Nucleation and Morphology of Cu6Sn5 Intermetallic at the Interface between Molten Sn-0.7Cu-0.2Cr Solder and Cu Substrate
Abstract
:1. Introduction
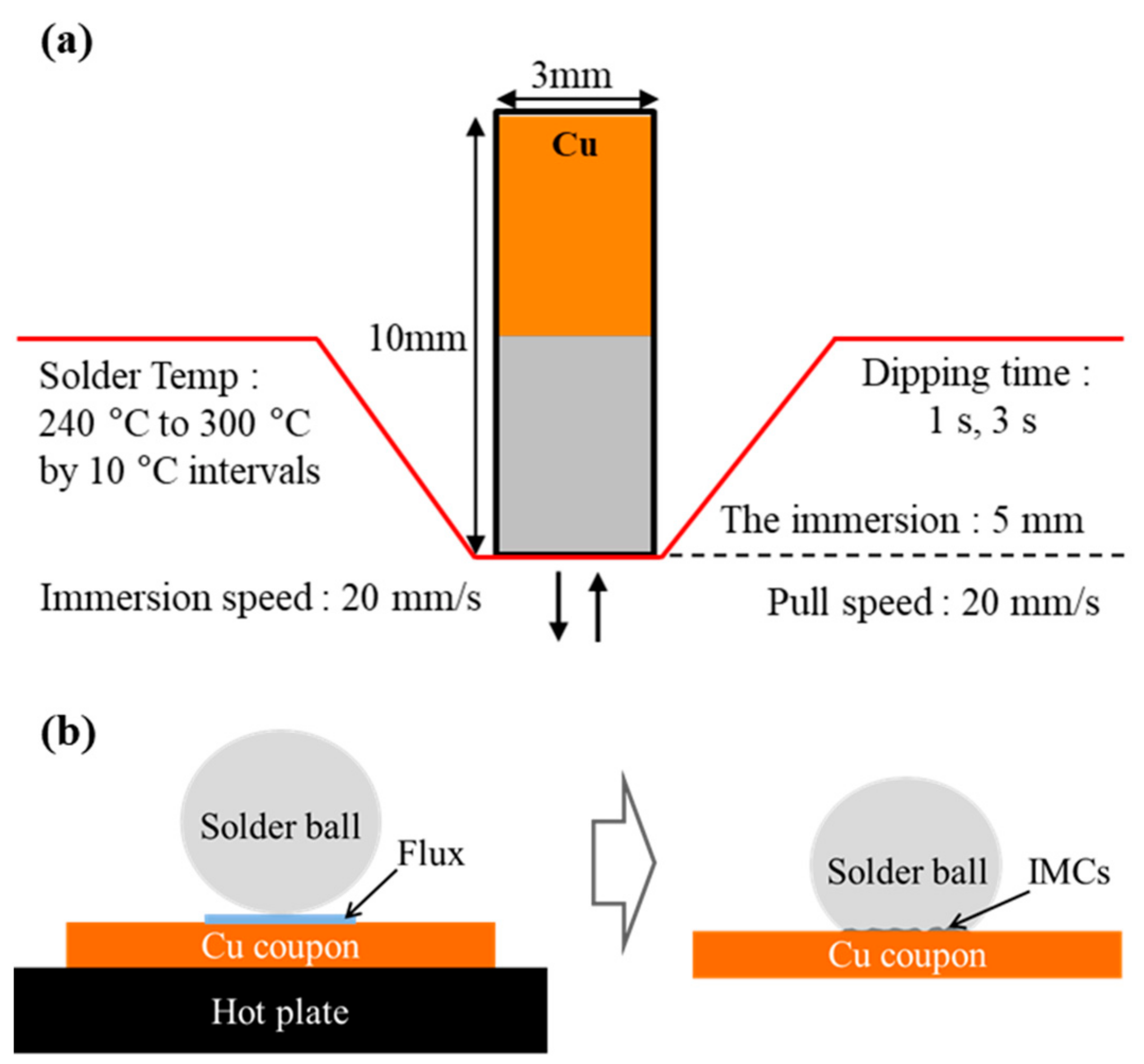
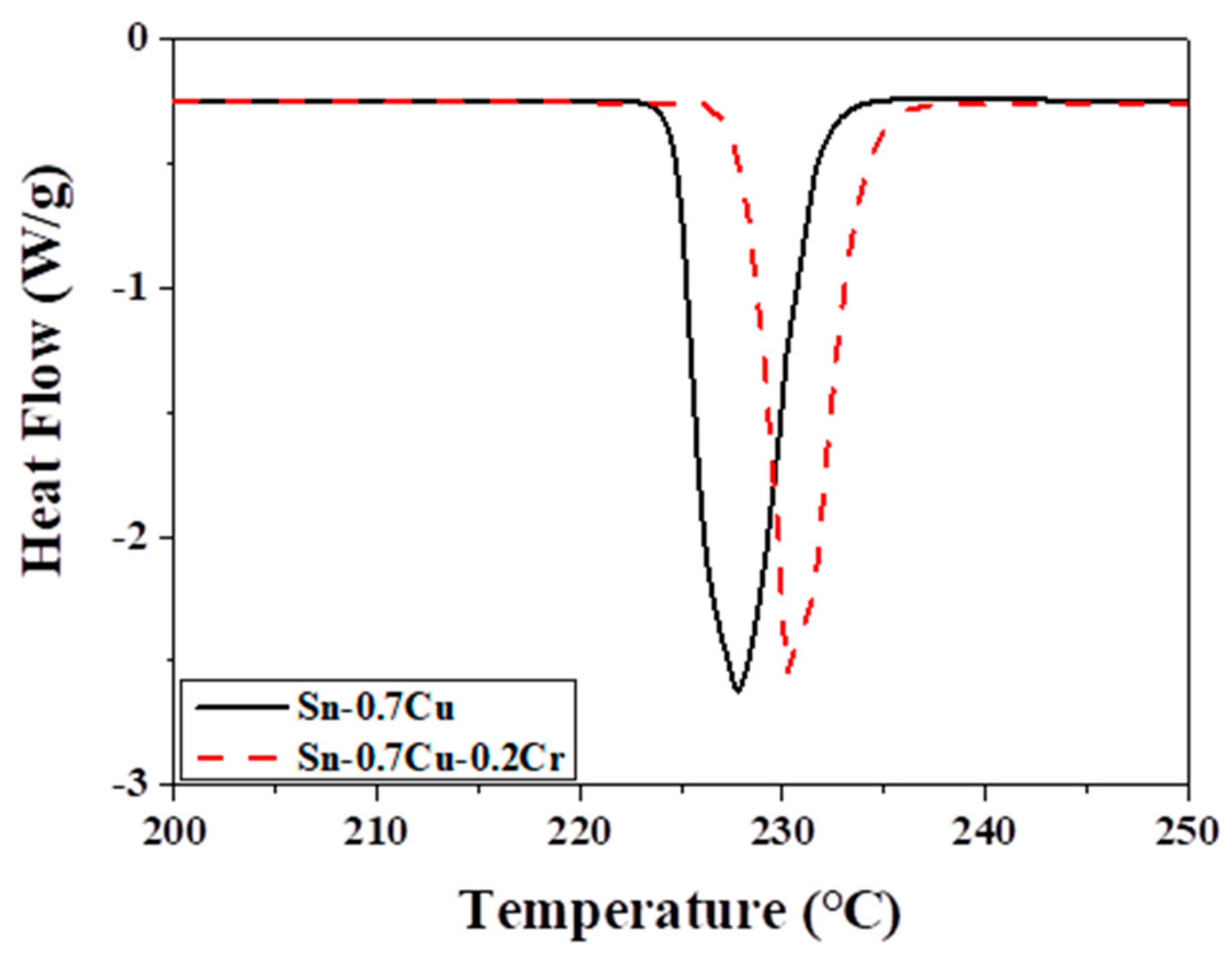
2. Materials and Methods
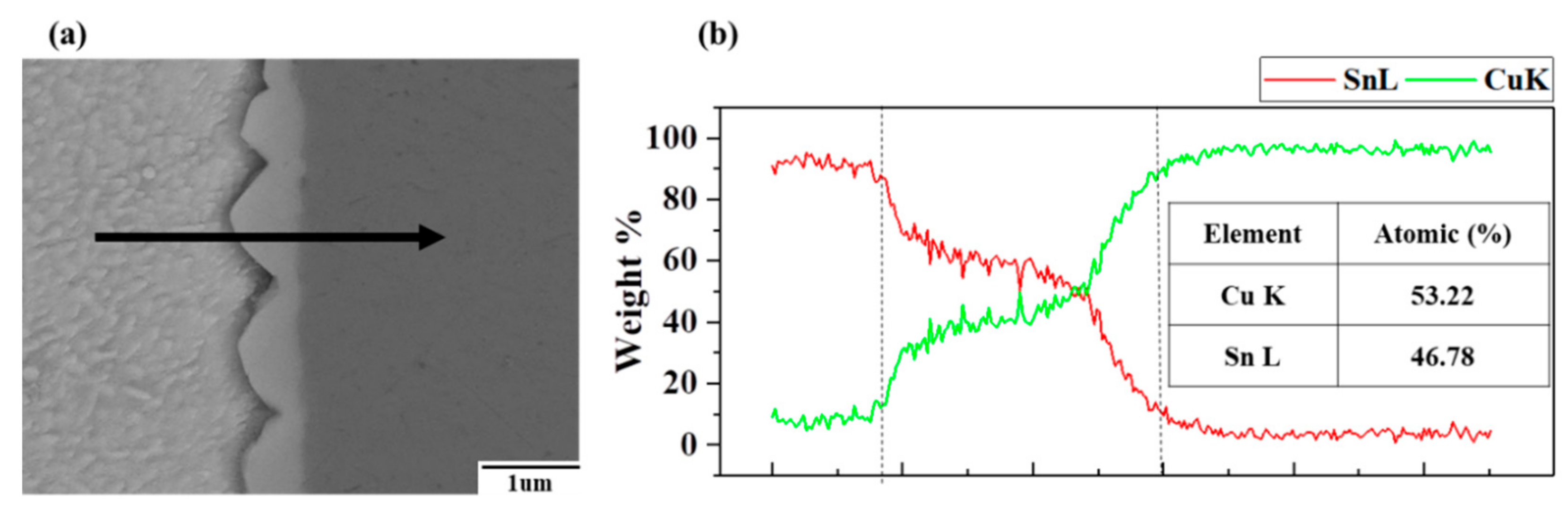
3. Results and Discussion
4. Conclusions
- (1)
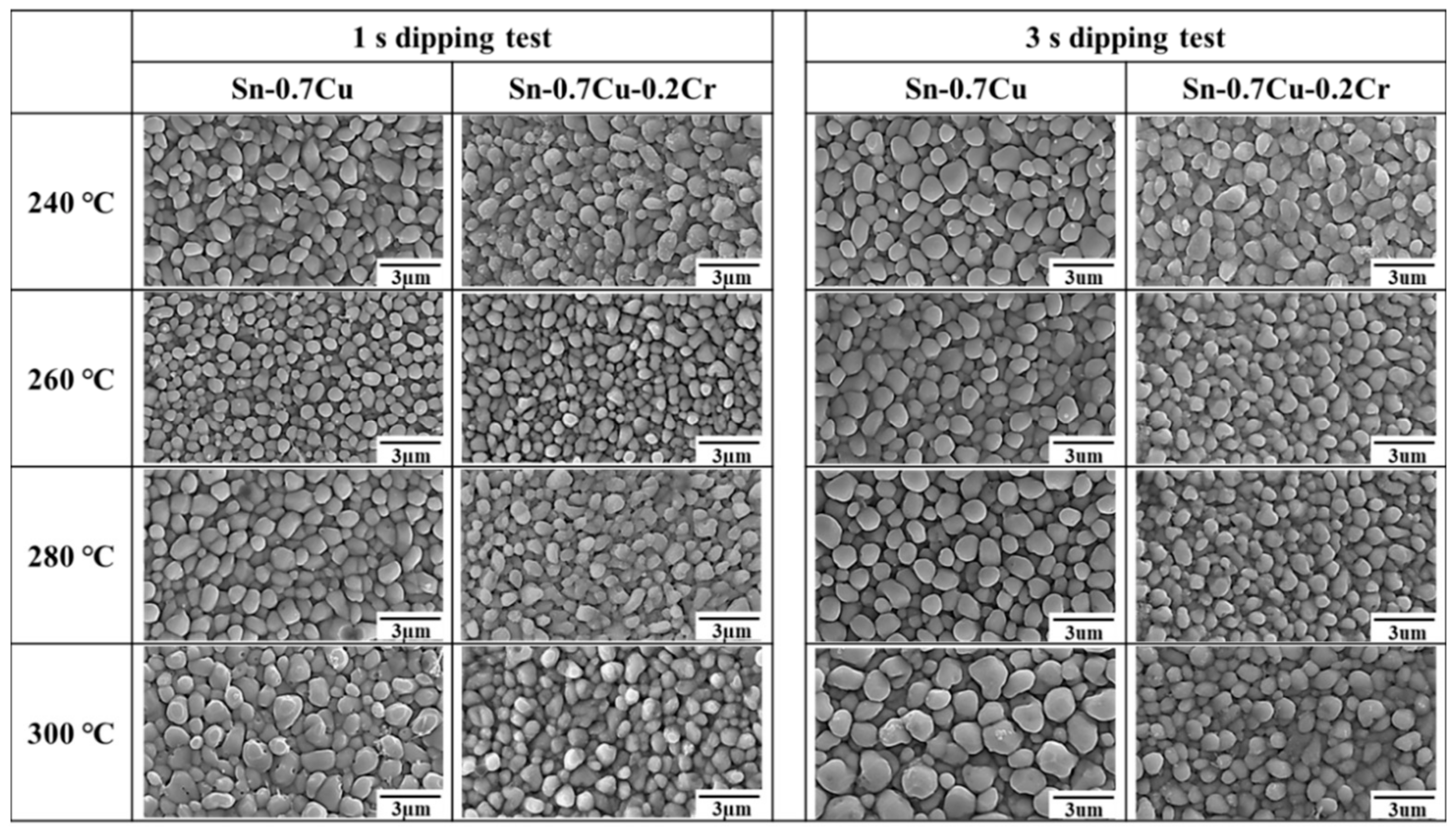
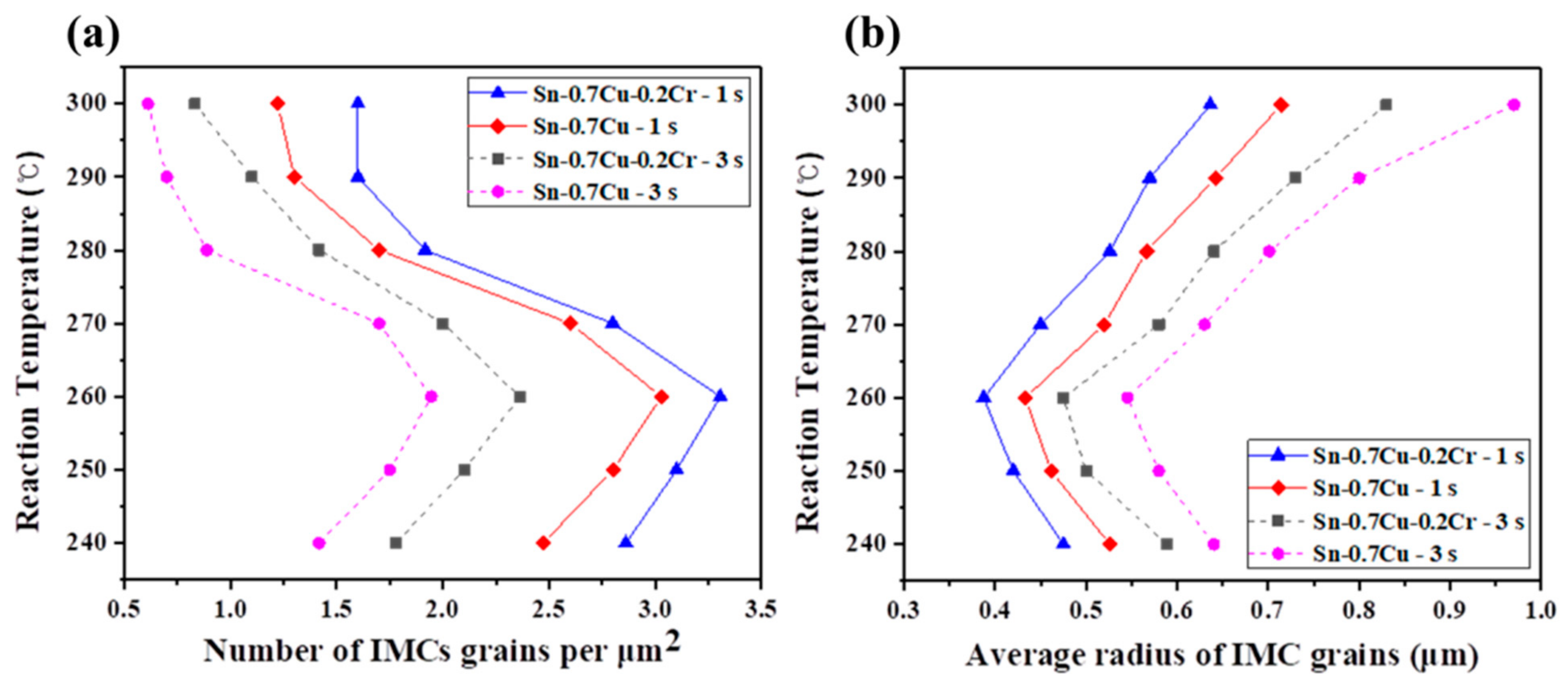
- The samples revealed that the grain size of the Cu6Sn5 IMCs in both solders was minimum at 260 °C. The grain size increased as the temperature increased to 280 and 300 °C. The grain size of the Cu6Sn5 IMCs in the Sn-0.7Cu-0.2Cr solder was lower than that of Cu6Sn5 IMCs in the Sn-0.7Cu solder, at all dipping times and temperatures. The addition of Cr decreased the supercooling degree and promoted nucleation, thereby lowering the particle size.
- (2)
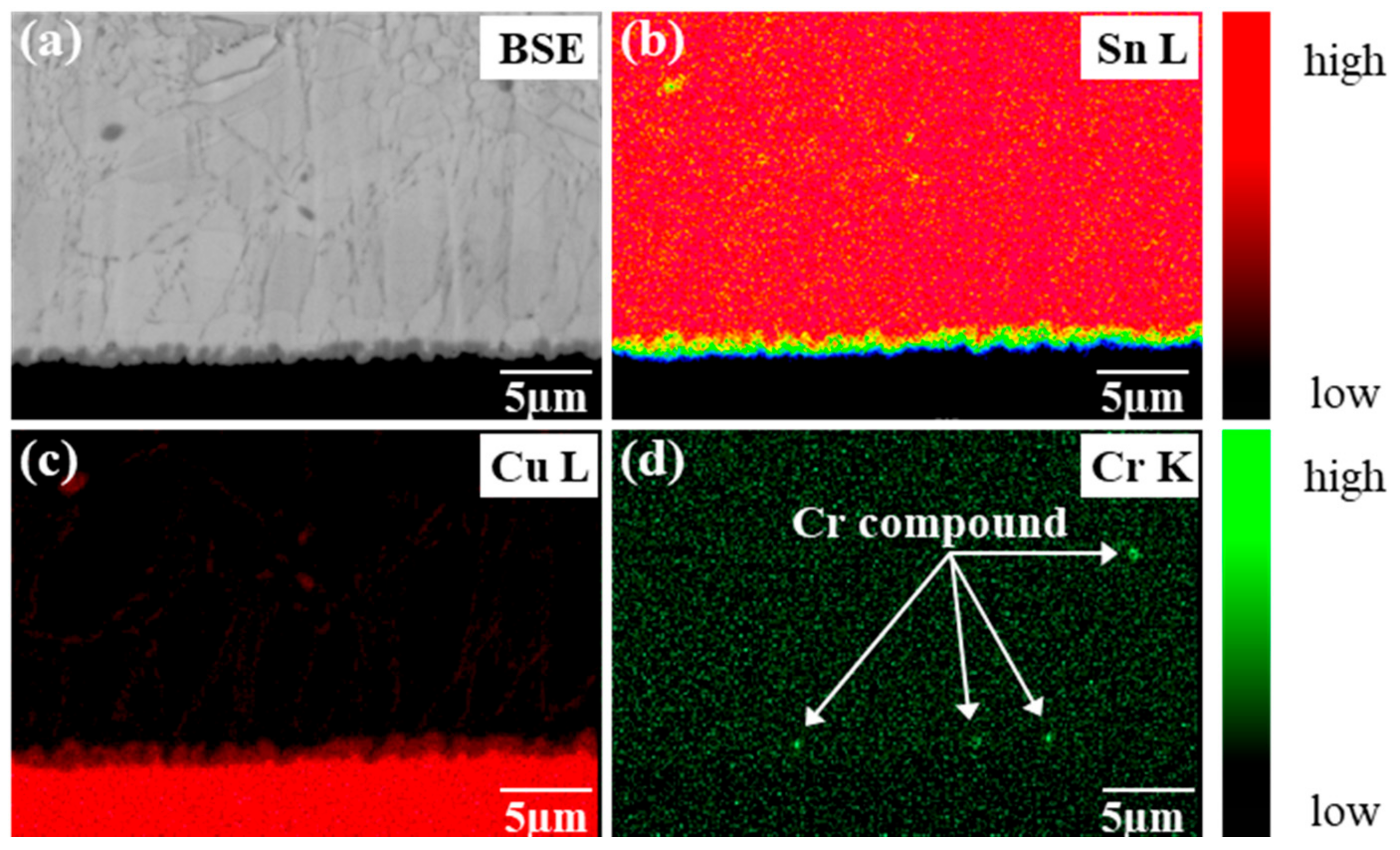
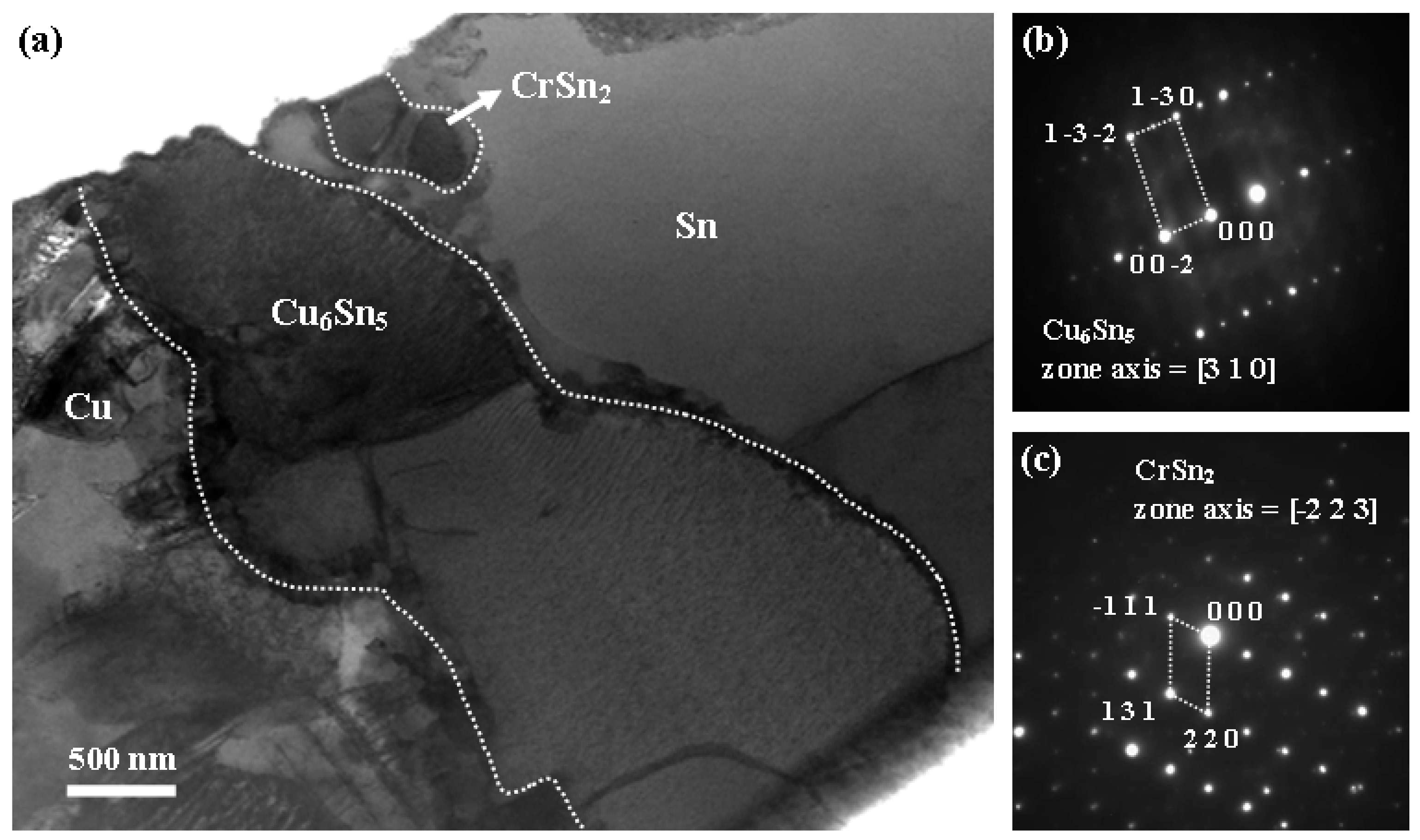
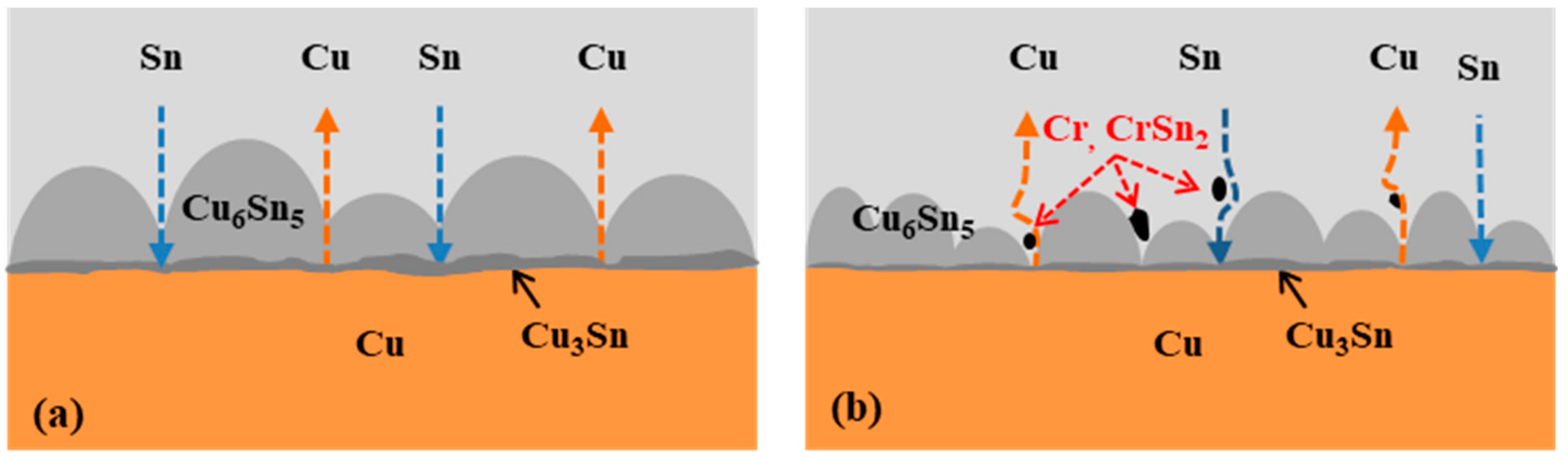
- The Sn-0.7Cu-0.2Cr solder/Cu joints suppressed IMC growth more effectively than the Sn-0.7Cu solder/Cu joints. This suppressed the interfacial IMC growth by interfering with the diffusion of Sn and Cu by CrSn2 IMC present at the interface.
- (3)
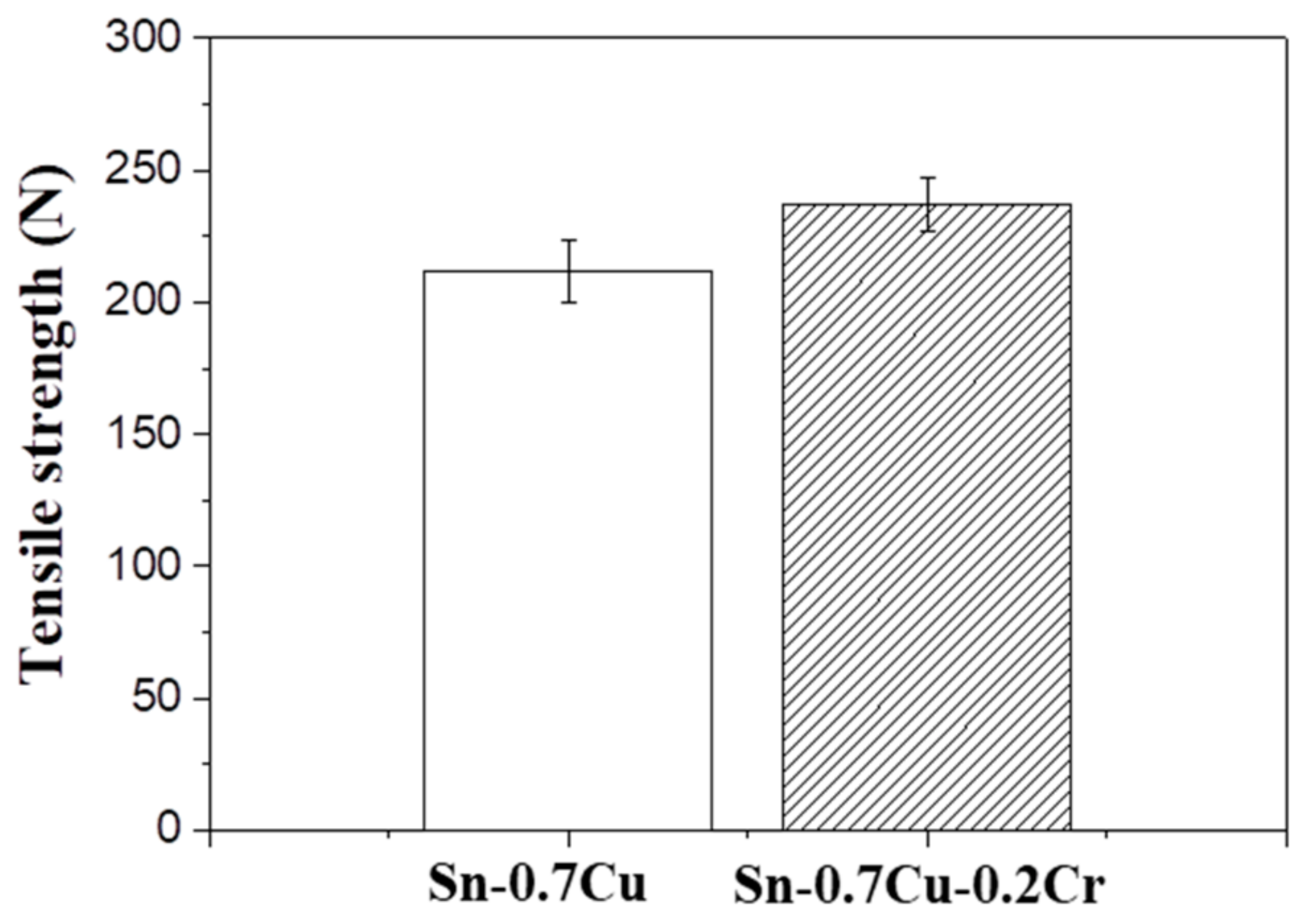
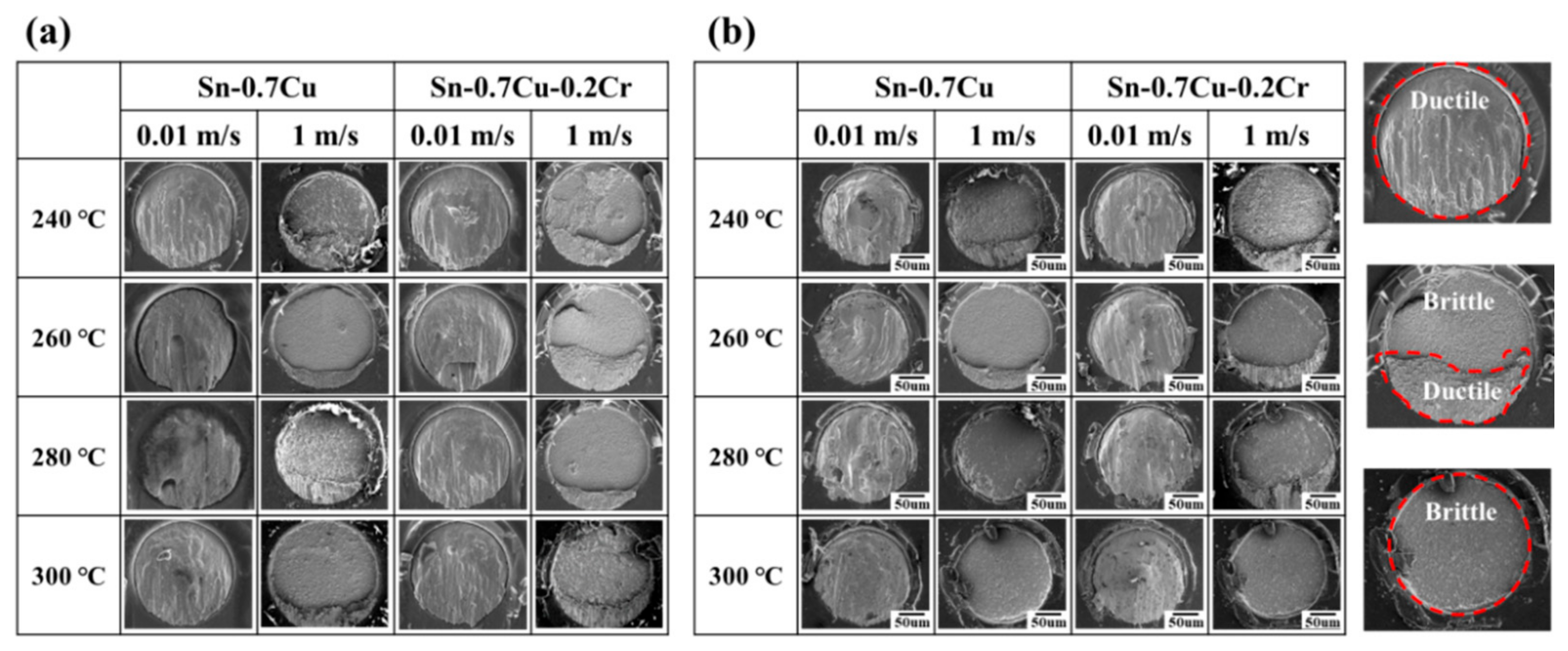
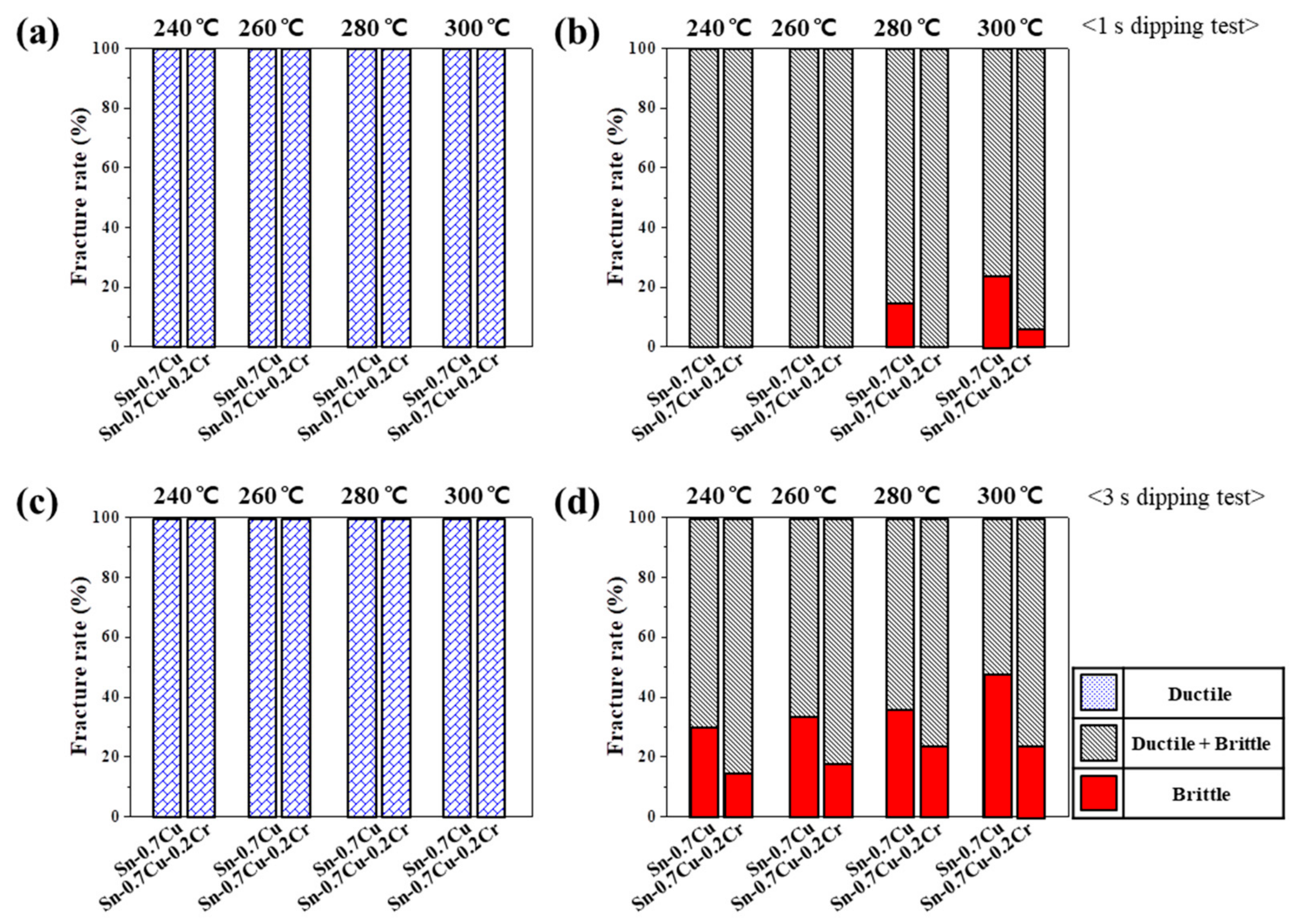
- In the shear test, the fracture occurred inside the solder at low shear rates, and at high shear rates at the solder/IMC interface or IMC/substrate interface. The results of the low shear strength tests revealed that the shear strengths of the Sn-0.7Cu-0.2Cr solder joints were higher than those of the Sn-0.7Cu solder joints. The high shear strength of the Sn-0.7Cu-0.2Cr solder joints was attributed to the shear strength of the CrSn2 compounds inside and near the surface of the solder. In the high-speed shear test, the brittle fracture increased as the thickness of IMC increased.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, S.K. Lead (Pb)-free solders for electronic packaging. J. Electron. Mater. 1994, 23, 701–707. [Google Scholar] [CrossRef]
- Leng, E.P.; Ding, M.; Ling, W.T.; Amin, N.; Ahmad, I.; Lee, M.Y.; Haseeb, A.S.M.A. A study of SnAgNiCo vs Sn3.8AgO.7Cu C5 lead free solder alloy on mechanical strength of BGA solder joint. In Proceedings of the 2008 10th Electronics Packaging Technology Conference, Singapore, 9–12 December 2008; pp. 588–594. [Google Scholar]
- Kim, J.M.; Jeong, M.H.; Yoo, S.; Park, Y.B. Effect of interfacial microstructures on the bonding strength of Sn-3.0Ag-0.5Cu Pb-free solder bump. Jpn. J. Appl. Phys. 2012, 51, 05EE06. [Google Scholar] [CrossRef]
- Park, J.M.; Kim, S.H.; Jeong, M.H.; Park, Y.B. Effect of Cu-Sn intermetallic compound reactions on the Kirkendall void Growth characteristics in Cu/Sn/Cu microbumps. Jpn. J. Appl. Phys. 2014, 53, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Tu, K.N.; Thompson, R.D. Kinetics of interfacial reaction in bimetallic CuSn thin films. Acta Metall. 1982, 30, 947–952. [Google Scholar] [CrossRef]
- Deng, X.; Piotrowski, G.; Williams, J.J.; Chawla, N. Influence of Initial Morphology and Thickness of Cu6Sn5 and Cu3Sn Intermetallics on Growth and Evolution during Thermal Aging of Sn-Ag Solder/Cu Joints. J. Electron. Mater. 2003, 32, 1403–1413. [Google Scholar] [CrossRef] [Green Version]
- Mu, D.; Huang, H.; Nogita, K. Anisotropic mechanical properties of Cu6Sn5 and (Cu,Ni)6Sn5. Mater. Lett. 2012, 86, 46–49. [Google Scholar] [CrossRef]
- Deng, X.; Sidhu, R.S.; Johnson, P.; Chawla, N. Influence of reflow and thermal aging on the shear strength and fracture behavior of Sn-3.5Ag solder/Cu joints. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2005, 36, 55–64. [Google Scholar] [CrossRef]
- Chan, Y.C.; So, A.C.K.; Lai, J.K.L. Growth kinetic studies of Cu – Sn intermetallic compound and its effect on shear strength of LCCC SMT solder joints. Mater. Sci. Eng B 1998, 55, 5–13. [Google Scholar] [CrossRef]
- Limaye, P.; Labie, R.; Vandevelde, B.; Vandepitte, D.; Verlinden, B. Creep behavior of mixed SAC 405/ SnPb soldered assemblies in shear loading. In Proceedings of the 2007 9th Electronics Packaging Technology Conference, Singapore, 10–12 December 2007; pp. 703–712. [Google Scholar]
- Chen, X.; Li, M.; Ren, X.; Mao, D. Effects of alloying elements on the characteristics of Sn-Zn lead-free solder. In Proceedings of the 2005 6th International Conference on Electronic Packaging Technology, Shenzhen, China, 30 August–2 September 2005. [Google Scholar]
- Lin, K.L.; Liu, P.C.; Song, J.M. Wetting interaction between Pb-free Sn-Zn series solders and Cu, Ag substrates. In Proceedings of the 54th Electronic Components and Technology Conference, Las Vegas, NV, USA, 4 June 2004; Volume 2, pp. 1310–1313. [Google Scholar]
- Wang, J.; Wen, L.; Zhou, J.; Chung, M. Mechanical properties and joint reliability improvement of Sn-Bi alloy. In Proceedings of the 2011 IEEE 13th Electronics Packaging Technology Conference, Singapore, 7–9 December 2011; Volume 8823, pp. 492–496. [Google Scholar]
- Cho, M.G.; Kang, S.K.; Shih, D.Y.; Lee, H.M. Effects of minor additions of Zn on interfacial reactions of Sn-Ag-Cu and Sn-Cu solders with various Cu substrates during thermal aging. J. Electron. Mater. 2007, 36, 1501–1509. [Google Scholar] [CrossRef]
- Hu, F.Q.; Zhang, Q.K.; Jiang, J.J.; Song, Z.L. Influences of Ag addition to Sn-58Bi solder on SnBi/Cu interfacial reaction. Mater. Lett. 2018, 214, 142–145. [Google Scholar] [CrossRef]
- Li, J.Y.; Peng, J.; Wang, R.C.; Feng, Y.; Peng, C.Q. Effects of Co addition on shear strength and interfacial microstructure of Sn–Zn–(Co)/Ni joints. J. Mater. Sci. Mater. Electron. 2018, 29, 19901–19908. [Google Scholar] [CrossRef]
- Bang, J.; Yu, D.Y.; Ko, Y.H.; Son, J.H.; Nishikawa, H.; Lee, C.W. Intermetallic compound growth between Sn-Cu-Cr lead-free solder and Cu substrate. Microelectron. Reliab. 2019, 99, 62–73. [Google Scholar] [CrossRef]
- Santra, S.; Davies, T.; Matthews, G.; Liu, J.; Grovenor, C.R.M.; Speller, S.C. The effect of the size of NbTi filaments on interfacial reactions and the properties of InSn-based superconducting solder joints. Mater. Des. 2019, 176, 107836. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Jiang, X.; Li, Y. Effect of Ni addition to the Cu substrate on the interfacial reaction and IMC growth with Sn3.0Ag0.5Cu solder. Appl. Phys. A Mater. Sci. Process. 2018, 124, 315. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, M.; Wu, Y.; Du, J.; Lin, T.; He, P.; Huang, M.; Paik, K.W. A Study on the Optimization of Anisotropic Conductive Films for Sn-3Ag-0.5Cu-Based Flex-on-Board Application at a 250 °C Bonding Temperature. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 383–391. [Google Scholar] [CrossRef]
- Nogita, K.; Gourlay, C.M.; Nishimura, T. Cracking and phase stability in reaction layers between Sn-Cu-Ni solders and Cu substrates. Jom 2009, 61, 45–51. [Google Scholar] [CrossRef]
- Chen, S.W.; Wu, S.H.; Lee, S.W. Interfacial reactions in the Sn-(Cu)/Ni, Sn-(Ni)/Cu, and Sn/(Cu,Ni) systems. J. Electron. Mater. 2003, 32, 1188–1194. [Google Scholar] [CrossRef]
- Chen, S.W.; Chen, C.M.; Liu, W.C. Electric current effects upon the Sn\Cu and Sn\Ni interfacial reactions. J. Electron. Mater. 1998, 27, 1193–1198. [Google Scholar] [CrossRef]
- Park, M.S.; Arroyave, R. Formation and growth of intermetallic compound Cu6Sn5 at early stages in lead-free soldering. J. Electron. Mater. 2010, 39, 2574–2582. [Google Scholar] [CrossRef] [Green Version]
- Kotadia, H.R.; Mokhtari, O.; Clode, M.P.; Green, M.A.; Mannan, S.H. Intermetallic compound growth suppression at high temperature in SAC solders with Zn addition on Cu and Ni-P substrates. J. Alloy. Compd. 2012, 511, 176–188. [Google Scholar] [CrossRef]
- Yang, M.; Cao, Y.; Joo, S.; Chen, H.; Ma, X.; Li, M. Cu6Sn5 precipitation during Sn-based solder/Cu joint solidification and its effects on the growth of interfacial intermetallic compounds. J. Alloy. Compd. 2014, 582, 688–695. [Google Scholar] [CrossRef]
- Gagliano, R.A.; Ghosh, G.; Fine, M.E. Nucleation kinetics of Cu6Sn5 by reaction of molten tin with a copper substrate. J. Electron. Mater. 2002, 31, 1195–1202. [Google Scholar] [CrossRef]
- Wölpl, T.; Jeitschko, W. Crystal structures of VSn2, NbSn2 and CrSn2 with Mg2Cu-type structure and NbSnSb with CuAl2-type structure. J. Alloy. Compd. 1994, 210, 185–190. [Google Scholar] [CrossRef]
- Larsson, A.K.; Lidin, S. The crystal structures of the closely related CrSn2, NiCr5Sn12 and NiCr3Sn8 phases. J. Alloy. Compd. 1995, 221, 136–142. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, D.G.; Jung, S.B. Mechanical strength test method for solder ball joint in BGA package. Met. Mater. Int. 2005, 11, 121–129. [Google Scholar] [CrossRef]
- Kim, J.W.; Jang, J.K.; Ha, S.O.; Ha, S.S.; Kim, D.G.; Jung, S.B. Effect of high-speed loading conditions on the fracture mode of the BGA solder joint. Microelectron. Reliab. 2008, 48, 1882–1889. [Google Scholar] [CrossRef]
- Hu, X.; Xu, T.; Keer, L.M.; Li, Y.; Jiang, X. Shear strength and fracture behavior of reflowed Sn3.0Ag0.5Cu/Cu solder joints under various strain rates. J. Alloy. Compd. 2017, 690, 720–729. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Harsh service environment effects on the microstructure and mechanical properties of Sn–Ag–Cu-1 wt% nano-Al solder alloy. J. Mater. Sci. Mater. Electron. 2016, 27, 11273–11283. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, K.; Shi, B.; Wang, H.; Zhao, D.; Sun, M.; Zhang, C. The interface microstructure and shear strength of Sn2.5Ag0.7Cu0.1RExNi/Cu solder joints under thermal-cycle loading. Metals 2019, 9, 518. [Google Scholar] [CrossRef] [Green Version]
- Qiu, H.; Hu, X.; Li, S.; Wan, Y.; Li, Q. Shear strength and fracture surface analysis of lead-free solder joints with high fraction of IMCs. Vacuum 2020, 180, 109611. [Google Scholar] [CrossRef]
- Yoon, J.W.; Noh, B.I.; Lee, Y.H.; Lee, H.S.; Jung, S.B. Effects of isothermal aging and temperature-humidity treatment of substrate on joint reliability of Sn-3.0Ag-0.5Cu/OSP-finished Cu CSP solder joint. Microelectron. Reliab. 2008, 48, 1864–1874. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, J.; Yu, D.-Y.; Kim, M.-S.; Ko, Y.-H.; Byun, D.-J.; Bang, J. Nucleation and Morphology of Cu6Sn5 Intermetallic at the Interface between Molten Sn-0.7Cu-0.2Cr Solder and Cu Substrate. Metals 2021, 11, 210. https://doi.org/10.3390/met11020210
Son J, Yu D-Y, Kim M-S, Ko Y-H, Byun D-J, Bang J. Nucleation and Morphology of Cu6Sn5 Intermetallic at the Interface between Molten Sn-0.7Cu-0.2Cr Solder and Cu Substrate. Metals. 2021; 11(2):210. https://doi.org/10.3390/met11020210
Chicago/Turabian StyleSon, Junhyuk, Dong-Yurl Yu, Min-Su Kim, Yong-Ho Ko, Dong-Jin Byun, and Junghwan Bang. 2021. "Nucleation and Morphology of Cu6Sn5 Intermetallic at the Interface between Molten Sn-0.7Cu-0.2Cr Solder and Cu Substrate" Metals 11, no. 2: 210. https://doi.org/10.3390/met11020210





