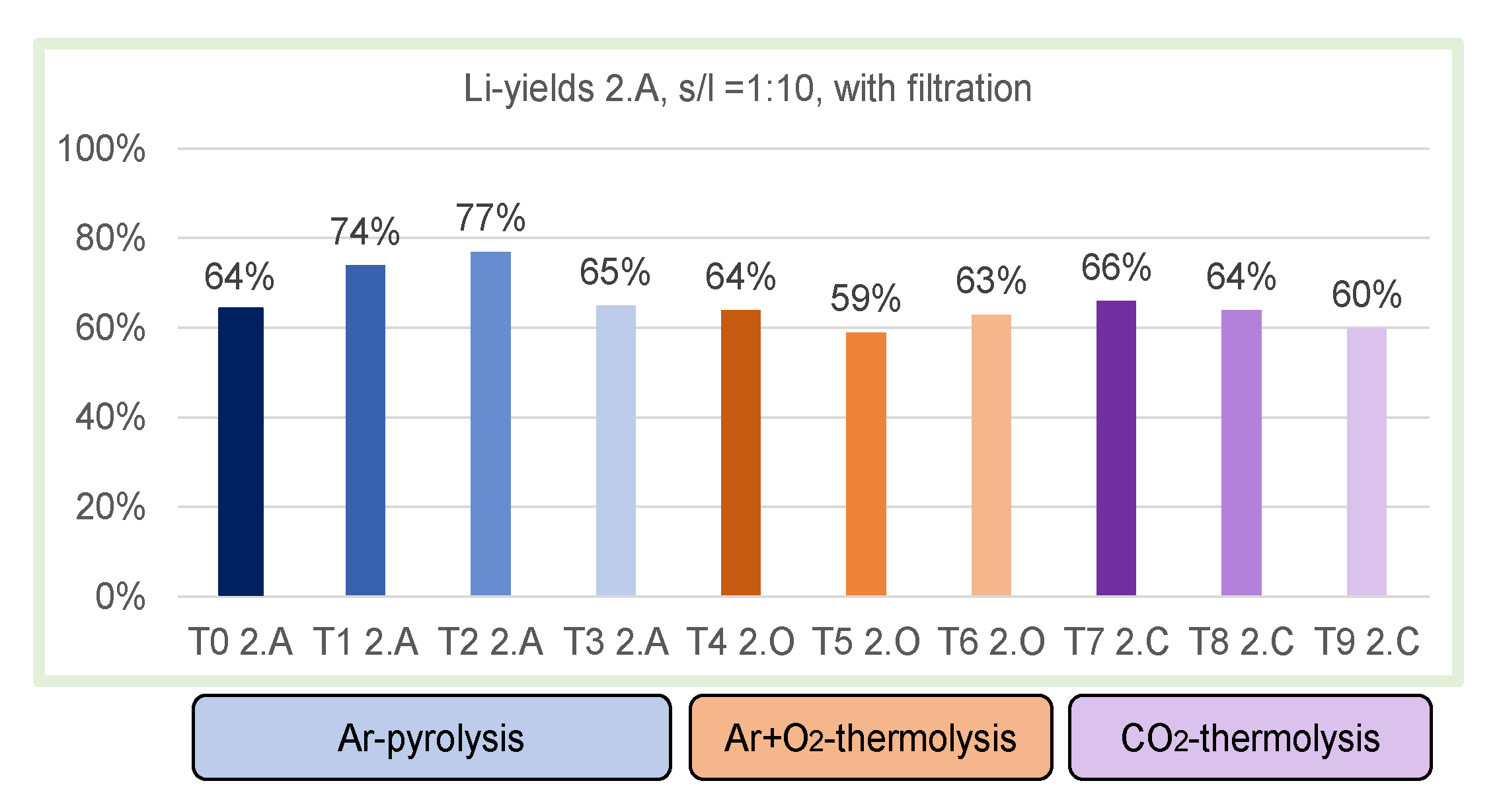
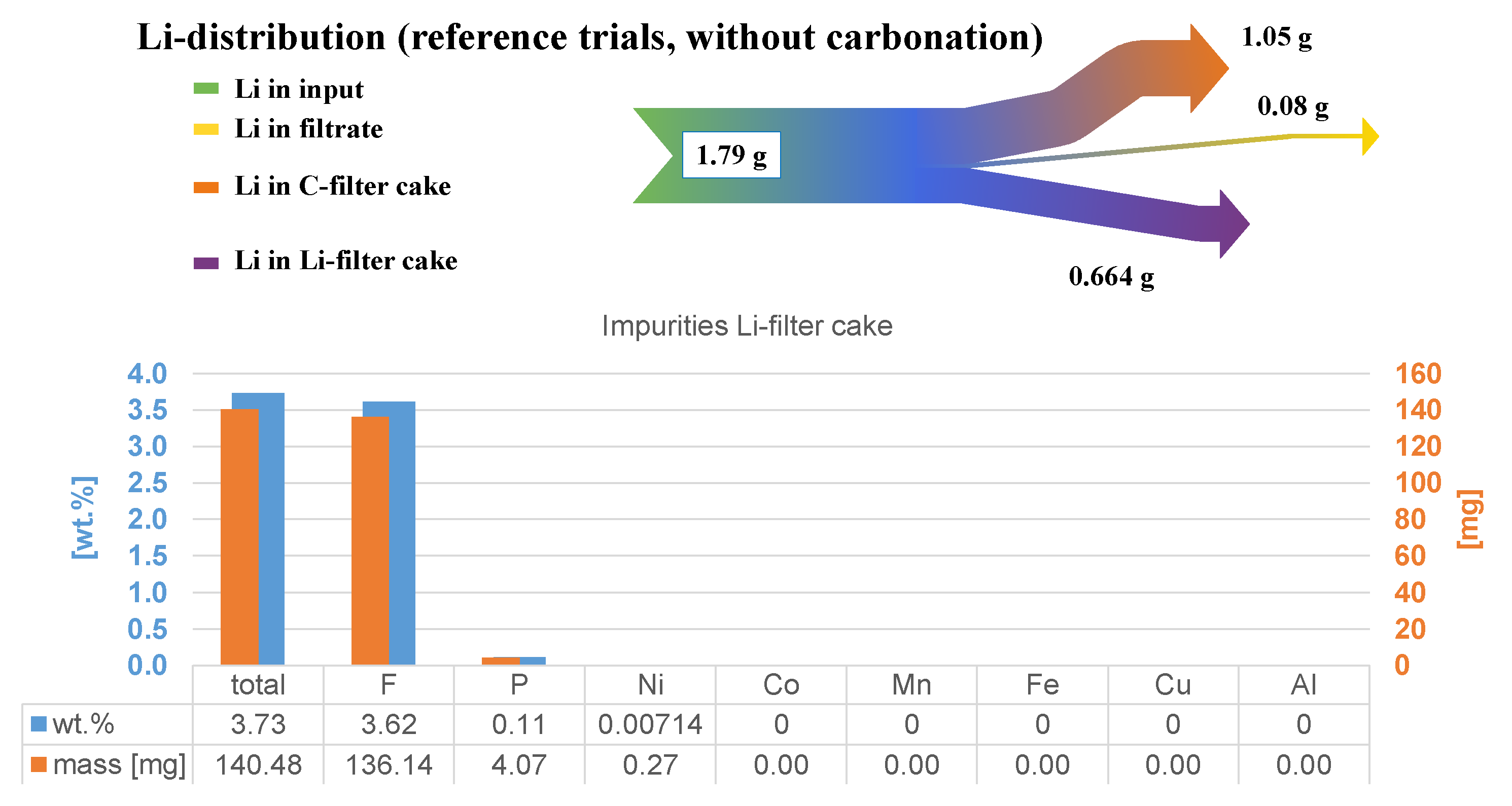
3.1. Neutral Leaching in Deionized H2O
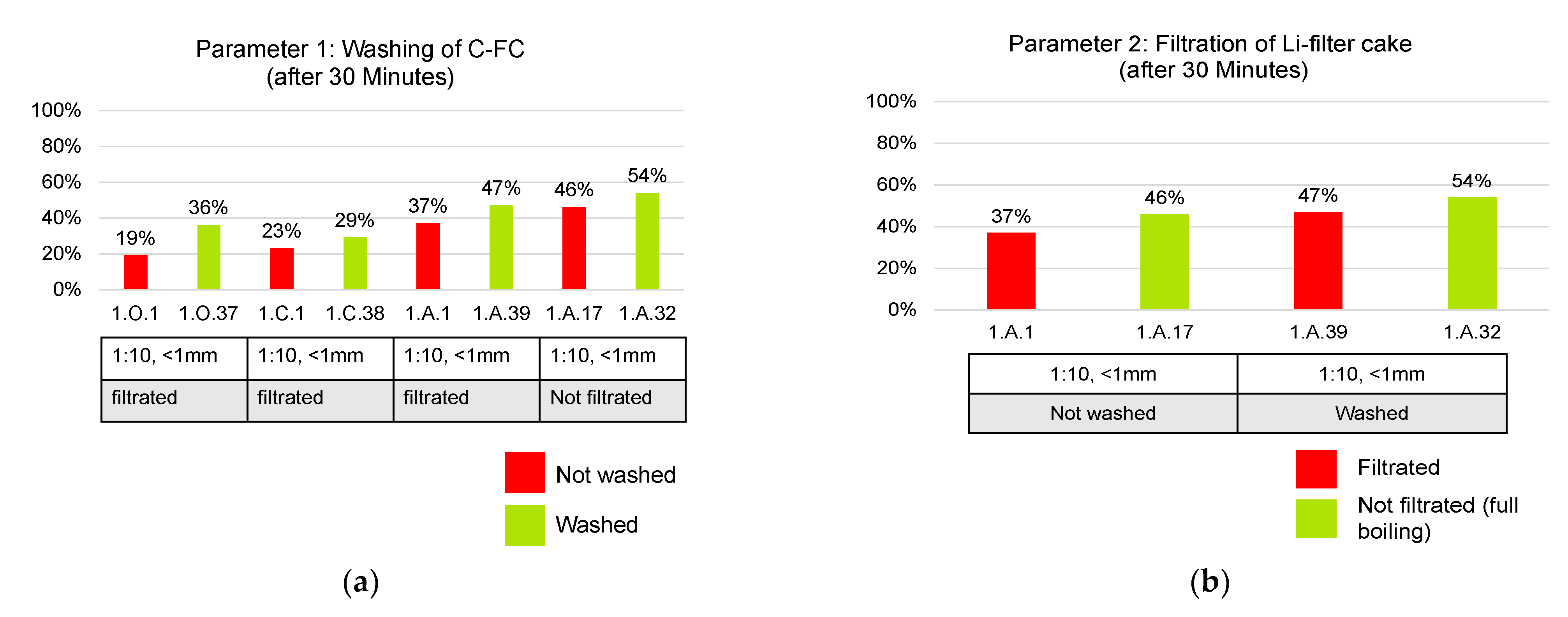
- Washing of C-filter cake with deionized water: if this parameter is performed, it is important to keep the washing volume constant. In this study, 200 mL of deionized water are used;
- Filtration of Li-filter cake or full boiling: full boiling describes the removal of H2O in the laboratory beaker. Filtration stands for filtering the precipitating Li2CO3 at a minimum liquid volume. Hence, there are losses in the residual filtrate. Filtration is conventionally used after acidic leaching to avoid a co-precipitation of acid components and chemical additives;
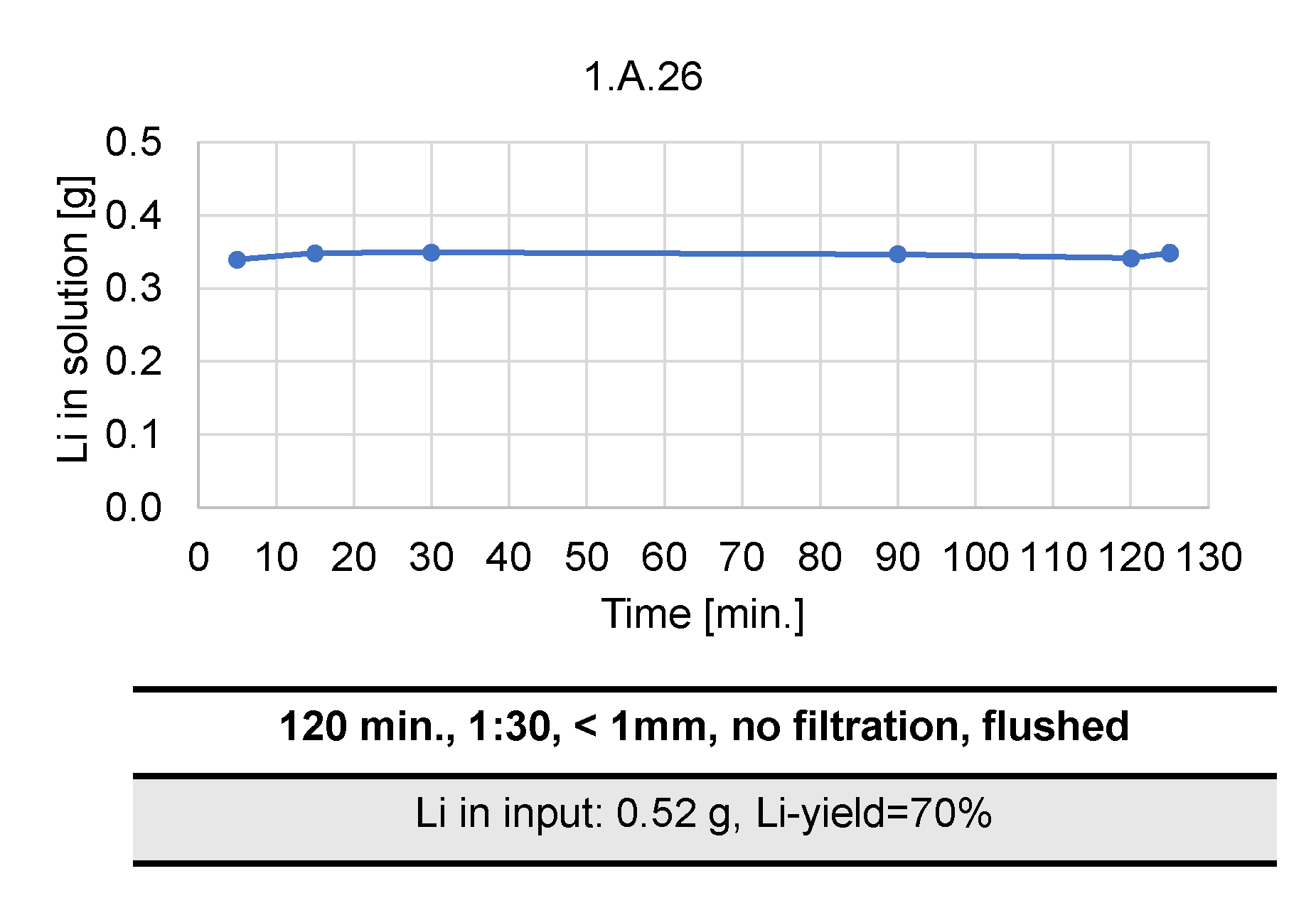
- Leaching time: 5, 30, 90 and 120 min;
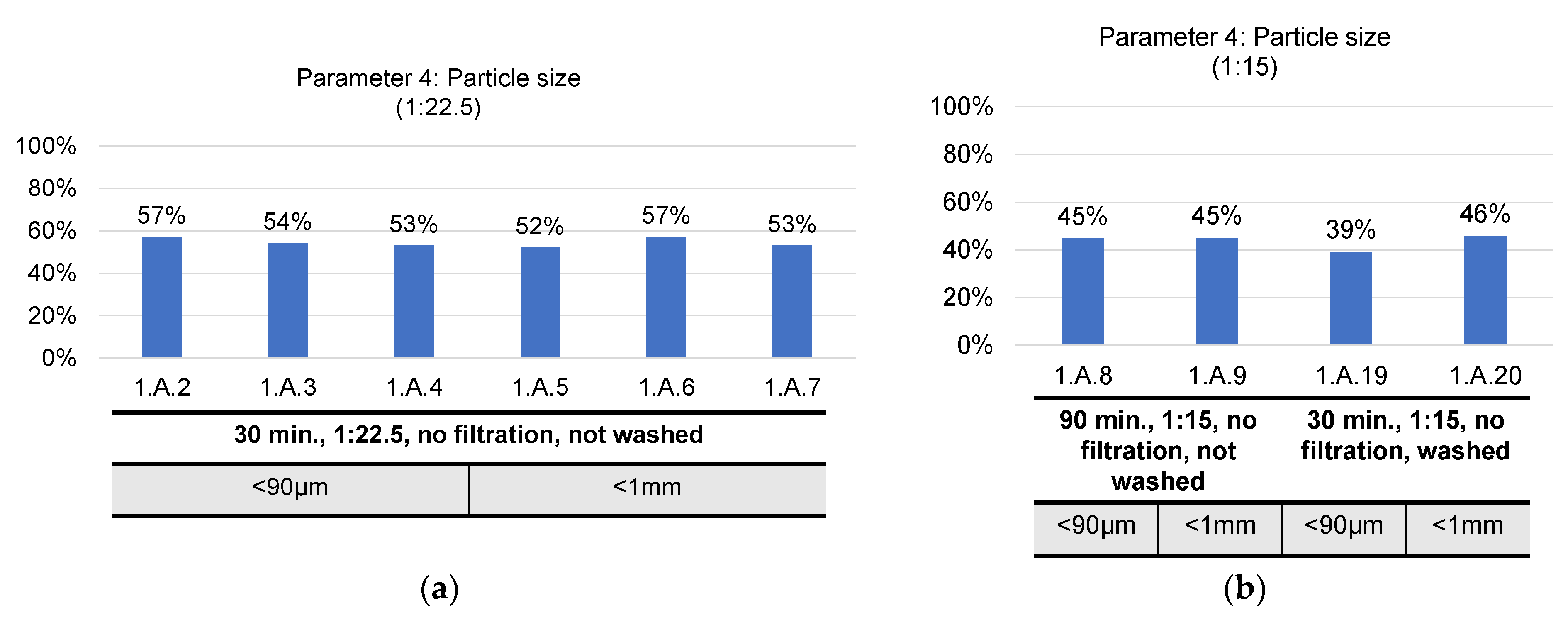
- Particle size of heat-treated black mass: <1 mm vs. <90 µm. The particles <90 µm are obtained by additional grinding of the heat-treated black mass;
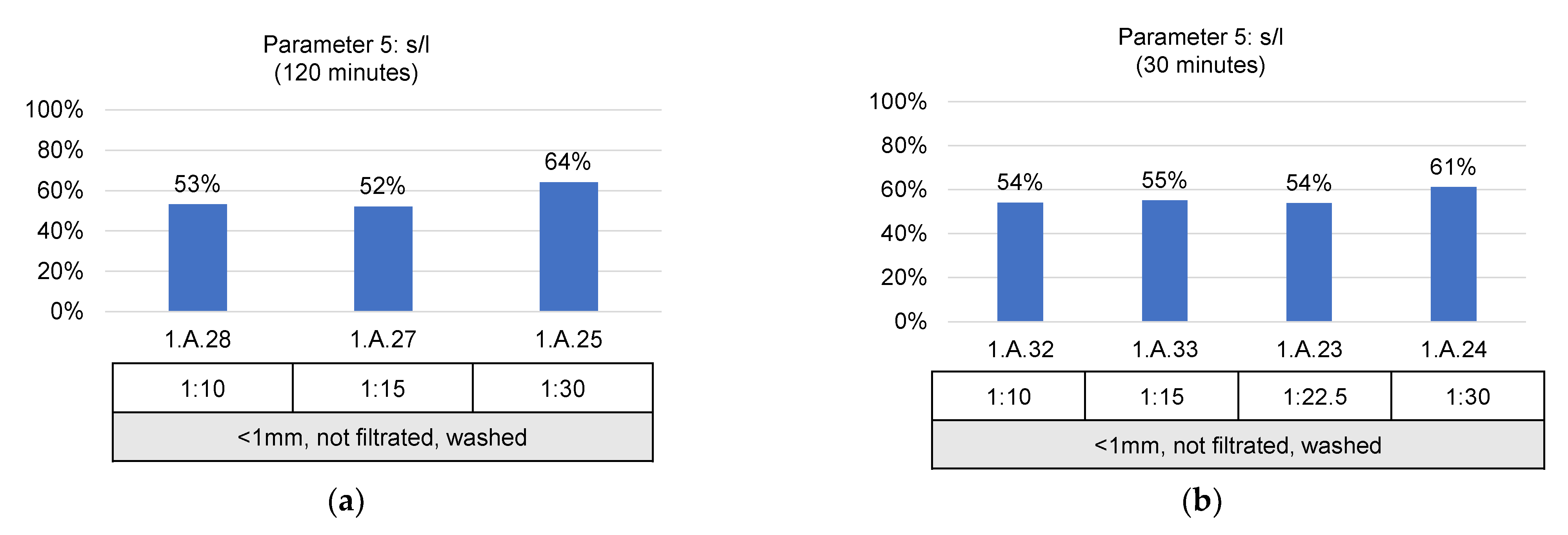
- Solid/liquid ratio (g/mL): 1:10, 1:15, 1:22.5 and 1:30;
- Pyrolysis temperature: 501 vs. 603 °C in Ar-pyrolysis
- 1.
- Washing of C-filter cake with deionized water:
- 2.
- Filtration of Li-filter cake or full boiling:
- 3.
- Leaching time:
- 4.
- Particle size of heat-treated black mass:
- 5.
- Solid/liquid ratio (g/mL):
- 6.
- Pyrolysis temperature:
3.2. Carbonation by Supercritical CO2
4. Discussion
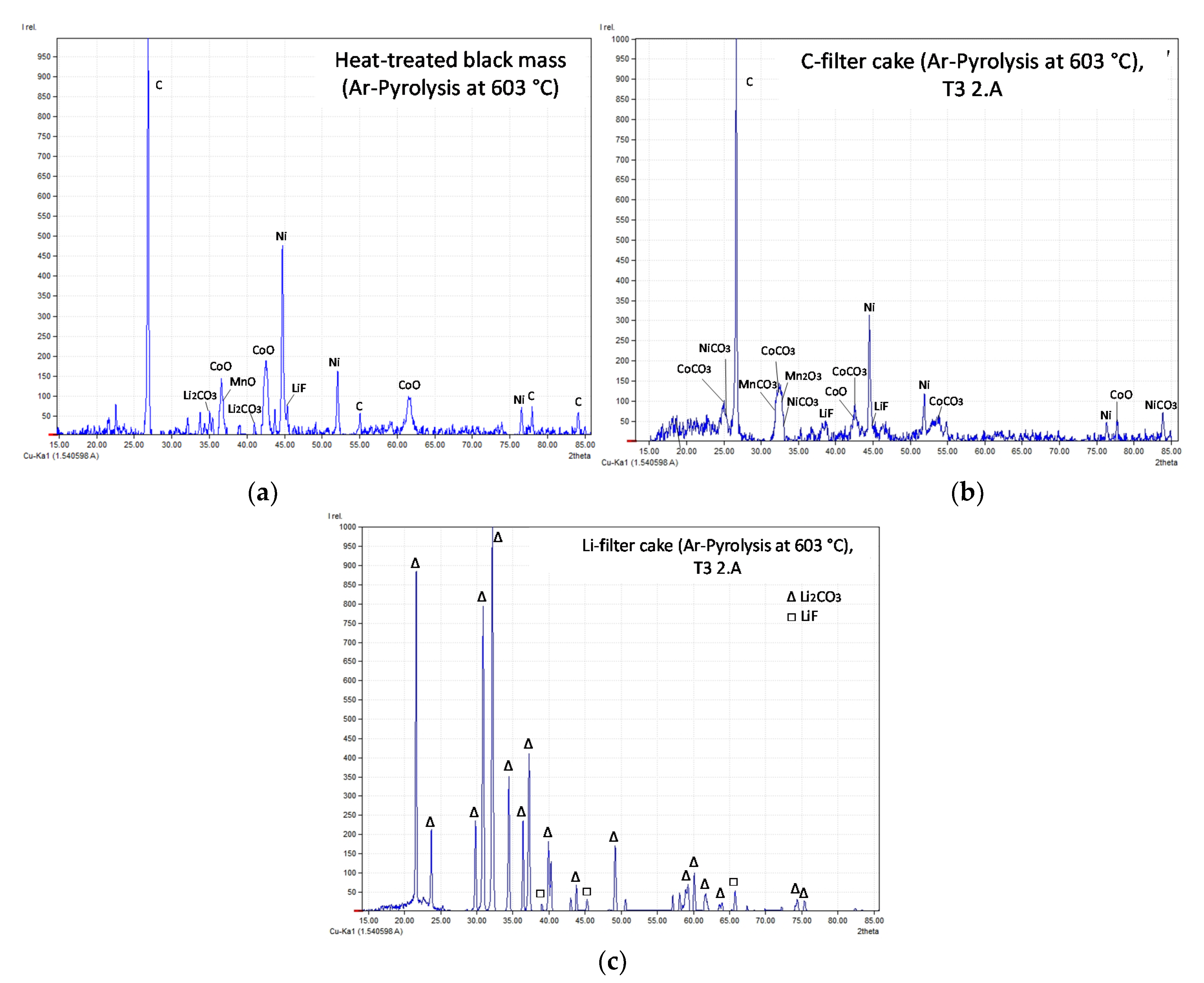
- When leaching heat-treated black mass in H2O, the solution is basic. This can be attributed to the dissolution of basic phases in the liquid. → LiF and Li2CO3 could be detected in the heat-treated black mass by XRD; both phases are slightly soluble and therefore are responsible for the elevated pH-value. Although LiOH and Li2O could not be detected via XRD-analysis in the heat-treated black mass, they may be present in small amounts since the SEI-layers consist of Li2CO3, LiF, LiOH and Li2O [93]. However, it was shown that LiF decomposes to HF and LiOH in aqueous solutions, which indicates Li+ + OH− in the solution.
- When leaching heat-treated black mass in H2O and adding CO2-gas, the pH value of the solution decreases to 7–8. Mechanisms are in place, which can be attributed to CO2 and which are leading to a higher lithium leaching efficiency. In the following, hypotheses for the underlying mechanisms are stated:
- The formation of carbonic acid and thus the formation of CO32− and HCO3− as acidic leaching agents. CO2 is added to a basic solution; it reacts acidic by the release of H+ ions. This pH-value decrease can be responsible for a higher leaching efficiency by creating quasi-acidic leaching conditions similar to conventional hydrometallurgy.
- Recombination of Li+, stemming from non-lithium carbonate phases like LiF, with present CO32− or HCO3−. This would entail the following suggested equations (see Equations (19) and (20), schematically shown in Figure 22):Li+ + CO32− → Li2CO3Li+ + HCO3− → LiHCO3
- A combination of both mentioned mechanisms. In this way, the dissolution of lithium phases in the heat-treated black mass is promoted by CO2, more lithium ions can be formed to Li2CO3, and this effect is also promoted by the increased operating temperatures and arising excess pressure.
5. Conclusions
- Conventional lithium carbonation, e.g., by Na2CO3, is avoided, and no further chemicals are required, making lithium recovery more environmentally friendly;
- Subsequent treating the C-filter cake hydrometallurgically for metal extraction (Ni, Co,…) requires fewer leaching agents because the input mass is reduced, and hence, fewer additives for pH-adjustments are needed;
- Moreover, in comparison to conventional hydrometallurgical lithium recovery, the liquid volume can be fully evaporated (filtration vs. full boiling). Hereby, no lithium remains in the solution. This is possible since no enhancement of salinity is caused in “ESLR”;
- Lithium losses in various byproducts of chemical solution purification and metal winning steps are avoided;
- Costly lithium extraction from a pyrometallurgy treatment and hydrometallurgical purifying of slags is also avoided.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Richa, K. Economies of scale for future lithium-ion battery recycling infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustain. Metall. 2019, 5, 402–416. [Google Scholar] [CrossRef]
- Boyden, A.; Soo, V.K.; Doolan, M. The Environmental Impacts of Recycling Portable Lithium-Ion Batteries. Procedia CIRP 2016, 48, 188–193. [Google Scholar] [CrossRef]
- Zhao, S.; He, W.; Li, G. Recycling Technology and Principle of Spent Lithium-Ion Battery. In Recycling of Spent Lithium-Ion Batteries; An, L., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–26. ISBN 978-3-030-31833-8. [Google Scholar]
- Becker, J.; Beverungen, D.; Winter, M.; Menne, S. Umwidmung und Weiterverwendung von Traktionsbatterien; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2019; ISBN 978-3-658-21020-5. [Google Scholar]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling lithium-ion batteries from electric vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Pinegar, H.; Smith, Y.R. Recycling of End-of-Life Lithium-Ion Batteries, Part II: Laboratory-Scale Research Developments in Mechanical, Thermal, and Leaching Treatments. J. Sustain. Metall. 2020, 6, 142–160. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and environmental aspects in recycling lithium-ion batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Peters, J.F.; Simon, B.; Rodriguez-Garcia, G.; Weil, M. Building a common base for LCA benchmarking of Li-Ion batteries. In Proceedings of the SETAC 26th Annual Meeting, Nantes, France, 22–26 May 2016. [Google Scholar]
- Dunn, J.B.; Gaines, L.; Kelly, J.C.; Gallagher, K.G. Life Cycle Analysis Summary for Automotive Lithiumion Battery Production and Recycling. In Rewas 2016: Towards Materials Resource Sustainability; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 73–79. [Google Scholar]
- Martens, H.; Goldmann, D. Recyclingtechnik; Fachbuch für Lehre und Praxis, 2. Aufl; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2016; ISBN 978-3-658-02786-5. [Google Scholar]
- Diekmann, J.; Sander, S.; Sellin, G.; Petermann, M.; Kwade, A. Crushing of Battery Modules and Cells. In Recycling of Lithium-Ion Batteries; Kwade, A., Diekmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 127–138. ISBN 978-3-319-70571-2. [Google Scholar]
- Sojka, R.T. Sichere Aufbereitung von Lithium-basierten Batterien durch thermische Konditionierung. Safe treatment of Lithium-based Batteries through Thermal Conditioning. In Recycling und Sekundärrohstoffe, Band 13; Thomé-Kozmiensky, E., Holm, O., Friedrich, B., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Nietwerder, Germany, 2020; pp. 506–523. ISBN 978-3-944310-51-0. [Google Scholar]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef]
- Jiang, H.; Emmett, R.K.; Roberts, M.E. Thermally induced deactivation of lithium-ion batteries using temperature-responsive interfaces. Ionics 2019, 25, 2453–2457. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects. Waste Manag. 2015, 45, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, G.; Ebin, B.; St. J. Foreman, M.R.; Steenari, B.-M.; Petranikova, M. Chemical Transformations in Li-Ion Battery Electrode Materials by Carbothermic Reduction. ACS Sustain. Chem. Eng. 2019, 7, 13668–13679. [Google Scholar] [CrossRef]
- Ekberg, C.; Petranikova, M. Lithium Batteries Recycling. In Lithium Process Chemistry. Resources, Extraction, Batteries, and Recycling; Chagnes, A., Światowska, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–267. ISBN 9780128014172. [Google Scholar]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef]
- Träger, T.; Friedrich, B.; Weyhe, R. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar] [CrossRef]
- Stallmeister, C.; Schwich, L.; Friedrich, B. Early-Stage Li-Removal-Vermeidung von Lithiumverlusten im Zuge der Thermischen und Chemischen Recyclingrouten von Batterien. In Recycling und Sekundärrohstoffe, Band 13; Thomé-Kozmiensky, E., Holm, O., Friedrich, B., Goldmann, D., Eds.; Thomé-Kozmiensky Verlag GmbH: Nietwerder, Germany, 2020; pp. 545–557. ISBN 978-3-944310-51-0. [Google Scholar]
- Vest, M. Weiterentwicklung des Pyrometallurgischen IME Recyclingverfahrens für Li-Ionen Batterien von Elektrofahrzeugen. Ph.D. Thesis, Shaker Verlag GmbH, Aachen, Germany, 2016. [Google Scholar]
- Wang, H.; Friedrich, B. Development of a Highly Efficient Hydrometallurgical Recycling Process for Automotive Li–Ion Batteries. J. Sustain. Metall. 2015, 1, 168–178. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, N.; Jie, Y.; Hu, F.; Li, Y.; Wilson, B.P.; Xi, Y.; Lai, Y.; Yang, S. Toxicity Identification and Evolution Mechanism of Thermolysis-Driven Gas Emissions from Cathodes of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 18228–18235. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Yang, Y.; Song, S.; Lei, S.; Sun, W.; Hou, H.; Jiang, F.; Ji, X.; Zhao, W.; Hu, Y. A process for combination of recycling lithium and regenerating graphite from spent lithium-ion battery. Waste Manag. 2019, 85, 529–537. [Google Scholar] [CrossRef]
- Or, T.; Gourley, S.W.D.; Kaliyappan, K.; Yu, A.; Chen, Z. Recycling of mixed cathode lithium-ion batteries for electric vehicles: Current status and future outlook. Carbon Energy 2020, 61, 1. [Google Scholar] [CrossRef]
- Essay, P.; Foxworth, C.; Strauss, C.; Torres, A. Corby Anderson. Hydrometallurgical Recovery of Materials from Lithium-ion Batteries. 2012. Available online: https://www.researchgate.net/publication/302285317_Hydrometallurgical_Recovery_of_Materials_from_Lithium-ion_Batteries (accessed on 19 January 2021).
- Liu, P.; Xiao, L.; Chen, Y.; Tang, Y.; Wu, J.; Chen, H. Recovering valuable metals from LiNixCoyMn1-x-yO2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching. J. Alloys Compd. 2019, 783, 743–752. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Li, M.; Chen, R.; Wu, F.; Amine, K.; Lu, J. The Recycling of Spent Lithium-Ion Batteries: A Review of Current Processes and Technologies. Electrochem. Energy Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. Economical recycling process for spent lithium-ion batteries and macro- and micro-scale mechanistic study. J. Power Sources 2018, 377, 70–79. [Google Scholar] [CrossRef]
- Barik, S.P.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, T. Hydrometallurgical process for the recovery of metal values from spent lithium-ion batteries in citric acid media. Waste Manag. Res. 2014, 32, 1083–1093. [Google Scholar] [CrossRef]
- Wang, R.-C.; Lin, Y.-C.; Wu, S.-H. A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 2009, 99, 194–201. [Google Scholar] [CrossRef]
- Nayl, A.A.; Elkhashab, R.A.; Badawy, S.M.; El-Khateeb, M.A. Acid leaching of mixed spent Li-ion batteries. Arab. J. Chem. 2017, 10, S3632–S3639. [Google Scholar] [CrossRef]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, X.; Zheng, X.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Lithium Carbonate Recovery from Cathode Scrap of Spent Lithium-Ion Battery: A Closed-Loop Process. Environ. Sci. Technol. 2017, 51, 1662–1669. [Google Scholar] [CrossRef]
- Stopic, S.; Dertmann, C.; Modolo, G.; Kegler, P.; Neumeier, S.; Kremer, D.; Wotruba, H.; Etzold, S.; Telle, R.; Rosani, D.; et al. Synthesis of Magnesium Carbonate via Carbonation under High Pressure in an Autoclave. Metals 2018, 8, 993. [Google Scholar] [CrossRef]
- Rahmani, O.; Highfield, J.; Junin, R.; Tyrer, M.; Pour, A.B. Experimental Investigation and Simplistic Geochemical Modeling of CO₂ Mineral Carbonation Using the Mount Tawai Peridotite. Molecules 2016, 21, 353. [Google Scholar] [CrossRef] [PubMed]
- Daval, D.; Martinez, I.; Guigner, J.-M.; Hellmann, R.; Corvisier, J.; Findling, N.; Dominici, C.; Goffe, B.; Guyot, F. Mechanism of wollastonite carbonation deduced from micro- to nanometer length scale observations. Am. Mineral. 2009, 94, 1707–1726. [Google Scholar] [CrossRef]
- Haug, T.A. Dissolution and Carbonation of Mechanically Activated Olivine. Investigating CO2 Sequestration Possibilities. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2010. [Google Scholar]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Zhang, W.; Chen, Y.; Wang, C. Efficient and economical recovery of lithium, cobalt, nickel, manganese from cathode scrap of spent lithium-ion batteries. J. Clean. Prod. 2018, 204, 437–446. [Google Scholar] [CrossRef]
- Kunzler, C.; Alves, N.; Pereira, E.; Nienczewski, J.; Ligabue, R.; Einloft, S.; Dullius, J. CO2 storage with indirect carbonation using industrial waste. Energy Procedia 2011, 4, 1010–1017. [Google Scholar] [CrossRef]
- Jandová, J.; Dvorák, P.; Kondás, J.; Havlák, L. Recovery of Lithium from Waste Materials. Ceram.-Silik. 2012, 56, 50–54. [Google Scholar]
- Hönisch, B.; Ridgwell, A.; Schmidt, D.N.; Thomas, E.; Gibbs, S.J.; Sluijs, A.; Zeebe, R.; Kump, L.; Martindale, R.C.; Greene, S.E.; et al. The geological record of ocean acidification. Science 2012, 335, 1058–1063. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC handbook of chemistry and physics. In A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2017; ISBN 978-1-4987-5429-3. [Google Scholar]
- Yi, W.-T.; Yan, C.-Y.; Ma, P.-H. Kinetic study on carbonation of crude Li2CO3 with CO2-water solutions in a slurry bubble column reactor. Korean J. Chem. Eng. 2011, 28, 703–709. [Google Scholar] [CrossRef]
- Yi, W.-T.; Yan, C.-Y.; Ma, P.-H. Crystallization kinetics of Li2CO3 from LiHCO3 solutions. J. Cryst. Growth 2010, 312, 2345–2350. [Google Scholar] [CrossRef]
- Trogdon-Stout, D. Chemistry. In Essential Practice for Key Science Topics; The 100+ series; Carson Dellosa Publishing Group: Greensboro, NC, USA, 2015; ISBN 1483817091. [Google Scholar]
- European Chemicals Agency ECHA. Lithium Carbonate (EC number: 209-062-5|CAS Number: 554-13-2). Hydrolysis. European Union. 2010. Available online: https://echa.europa.eu/de/registration-dossier/-/registered-dossier/15034/5/2/3#sLinkToRelevantStudyRecord (accessed on 13 June 2020).
- Wawra, E.; Dolznig, H.; Müllner, E. Chemie verstehen. In Allgemeine Chemie für Mediziner und Naturwissenschafter, 5. Aufl; UTB: Medizin, Naturwissenschaften, 2009; ISBN 9783825282059. [Google Scholar]
- House, J.E. Inorganic Chemistry, 2nd ed.; Elsevier: San Diego, CA, USA, 2020; ISBN 978-0-12-814369-8. [Google Scholar]
- Perry, D.L. Handbook of Inorganic Compounds; CRC Press: Hoboken, NJ, USA, 2011; ISBN 978-1-4398-1461-1. [Google Scholar]
- Groult, H.; Nakajima, T. Fluorinated Materials for Energy Conversion, 1. Aufl.; Elsevier: Amsterdam, The Netherlands; San Diego, CA, USA; Oxford, UK, 2005; ISBN 9780080444727. [Google Scholar]
- Scholz, F.; Kahlert, H. Chemische Gleichgewichte in der Analytischen Chemie; Springer: Berlin/Heidelberg, Germany, 2020; ISBN 978-3-662-61106-7. [Google Scholar]
- Binnewies, M.; Finze, M.; Jäckel, M.; Schmidt, P.; Willner, H.; Rayner-Canham, G. Allgemeine und Anorganische Chemie; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-45066-6. [Google Scholar]
- Lew, K. Acids and bases. In Essential Chemistry; Chelsea House: New York, NY, USA, 2009; ISBN 9781438116617. [Google Scholar]
- Sigma-Aldrich Co. Safety Data Sheet according to Regulation (EC) No. 1907/2006. Lithium Fluoride (CAS-No.: 7789-24-4). 2018. Available online: https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=DE&language=EN-generic&productNumber=449903&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Fterm%3DLiF%26interface%3DAll_DE%26N%3D0%26mode%3Dmatch%26lang%3Dde%26region%3DDE%26focus%3Dproduct (accessed on 29 December 2020).
- Shimonishi, Y.; Zhang, T.; Imanishi, N.; Im, D.; Lee, D.J.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. A study on lithium/air secondary batteries—Stability of the NASICON-type lithium ion conducting solid electrolyte in alkaline aqueous solutions. J. Power Sources 2011, 196, 5128–5132. [Google Scholar] [CrossRef]
- Bi, X.; Wang, R.; Lu, J. Li-Air Batteries: Discharge products. In Metal-Air Batteries. Fundamentals and Applications; Zhang, X.-B., Ed.; Wiley-VCH: Weinheim, Germany, 2018; ISBN 3527807667. [Google Scholar]
- Ham, B.M.; MaHam, A. Analytical chemistry. In A Chemist and Laboratory Technician’s Toolkit; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781119069690. [Google Scholar]
- Zilberman, P. The CO2 Absorber Based on LiOH. Acta Medica Marisiensis 2015, 61, 4–6. [Google Scholar] [CrossRef]
- Johnson, G.K.; Grow, R.T.; Hubbard, W.N. The enthalpy of formation of lithium oxide (Li2O). J. Chem. Thermodyn. 1975, 7, 781–786. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Vishvakarma, S.; Dhawan, N. Recovery of Cobalt and Lithium Values from Discarded Li-Ion Batteries. J. Sustain. Metall. 2019, 5, 204–209. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Recycling metals from lithium ion battery by mechanical separation and vacuum metallurgy. J. Hazard. Mater. 2017, 338, 124–131. [Google Scholar] [CrossRef]
- Kuzuhara, S.; Ota, M.; Tsugita, F.; Kasuya, R. Recovering Lithium from the Cathode Active Material in Lithium-Ion Batteries via Thermal Decomposition. Metals 2020, 10, 433. [Google Scholar] [CrossRef]
- Wang, J.-P.; Pyo, J.-J.; Ahn, S.-H.; Choi, D.-H.; Lee, B.-W.; Lee, D.-W. A Study on the Recovery of Li2CO3 from Cathode Active Material NCM(LiNiCoMnO2) of Spent Lithium Ion Batteries. J. Korean Powder Metall. Inst. 2018, 25, 296–301. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Novel Approach for in Situ Recovery of Lithium Carbonate from Spent Lithium Ion Batteries Using Vacuum Metallurgy. Environ. Sci. Technol. 2017, 51, 11960–11966. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal treatment process for the recovery of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; St J Foreman, M.R.; Steenari, B.-M.; Petranikova, M. Incineration of EV Lithium-ion batteries as a pretreatment for recycling—Determination of the potential formation of hazardous by-products and effects on metal compounds. J. Hazard. Mater. 2020, 393, 122372. [Google Scholar] [CrossRef] [PubMed]
- Peters, L.; Friedrich, B. Proven Methods for Recovery of Lithium from Spent Batteries; DERA Workshop Lithium: Berlin, Germany, 2017. [Google Scholar]
- Petranikova, M. Spracovanie Pouţitých Prenosných Lítiových Akumulátorov. Ph.D. Thesis, Technical University of Košice, Košice, Slovakia, 2012. [Google Scholar]
- Mosqueda, H.A.; Vazquez, C.; Bosch, P.; Pfeiffer, H. Chemical Sorption of Carbon Dioxide (CO2) on Lithium Oxide (Li2O). Chem. Mater. 2006, 18, 2307–2310. [Google Scholar] [CrossRef]
- Ueda, S.; Inoue, R.; Sasaki, K.; Wakuta, K.; Ariyama, T. CO2 Absorption and Desorption Abilities of Li2O–TiO2 Compounds. ISIJ Int. 2011, 51, 530–537. [Google Scholar] [CrossRef][Green Version]
- Sloop, S.E.; Parker, R. System and Method for Processing an End-of-Life or Reduced Performance Energy Storage and/or Conversion Device Using a Supercritical Fluid (US 8,067,107 B2). 2011. Available online: https://patentimages.storage.googleapis.com/1f/5d/1b/69963cd0c16466/US8067107.pdf (accessed on 1 April 2020).
- Nowak, S.; Winter, M. The Role of Sub- and Supercritical CO2 as “Processing Solvent” for the Recycling and Sample Preparation of Lithium Ion Battery Electrolytes. Molecules 2017, 22, 403. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Q.; Chen, B.; Shi, X.; Liao, T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Bertau, M.; Voigt, W.; Schneider, A.; Martin, G. Lithiumgewinnung aus anspruchsvollen Lagerstätten: Zinnwaldit und magnesiumreiche Salzseen. Chem. Ing. Tech. 2017, 89, 64–81. [Google Scholar] [CrossRef]
- Liu, Y. Analysis on Extraction Behaviour of Lithium-ion Battery Electrolyte Solvents in Supercritical CO2 by Gas Chromatography. Int. J. Electrochem. Sci. 2016, 11, 7594–7604. [Google Scholar] [CrossRef]
- Grützke, M.; Mönnighoff, X.; Horsthemke, F.; Kraft, V.; Winter, M.; Nowak, S. Extraction of lithium-ion battery electrolytes with liquid and supercritical carbon dioxide and additional solvents. RSC Adv. 2015, 5, 43209–43217. [Google Scholar] [CrossRef]
- Rothermel, S.; Grützke, M.; Mönnighoff, X.; Winter, M.; Nowak, S. Electrolyte Extraction—Sub and Supercritical CO2. In Recycling of Lithium-Ion Batteries; Kwade, A., Diekmann, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 177–185. ISBN 978-3-319-70571-2. [Google Scholar]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A direct recycling case study from a lithium-ion battery recall. Sustain. Mater. Technol. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Rothermel, S.; Evertz, M.; Kasnatscheew, J.; Qi, X.; Grützke, M.; Winter, M.; Nowak, S. Graphite Recycling from Spent Lithium-Ion Batteries. ChemSusChem 2016, 9, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Bertau, M.; Martin, G. Integrated Direct Carbonation Process for Lithium Recovery from Primary and Secondary Resources. Mater. Sci. Forum 2019, 959, 69–73. [Google Scholar] [CrossRef]
- Bertau, M.; Martin, G.; Pätzold, C. Verfahren zur Gewinnung von Lithiumcarbonat aus lithiumhaltigen Batterierückständen mittels CO2-Behandlung. Deutsche Patentanmeldung (DE102016208407A1), 23 November 2017. [Google Scholar]
- Stopic, S.; Dertmann, C.; Koiwa, I.; Kremer, D.; Wotruba, H.; Etzold, S.; Telle, R.; Knops, P.; Friedrich, B. Synthesis of Nanosilica via Olivine Mineral Carbonation under High Pressure in an Autoclave. Metals 2019, 9, 708. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Penciner, J. The Anode/Electrolyte Interface. In Handbook of Battery Materials, 2. Aufl.; Daniel, C., Besenhard, J.O., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp. 479–524. ISBN 978-3-527-32695-2. [Google Scholar]










| Phase | Solubility at 20 °C | Solubility at 100 °C |
|---|---|---|
| LiOH | 110 g/L 1 | 161 g/L 1 |
| LiF | 1.2 g/L 1 | 1.34 g/L 1 |
| LiHCO3 | 55 g/L 2 | 57.4 g/L 1 |
| Li2CO3 | 13.3 g/L 1 | 7.2 g/L 1 |
| Al | Co | Cu | F | Fe | Li | Mn | P | C | Ni |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | |||||||||
| 2.10 | 11.7 | 0.88 | 4.10 | 0.00 | 3.69 | 8.91 | 0.44 | 33.9 | 11.5 |
| Element | C | O | F | P | Mn | Co | Ni | Al |
|---|---|---|---|---|---|---|---|---|
| Unit | wt.% | |||||||
| Spectrum 38 | 31.9 | 7.35 | <1 wt.% | <1 wt.% | <1 wt.% | <1 wt.% | <1 wt.% | 58.28 |
| Spectrum 16 | 32.83 | 12.1 | 21.9 | 1.81 | 8.59 | 11.38 | 10.62 | <1 wt.% |
| Spectrum 33 | 46.46 | 33.51 | 1.47 | <1 wt.% | <1 wt.% | <1 wt.% | <1 wt.% | 17.95 |
| Pyrolysis Conditions | ||||
|---|---|---|---|---|
| Ar-Atmosphere | 95 % Ar + 5 % O2-Atmosphere | CO2-Atmosphere | ||
| Autoclave conditions | H2O-leaching (reference trials) | 1.A | 1.O | 1.C |
| SCCO2 + H2O | 2.A | 2.O | 2.C | |
| Ar + H2O | 3.A | n/a | n/a | |
| CO2 + dry autoclave | 4.A | n/a | n/a | |
| Solid/Liquid Ratio (s/l) (g/mL) | H2O in Autoclave | Autoclave Gas | Washing C-Filter Cake with H2O | |
|---|---|---|---|---|
| T10 | 1:15 | yes | Ar | no |
| T11 | 1:15 | yes | Ar | no |
| T12 | 1:15 | yes | Ar | no |
| T13 | 1:15 | yes | Ar | no |
| T14 | 1:15 | yes | CO2 | no |
| T15 | 1:15 | yes | CO2 | yes |
| T16 | 1:15 | yes | CO2 | no |
| T17 | 1:30 | no | CO2 | no |
| T18 | 1:30 | yes | CO2 | yes |
| T19 | 1:30 | yes | CO2 | yes |
| T20 | 1:30 | no | CO2 | yes |
| T21 | 1:15 | yes | CO2 | no |
| T22 | 1:30 | yes | CO2 | no |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwich, L.; Schubert, T.; Friedrich, B. Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals 2021, 11, 177. https://doi.org/10.3390/met11020177
Schwich L, Schubert T, Friedrich B. Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals. 2021; 11(2):177. https://doi.org/10.3390/met11020177
Chicago/Turabian StyleSchwich, Lilian, Tom Schubert, and Bernd Friedrich. 2021. "Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation" Metals 11, no. 2: 177. https://doi.org/10.3390/met11020177
APA StyleSchwich, L., Schubert, T., & Friedrich, B. (2021). Early-Stage Recovery of Lithium from Tailored Thermal Conditioned Black Mass Part I: Mobilizing Lithium via Supercritical CO2-Carbonation. Metals, 11(2), 177. https://doi.org/10.3390/met11020177







