Performance Analysis of Electrochemical Micro Machining of Titanium (Ti-6Al-4V) Alloy under Different Electrolytes Concentrations
Abstract
:1. Introduction
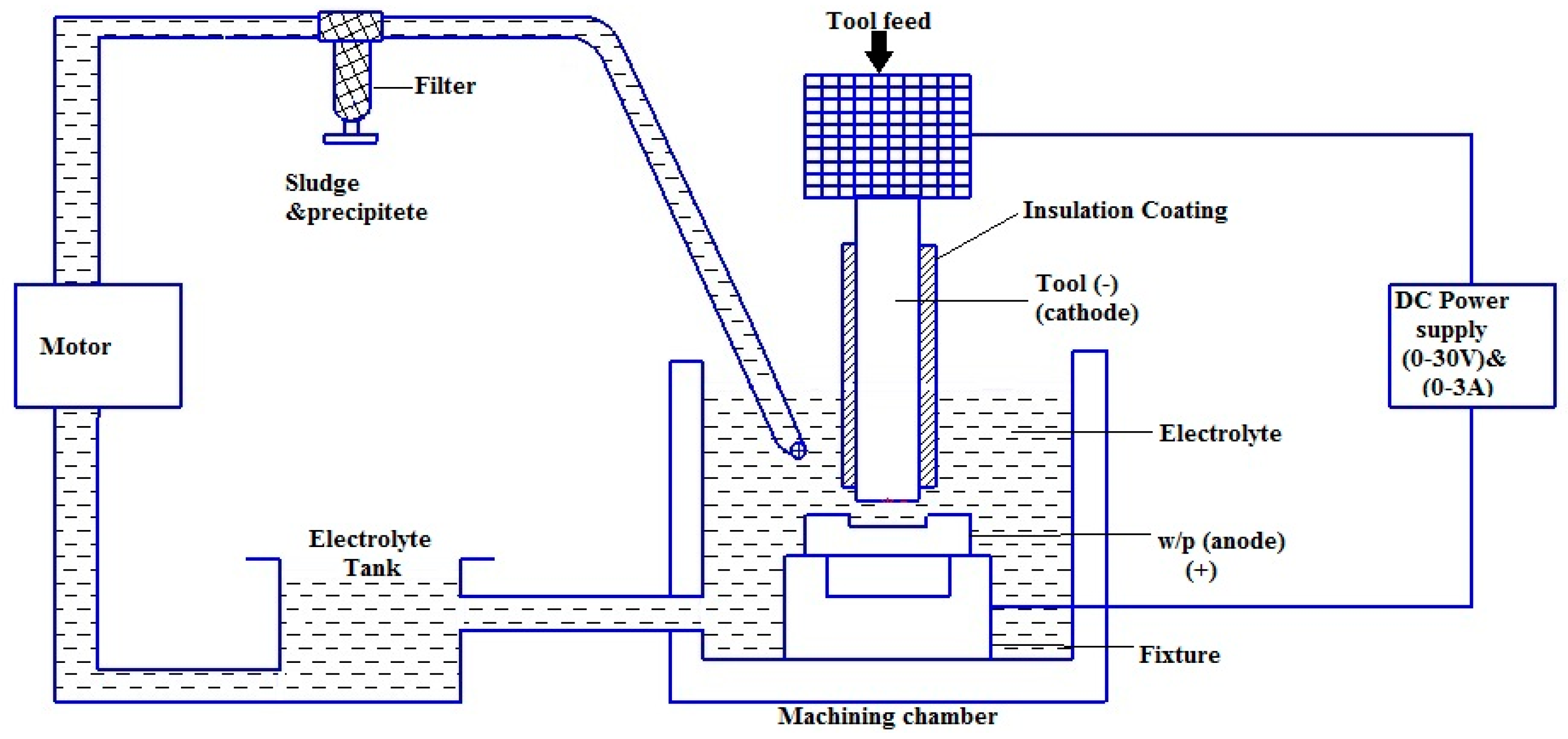
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Electrolytes Andprocess Parameters on MRR
3.2. Effect of Electrolytes Andprocess Parameters on Overcut
3.3. Effect of Electrolytes Andprocess Parameters on Conicity
3.4. Effect of Electrolytes and Process Parameters on Circularity at Entry and Exit
3.5. SEM Analysis of Machined Surface
4. Conclusions
- NaCl + NaNO3 combined electrolyte can provide 56% and 24% higher MRR than NaCl + Glycerol and NaCl + Citric acid, respectively, due to the dominance of the Sodium ions present in the electrolyte since it possesses higher reactive nature;
- NaCl + Glycerol mixed electrolyte creates 40% and 22% lower Overcut than electrolyte combinations NaCl + NaNO3 and NaCl + Citric acid, respectively, due to the presence of glycerol, which acts as a good chelating agent by increasing the bonding of ions;
- NaCl + Citric Acid can improve reduce the conicity by 70% and 39% compared to that of NaCl + NaNO3 and NaCl + Glycerol due to the presence of citric acid, which reduces the smutting around the machining surface;
- From the SEM analysis, it has been inferred that the NaCl + C3H8O3 electrolyte produces better micro hole over the workpiece surface compared to other electrolytes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suresh, H.S.; Sudhir, G.B.; Dayanand, S.B. Analysis of electrochemical machining process parameters affecting material removal rate of hastelloy C-276. Int. J. Adv. Res. Sci. Eng. Technol. 2014, 5, 18–23. [Google Scholar]
- Haisch, T.; Mittemeijer, E.; Schultze, J.W. Electrochemical machining of the steel 100Cr6 in aqueous NaCl and NaNO3 solutions: Microstructure of surface films formedby carbides. Electrochim. Acta 2001, 47, 235–241. [Google Scholar] [CrossRef]
- Huo, J.; Liu, S.; Wang, Y.; Muthuramalingam, T.; Pi, V.N. A Influence of process factors on surface measures on electrical discharge machined stainless steel using TOPSIS. Mater. Res. Express 2019, 6, 086507. [Google Scholar] [CrossRef]
- Rajurkar, K.P.; Sundaram, M.M.; Malshe, A.P. Review of electrochemical and electro discharge machining. Procedia CIRP 2013, 6, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Chiou, Y.C.; Lee, R.T.; Chen, T.J.; Chiou, J.M. Fabrication of high aspect ratio micro-rod using a novel electrochemical micromachining method. Precis. Eng. 2012, 36, 193–202. [Google Scholar] [CrossRef]
- Mileham, A.R.; Jones, R.M.; Harvey, S.J. Changes of valency state during Electrochemical Machining Production Points. Precis. Eng. 1982, 4, 168–170. [Google Scholar] [CrossRef]
- Sen, M.; Shan, H.S. A review of electrochemical macro-to micro-hole drilling processes. Int. J. Mach. Tool. Manuf. 2005, 45, 137–152. [Google Scholar] [CrossRef]
- Rajurkar, K.P.; Zhu, D.; Wei, B. Minimization of machining allowance in electrochemical machining. Ann. CIRP 1998, 47, 165–168. [Google Scholar]
- Thanigaivelan, R.; Arunachalam, R.M.; Karthikeyan, B.; Loganathan, P. Electrochemical micromachining of stainless steel with acidified sodium nitrate electrolyte. Procedia CIRP 2013, 6, 351–355. [Google Scholar] [CrossRef] [Green Version]
- De-Silva, A.K.M.; Altena, H.S.J.; McGeough, J.A. Influence of electrolyte concentration on copying accuracy of precision-ECM. CIRP Ann. Manuf. Technol. 2013, 52, 165–168. [Google Scholar] [CrossRef]
- Ayyappan, S.; Sivakumar, K. Experimental investigation on theperformance improvement of electrochemical machining process using oxygen-enriched electrolyte. Int. J. Adv. Manuf. Technol. 2014, 75, 479–487. [Google Scholar] [CrossRef]
- Tang, L.; Yang, S. Experimental investigation on the electrochemicalmachining of 00Cr12Ni9Mo4Cu2 material and multi-objective parameters optimization. Int. J. Adv. Manuf. Technol. 2013, 67, 2909–2916. [Google Scholar] [CrossRef]
- Kirchner, V.; Cagnon, L.; Schuster, R.; Etrl, G. Electrochemical machining of stainless steel microelements with ultrashort voltage pulses. Appl. Phys. Lett. 2008, 79, 1721–1723. [Google Scholar] [CrossRef] [Green Version]
- Huaiqian, B.; Jiawen, X.; Ying, L. Aviation-oriented Micromachining Technology Micro-ECM in pure Water. Chin. J. Aeronaut. 2008, 218, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, N.; Ito, Y.; Natsu, W. Electrochemical Machining of Tungsten Carbide Alloy Micro-pin with NaNO3 Solution. Int. J. Precis. Eng. Manuf. 2012, 13, 2075–2078. [Google Scholar] [CrossRef]
- Spieser, A.; Ivanov, A. Recent developments and research challenges in electrochemical micromachining (μECM). Int. J. Adv. Manuf. Technol. 2013, 69, 563–581. [Google Scholar] [CrossRef]
- Rajurkar, K.P.; Zhu, D.; McGeough, J.A.; Kozak, J.; Silva, A.D. New developments in electro-chemical machining. Ann. CIRP 1999, 48, 567–579. [Google Scholar] [CrossRef]
- Kozak, J.; Rajurkar, K.P.; Makkar, Y. Study of pulse electrochemical micromachining. J. Manuf. Proc. 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Geethapriyan, T.; Kalaichelvan, K.; Muthuramalingam, T. Influence of coated tool electrode on drilling Inconel alloy 718 in Electrochemical micro machining. Procedia CIRP 2016, 46, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Ginestra, P.; Ferraro, R.M.; Hauber, K.Z.; Abeni, A.; Giliani, S.; Ceretti, E. Selective Laser Melting and Electron Beam Melting of Ti6Al4V for Orthopedic Applications: A Comparative Study on the Applied Building Direction. Materials 2020, 13, 5584. [Google Scholar] [CrossRef]
- Lowther, M.; Louth, S.; Davey, A.; Hussain, A.; Ginestra, P.; Carter, L.; Eisenstein, N.; Grover, L.; Cox, S. Clinical, industrial, and research perspectives on powder bed fusion additively manufactured metal implants. Addit. Manuf. 2019, 28, 565–584. [Google Scholar] [CrossRef] [Green Version]
- Davydov, A.D.; Kabanova, T.B.; Volgin, V.M. Electrochemical Machining of Titanium. Review. Russ. J. Electrochem. 2017, 53, 941–965. [Google Scholar] [CrossRef]
- Geethapriyan, T.; Muthuramalingam, T.; Kalaichelvan, K. Influence of Process Parameters on Machinability of Inconel 718 by Electrochemical Micromachining Process using TOPSIS Technique. Arab. J. Sci. Eng. 2019, 44, 7945–7955. [Google Scholar] [CrossRef]
- Muthuramalingam, T.; Ramamurthy, A.; Moiduddin, K.; Alkindi, M.; Ramalingam, S.; Alghamdi, O. Enhancing the Surface Quality of Micro Titanium Alloy Specimen in WEDM Process by Adopting TGRA-Based Optimization. Materials 2020, 13, 1440. [Google Scholar]
- Senthilkumar, C.; Ganesan, G.; Karthikeyan, R. Study of electrochemical machining characteristics of Al/SiCp composites. Int. J. Adv. Manuf. Technol. 2009, 43, 256–263. [Google Scholar] [CrossRef]
- Geethapriyan, T.; Muthuramalingam, T.; Vasanth, S.; Thavamani, J.; Srinivasan, V.H. Influence of Nanoparticles-Suspended Electrolyte on Machinability of Stainless Steel 430 Using Electrochemical Micro-machining Process. Lect. Notes Mech. Eng. Adv. Manuf. Process. 2018, 433–440. [Google Scholar] [CrossRef]
- Mingcheng, G.; Yongbin, Z.; Lingchao, M. Electrochemical Micromachining of Square Holes in StainlessSteel in H2SO4. Int. J. Electrochem. Sci. 2019, 14, 414–426. [Google Scholar]
- Saxena, K.K.; Qian, J.; Reynaerts, D. A review on process capabilities of electrochemical micromachining and itshybrid variants. Int. J. Mach. Tool. Manuf. 2018, 127, 28–56. [Google Scholar] [CrossRef]
- Thakur, A.; Tak, M.; Mote, R.G. Electrochemical micromachining behavior on 17-4 PH stainless steel using different electrolytes. Procedia Manuf. 2019, 34, 355–361. [Google Scholar] [CrossRef]
- Leese, R.J.; Ivanov, A. Electrochemical micromachining: Anintroduction. Adv. Mech. Eng. 2016, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Geethapriyan, T.; Kalaichelvan, K.; Muthuramalingam, T.; Rajadurai, A. Performance Analysis of Process Parameters on Machining α-β Titanium Alloy in Electrochemical Micromachining Process. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 232, 1577–1589. [Google Scholar] [CrossRef]
- Tang, L.; Li, B.; Yang, S.; Duan, Q.; Kang, B. The effect of electrolyte current density on the electrochemical machining S-03 material. Int. J. Adv. Manuf. Technol. 2014, 71, 1825–1833. [Google Scholar] [CrossRef]
- Chun, K.H.; Kim, S.H.; Lee, E.S. Analysis of the relationship between electrolyte characteristics and electrochemical machinability in PECM on invar (Fe-Ni) fine sheet. Int. J. Adv. Manuf. Technol. 2016, 87, 3009–3017. [Google Scholar] [CrossRef]







| Combination | Symbol | NaCl + NaNO3 | NaCl + C3H8O3 | NaCl + C6H8O7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Process Parameter | I | II | III | I | II | III | I | II | III | |
| Applied voltage(V) | V | 10 | 12 | 14 | 10 | 12 | 14 | 10 | 12 | 14 |
| Electrolyte Concentration (g/L) | EC | 10 + 7 | 10 + 9 | 10 + 3 | 10 + 4 | 10 + 7 | 10 + 8 | 10 + 16 | 10 + 5 | 10 + 3 |
| Micro-tool feed rate (µm/s) | MF | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 |
| Frequency (Hz) | F | 50 | 60 | 70 | 50 | 60 | 70 | 50 | 60 | 70 |
| Duty Cycle (%) | DC | 33 | 50 | 66 | 33 | 50 | 66 | 33 | 50 | 66 |
| S. No | V | EC | MF | F | DC | ||
|---|---|---|---|---|---|---|---|
| NaCl + NaNO3 | NaCl + C3H8O3 | NaCl + C6H8O7 | |||||
| 1 | 10 | 17 | 14 | 26 | 0.1 | 50 | 33 |
| 2 | 10 | 19 | 17 | 15 | 0.5 | 60 | 50 |
| 3 | 10 | 13 | 18 | 13 | 1 | 70 | 66 |
| 4 | 12 | 17 | 14 | 26 | 0.1 | 60 | 50 |
| 5 | 12 | 19 | 17 | 15 | 0.5 | 70 | 66 |
| 6 | 12 | 13 | 18 | 13 | 1 | 50 | 33 |
| 7 | 14 | 17 | 14 | 26 | 0.5 | 50 | 66 |
| 8 | 14 | 19 | 17 | 15 | 1 | 60 | 33 |
| 9 | 14 | 13 | 18 | 13 | 0.1 | 70 | 50 |
| 10 | 10 | 17 | 14 | 26 | 1 | 70 | 50 |
| 11 | 10 | 19 | 17 | 15 | 0.1 | 50 | 66 |
| 12 | 10 | 13 | 18 | 13 | 0.5 | 60 | 33 |
| 13 | 12 | 17 | 14 | 26 | 0.5 | 70 | 33 |
| 14 | 12 | 19 | 17 | 15 | 1 | 50 | 50 |
| 15 | 12 | 13 | 18 | 13 | 0.1 | 60 | 66 |
| 16 | 14 | 17 | 14 | 26 | 1 | 60 | 66 |
| 17 | 14 | 19 | 17 | 15 | 0.1 | 70 | 33 |
| 18 | 14 | 13 | 18 | 13 | 0.5 | 50 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangamani, G.; Thangaraj, M.; Moiduddin, K.; Mian, S.H.; Alkhalefah, H.; Umer, U. Performance Analysis of Electrochemical Micro Machining of Titanium (Ti-6Al-4V) Alloy under Different Electrolytes Concentrations. Metals 2021, 11, 247. https://doi.org/10.3390/met11020247
Thangamani G, Thangaraj M, Moiduddin K, Mian SH, Alkhalefah H, Umer U. Performance Analysis of Electrochemical Micro Machining of Titanium (Ti-6Al-4V) Alloy under Different Electrolytes Concentrations. Metals. 2021; 11(2):247. https://doi.org/10.3390/met11020247
Chicago/Turabian StyleThangamani, Geethapriyan, Muthuramalingam Thangaraj, Khaja Moiduddin, Syed Hammad Mian, Hisham Alkhalefah, and Usama Umer. 2021. "Performance Analysis of Electrochemical Micro Machining of Titanium (Ti-6Al-4V) Alloy under Different Electrolytes Concentrations" Metals 11, no. 2: 247. https://doi.org/10.3390/met11020247
APA StyleThangamani, G., Thangaraj, M., Moiduddin, K., Mian, S. H., Alkhalefah, H., & Umer, U. (2021). Performance Analysis of Electrochemical Micro Machining of Titanium (Ti-6Al-4V) Alloy under Different Electrolytes Concentrations. Metals, 11(2), 247. https://doi.org/10.3390/met11020247







