The formation of emission particles in the combustion chamber of a rotary tilting furnace under OEC conditions can be illustrated using simulation modeling with CFD simulations. Prieler et al. [
30] described skeletal reaction mechanisms for the CFD model of the OEC system which they used. Bhuiyan et al. [
31] modeled the impact of oxy/fuel combustion on radiative and convective heat transfer in a furnace. Cravero et al. [
32] used a CFD model for designing and developing innovative configurations in regenerative furnaces. Gómez et al. [
33] analyzed the formation of emissions at different oxygen concentrations in a boiler using a CFD model. Nieckele et al. [
34] described CFD methodology for simulation modeling of an industrial aluminum melting furnace. Zhou et al. [
35] performed numerical modeling of aluminum scrap processing in a rotary furnace. Khoei et al. [
36] also modeled aluminum recycling processes in a rotary furnace. Rimar et al. [
37] developed a simulation model of combustion for a heating furnace.
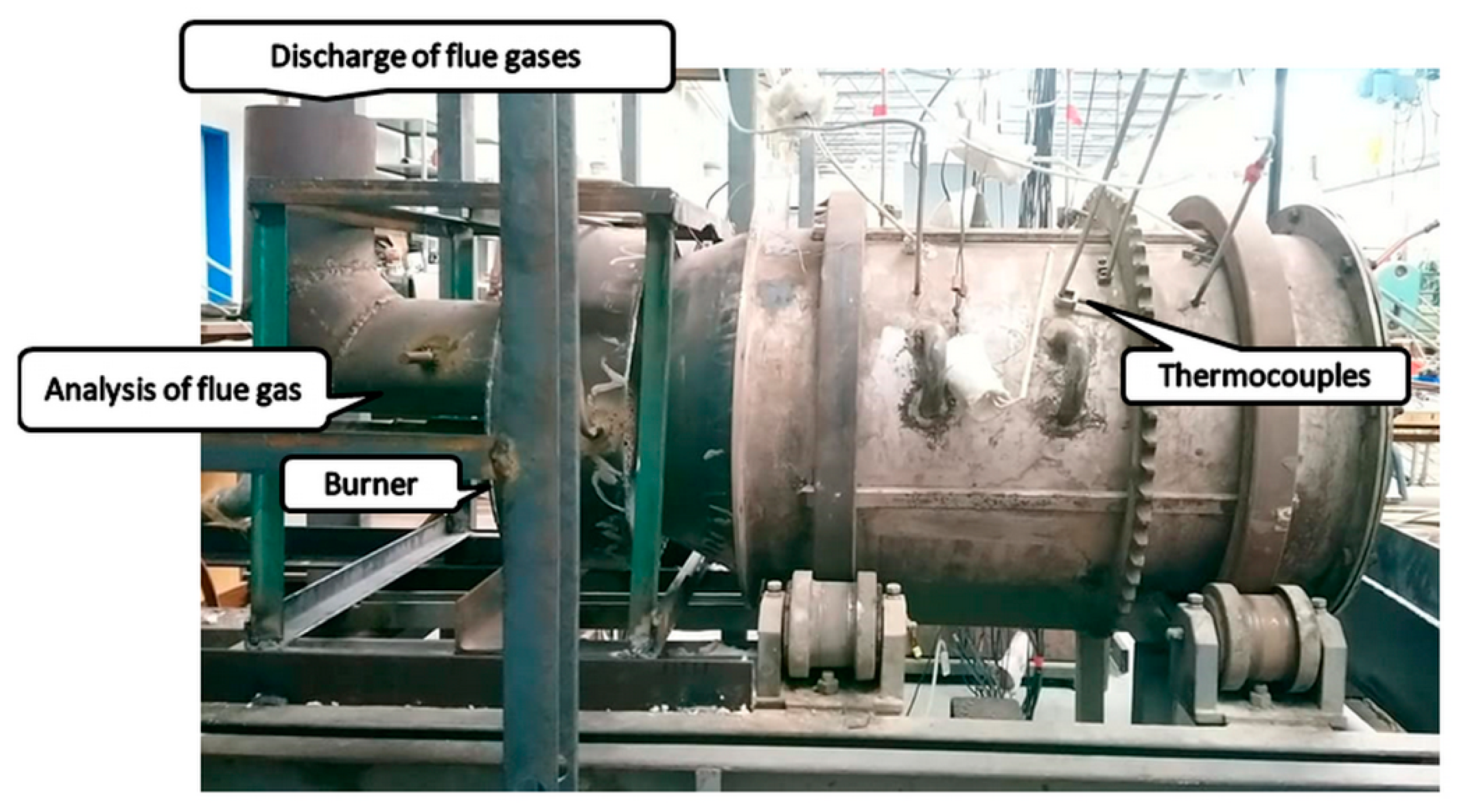
The simulation model of the laboratory-scale furnace shown in
Figure 11 was developed in Ansys software v19.2 (Ansys, Canonsburg, PA, USA). Modeling of the interaction of turbulence and chemistry was performed based on non-premixed combustion. In this type of combustion, the fuel and the oxidizer are not brought into contact until they are introduced into the reaction zone. The combustion process was described in terms of the Westbrook and Dryer (WD) global reaction mechanism, and the formation of NO
x emissions was calculated using the PDF transport equation. These reaction mechanisms are described in detail in paper [
4]. The chemical composition of natural gas, which was used in our laboratory measurement, consists of 95% methane. Our simulation model was therefore simplified, and the fuel was assumed to consist of 100% methane. Five-step methane/air WD global reaction mechanisms were used for describing the combustion process. This process was defined using a combination of finite rate chemistry and eddy dissipation models. The utilized methane/air WD global reaction mechanisms are defined in
Appendix B.
DesignModeler software was used to create the simulation model shown in
Figure 11. The model was divided into three domains. The main domain represents the fluid dynamics of the combustion chamber. It includes the above-mentioned model of interaction between turbulence and chemistry and boundary conditions for Inlet Fuel, Inlet Oxidizer, Outlet, and Opening. The boundary condition “Outlet” specifies the pressure condition of the stack, which in our case was defined as negative pressure created by the exhaust fan. On the basis of experimental measurement, the pressure condition of the chimney was set as −5 Pa. The boundary condition “Opening” defines the area through which outside air penetrated into the combustion chamber, where the specified pressure was equal to atmospheric, and the air composition was 21% vol. O
2 and 79% vol. N
2.
The second and third domains represent the charge material and the furnace wall respectively. The boundary condition between individual domains was defined as “Interface”, where the heat transfer module was turned on. Turbulence in the simulation model was described with model k-ε realizable, and radiative heat transfer was described with model P1 (combustion chamber) and Monte Carlo (Charge material, Furnace Wall).
Figure 11 provides a visualization of the mesh of the CFD model. The total number of cells was 5050326. Further increase in mesh fineness did not yield any further change in simulation outputs. This model was created from tetrahedral, hexahedral and wedge cells. Meshing in the vicinity of the furnace walls and charge material was supported with the inflation function and the inflation option “Smooth transition”, a maximum of five layers and growth rate of 1.2. The worst value of
y+ was 12, observed in the stack area, while in other areas it ranged from 0 to 1.5. In order to simplify the simulation model it was assumed that the charge material would not melt. The heating of the charge material was defined as the specific heat capacity of aluminum (903 J·kg
−1·K
−1). The melting model would require transient simulation, which is time-consuming due to the increased complexity of the CFD model.
Results of Simulation Model
The above-mentioned setting of the simulation model allowed monitoring of mass concentration of emission component CO
2, CO, H
2, NO, and CH
4, depending on the change in oxygen enrichment concentration in the oxidizer. Distribution of the temperature field and velocity in the combustion chamber could also be monitored. Modeled results of CFD simulations were created for both burner types (see
Section 2). The results presented in
Figure 12,
Figure 13,
Figure 14 and
Figure 15 are modeled for 35% oxygen concentration in the oxidizer. Verification of the simulation model was carried out for all modeled concentrations and burner types in comparison with the results from experimental measurement.
Figure 12 presents a comparison of the distribution of temperature contours in the combustion chamber based on the change of burner type. The results of the simulation model confirm that the change in the diameter of the burner outlet orifice had a significant effect on the combustion process. When using OEC technology on the air/fuel burner, combustion takes place near the mouth of the burner, caused by high combustion flame speed and slow flow speed of the combustion mixture away from the burner. By optimizing the burner, the flame moves closer to the charge material. Results of the simulation modeling confirm that the flame temperature is lower in the case of the modified burner, which signals higher heat flux to the charge material. The achieved temperatures from the simulation model confirm the results of the experimental model presented in
Figure 6. Comparison results from CFD modeling with experimental results are presented in
Appendix A.
The model results shown in
Figure 13 quantify the formation of CO emissions. Due to the small difference between achieved mass concentrations of CO based on the type of burner, the contour range had to be rescaled from 0 to 0.0015 kg m
−3, which represents around 165 ppm CO. From the results of the simulation, it is evident that the area of CO formation is located specifically in the area of the flame. In the case of the air/fuel burner, a small part of the produced emissions is drawn into the stack by the exhaust fan. In the case of the optimized burner, the concentration of CO emissions drawn off into the stack is significantly lower.
The difference between temperatures achieved in the combustion chamber as presented in
Figure 12 has a significant impact on the formation of NO emissions.
Figure 14 presents the distribution of NO mass concentration contours. Improved heat transfer to the charge material due to a longer flame leads to more uniform distribution of the temperature field, and lower temperatures are achieved in the combustion chamber. This in turn reduces the formation of NO emissions. The results of our experimental measurement and simulation modeling confirm that optimization of the burner is a suitable way of decreasing the formation of NO emissions.
Circulation of flue gas in the combustion chamber is typical for the construction of a rotary tilting furnace. CFD modeling is a useful tool for predicting the zone of flue-gas circulation.
Figure 15 and
Figure 16 show the distribution of velocity fields in the combustion chamber depicted with vectors. In the case of an air/fuel burner, the circulation of flue gases takes place in the rear part of the combustion chamber. The density of flue gases in the rear part is higher due to the lower flue-gas temperature, causing fresh flue gas with higher temperature and with lower flue-gas density to be predominantly pushed upwards. This effect means that part of the thermal energy of the flue gas is not used to heat the charge material, but causes an increase in the production of NO emissions. In the case of a modified burner, the kinetic energy of the flue gas is higher and the above-mentioned problem with the circulation of flue gas does not exist.
Flue-gas velocity contours presented in
Figure 15 indicate that the flame and the produced fresh hot flue gas are partly repelled from the vicinity of the charge and diverted to the bulk of the furnace and partly to the stack. As a result, flue-gas flow velocity over the charge is lower than in the modified burner design, and a dead zone with a flue-gas vortex is formed in the rear part of the furnace. Modified burner design as documented in
Figure 16 results in a flame orientated directly to the charge with the highest temperatures as well as the highest flue-gas flow velocities being reached in the vicinity of the charge. Although a flue-gas vortex forms in the furnace with modified burner design as well, in contrast to the basic burner design it does not disturb the flue-gas flow patterns above the charge. Thus, heat exchange both by convection and radiation is enhanced with the modified burner, charge melting time is decreased and flue-gas temperature to the stack is lowered.
The data presented in
Figure 17 verify the CFD simulation model as its results are comparable with those from experimental measurement. Values used to construct this figure are listed in
Appendix C. Thus, the method used for simulation model creation in Ansys can be considered as correct. The results obtained are relevant and can be used to predict the distribution of temperature in the combustion chamber and also the formation of emission components depending on the type of burner.
Table 5 presents an overview of the emissions produced per melting cycle of charge material. The charge material was aluminum ingots. The time required to remelt the charge material was defined by the condition that the average temperature of the charge was 750 °C. The CO
2, CO, and NO values shown in
Table 5 were calculated based on Equation (2) from the results of the experimental measurements and fuel consumption presented in
Table 2.
The impact of the produced emission gases on the environment is different for each gas. The Global Warming Potential (GWP) concept was designed to standardize the impact of individual gases on the environment [
40,
41]. GWP is the heat absorbed by any greenhouse gas in the atmosphere, as a multiple of the heat which would be absorbed by the same mass of carbon dioxide (CO
2). GWP is 1 for CO
2. For other gases it depends on the particular gas. GWP for CO is 10 [
40], which means that 1 g CO is equal to 10 g CO
2. GWP for NO is 33 [
41]. CO and NO produced as emission gases were recalculated using the GWP constant, and the results are included in
Table 5 in the form of carbon dioxide equivalents (CO
2 eq).
The results presented in
Table 5 enable estimation of optimal furnace operation regarding both fuel consumption and environmental impact. Evaluating the fuel consumption alone (reflected in the amount of CO
2 emissions produced) could lead to the misleading conclusion that “the higher oxygen enrichment rate the better”. However, including the impact of produced CO and NO emissions minimizes the incremental environmental benefit of oxygen enrichment rates over 35% vol., regardless of whether OEC technology is applied solely or in combination with a modified burner. Thus, it can be concluded that oxygen enrichment up to 35% vol. suffices in any attempt to approach near-optimal furnace operation, leading to its almost lowest combined energy use and environmental impact. The combination of OEC technology and burner modification allows for a reduction of fuel consumption by around 10% and total CO
2 eq. emissions produced by around 15%, compared to OEC technology solely at around 35% vol. oxygen enrichment. The contribution of NO and CO emissions to the total GHG amount produced, expressed as CO
2 equivalents, reaches around 5% for OEC technology solely and just 1% in the case of OEC technology combined with burner modification. This highlights the environmental performance superiority of the modified burner over the standard burner in OEC applications in rotary furnaces for aluminum melting.