Synthesis of FeSi-Al2O3 Composites by Autowave Combustion with Metallothermic Reduction
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
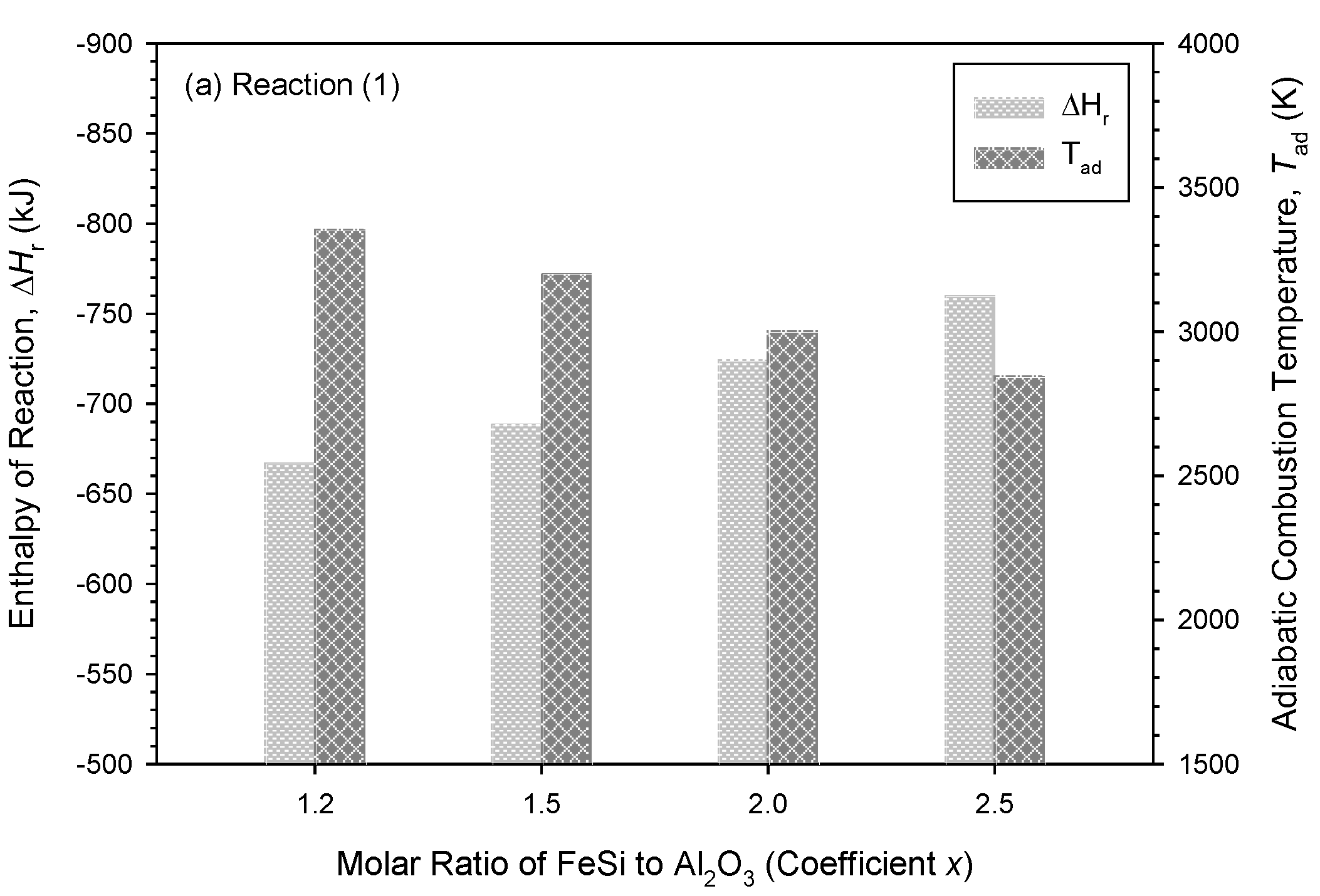
3.1. Thermodynamic Analysis
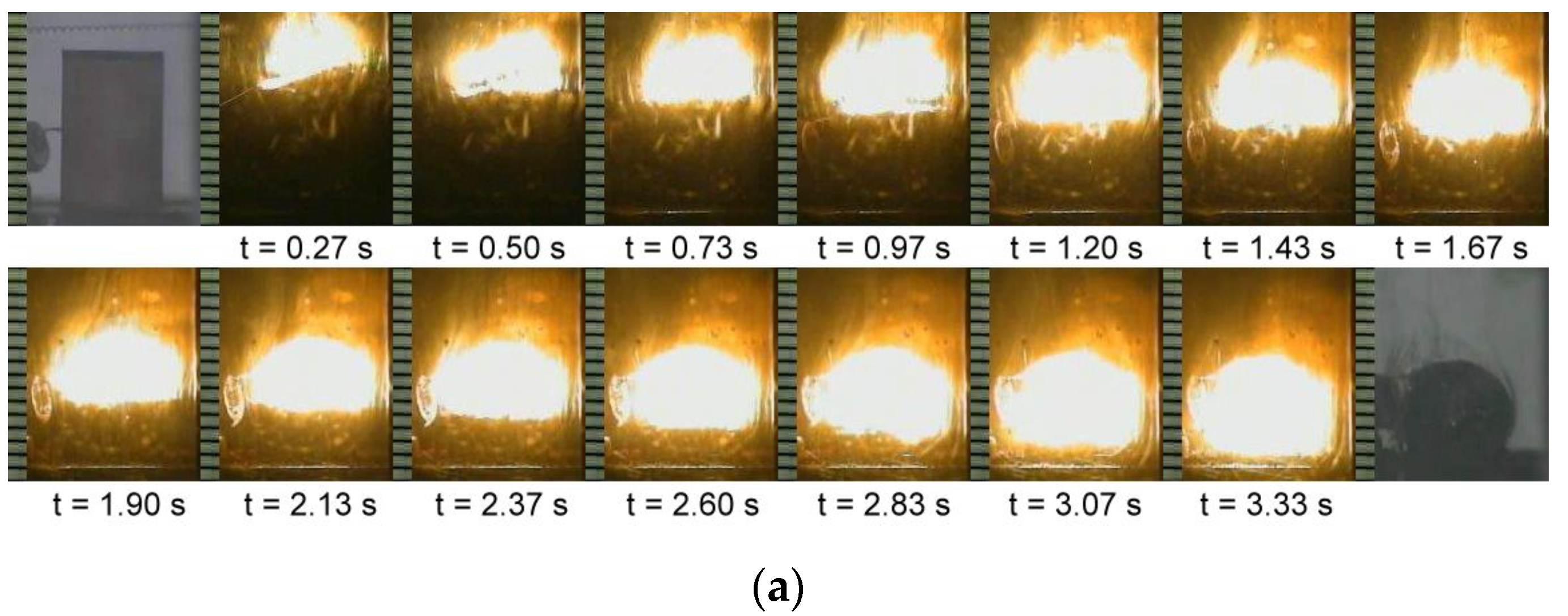
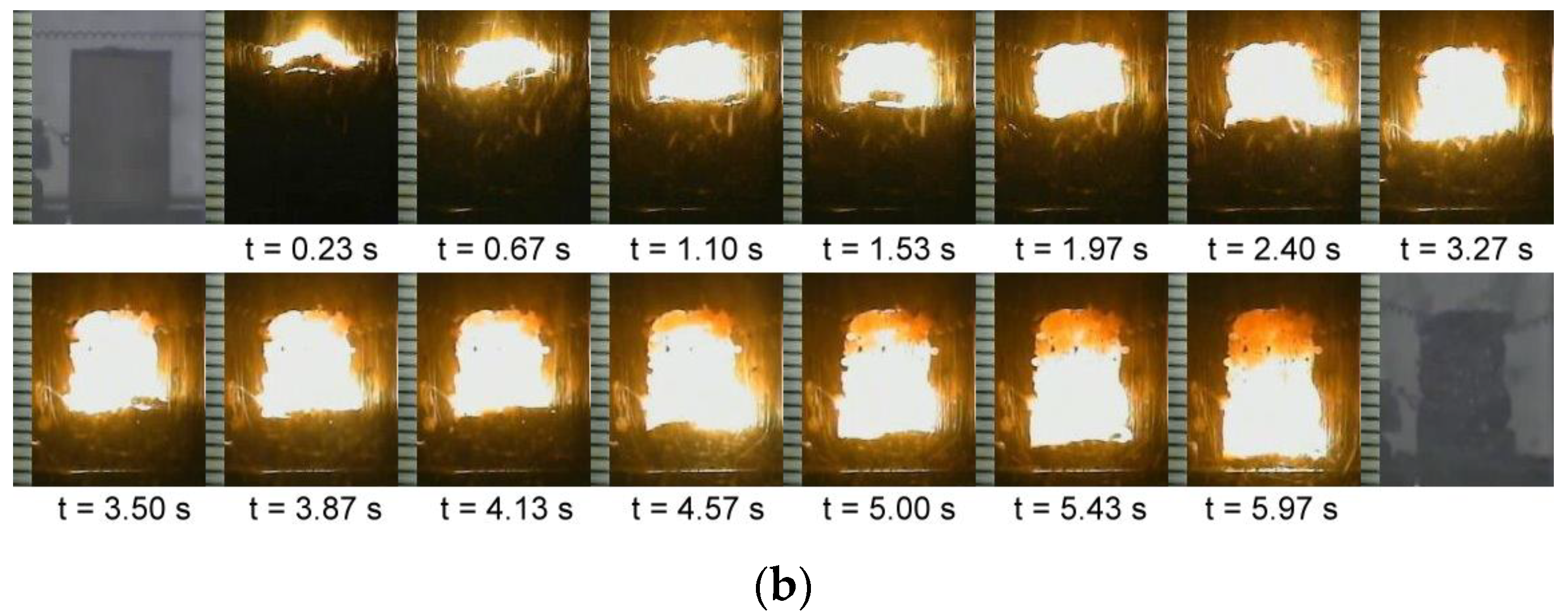
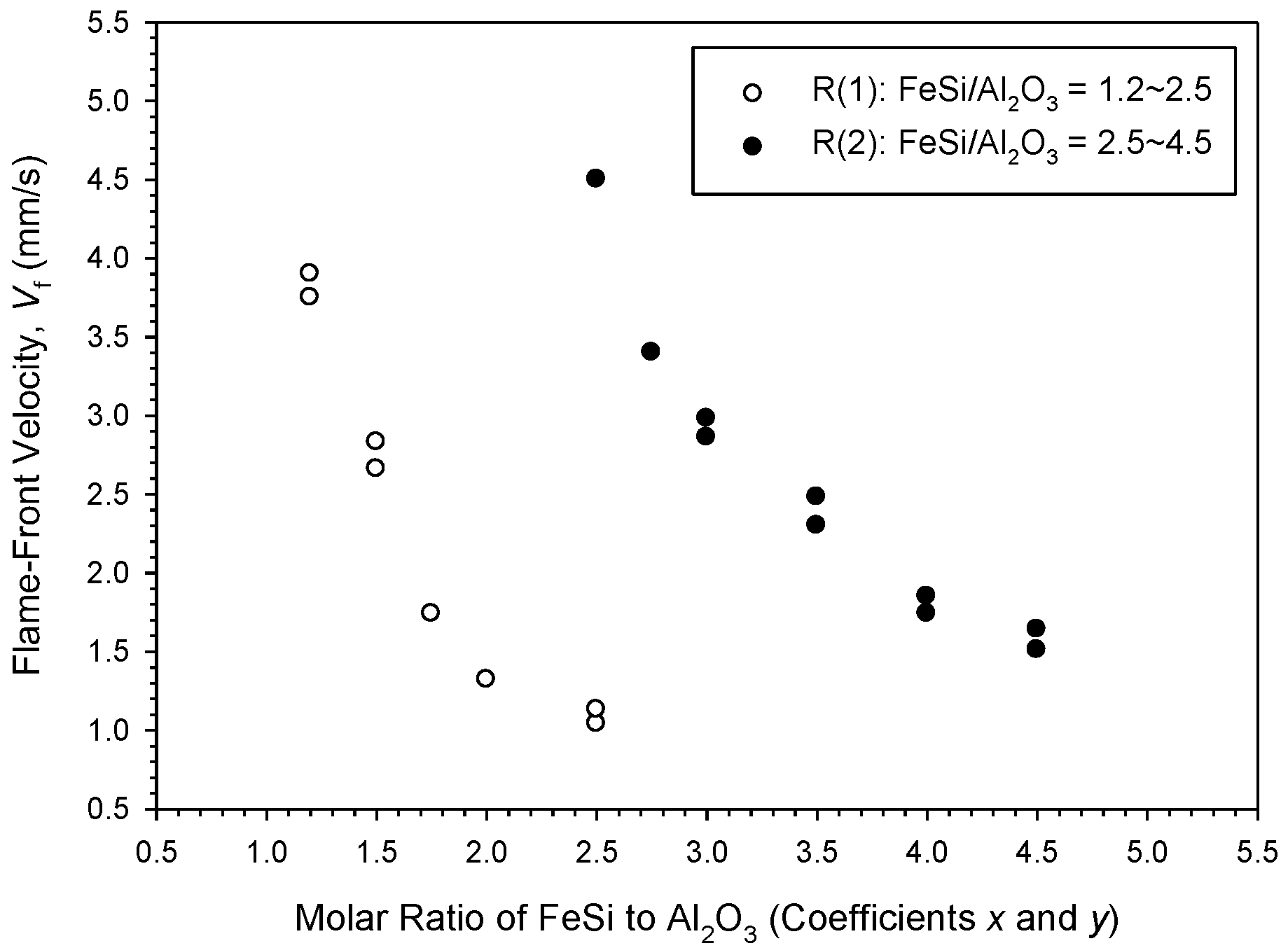
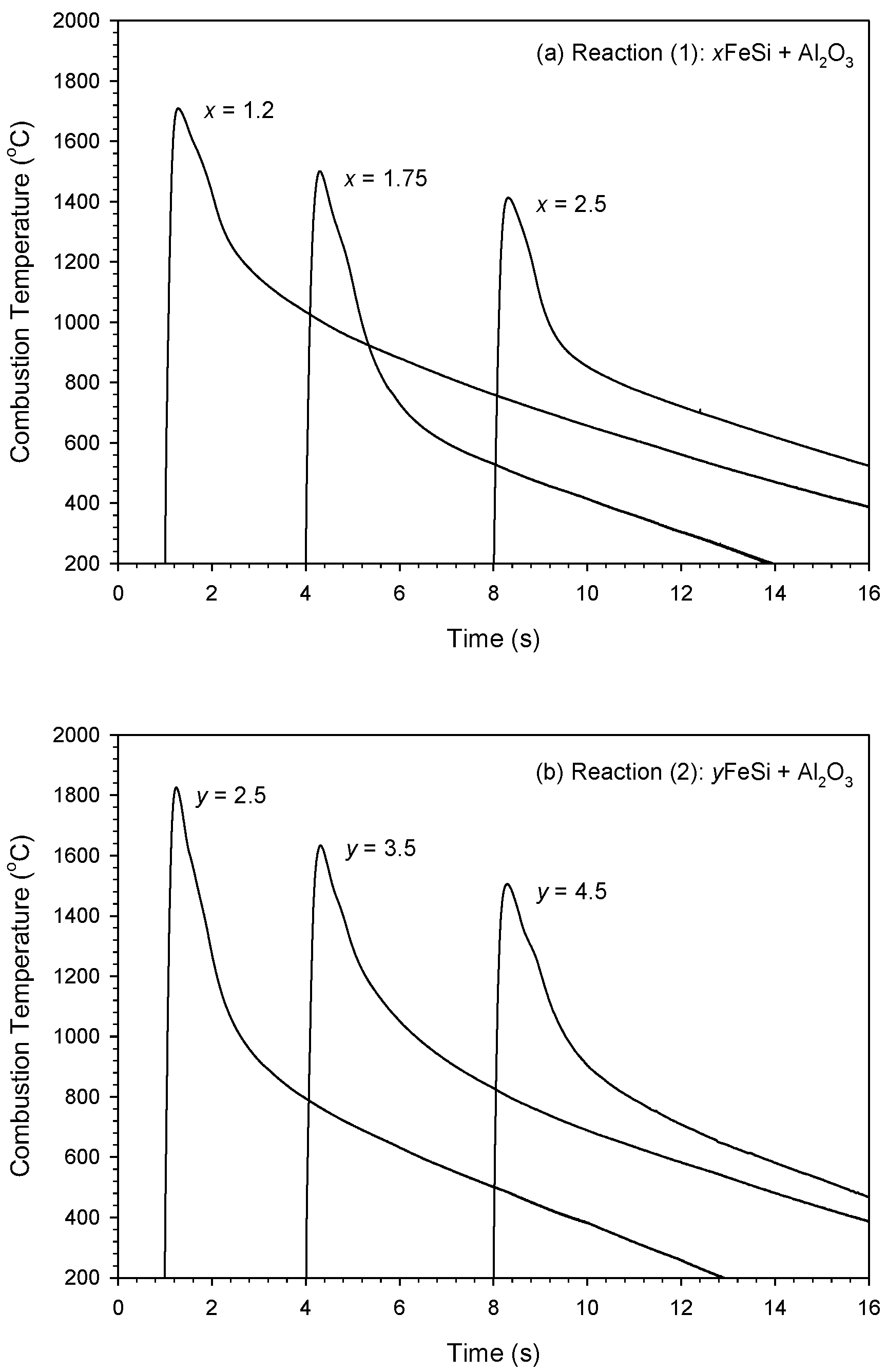
3.2. Self-Sustaining Combustion Wave Kinetics
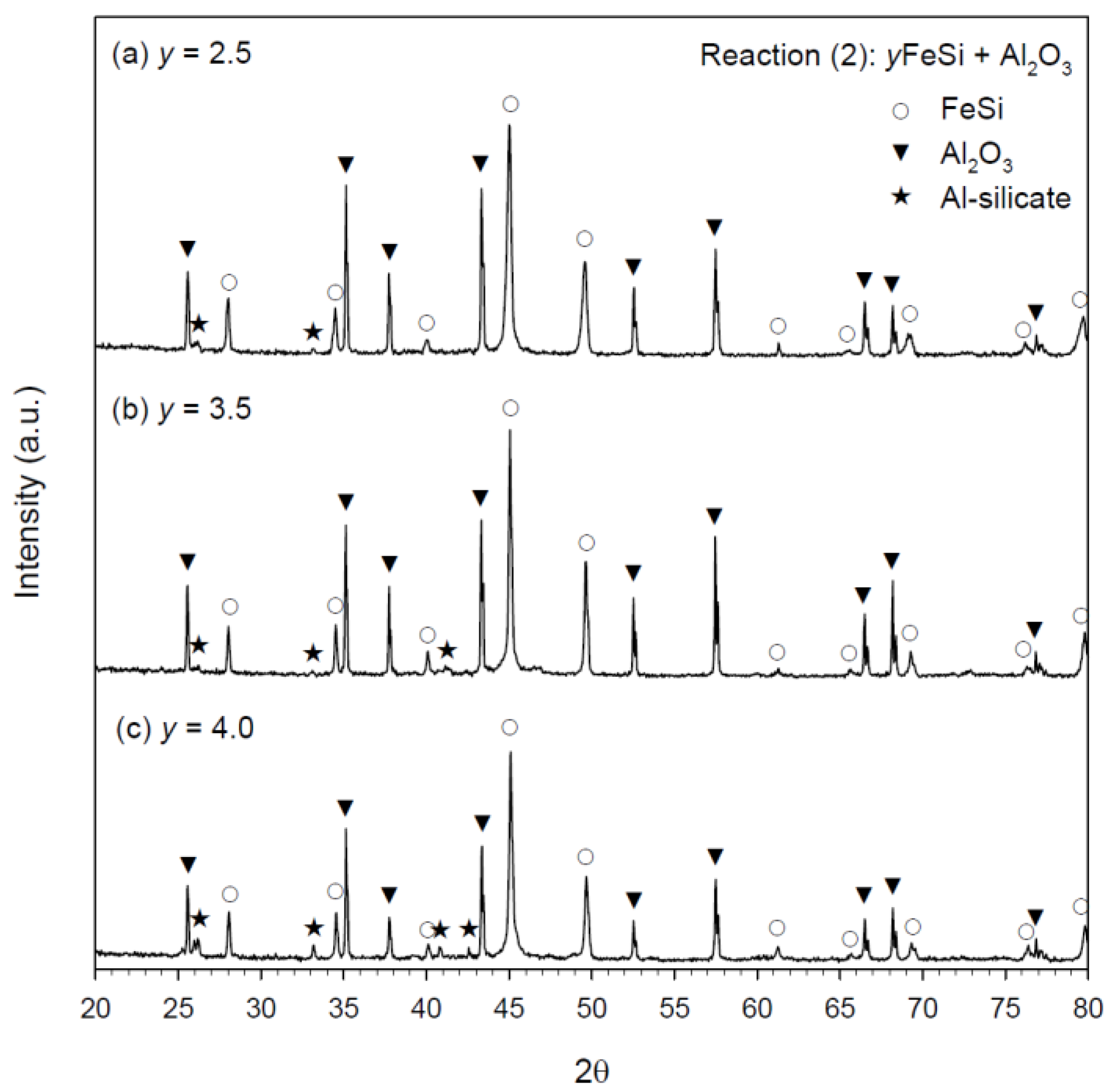
3.3. Phase Constituents of Synthesized Products
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okamoto, H. Desk Handbook: Phase Diagrams for Binary Alloys; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Dahal, N.; Chikan, V. Phase-Controlled synthesis of iron silicide (Fe3Si and FeSi2) nanoparticles in solution. Chem. Mater. 2010, 22, 2892–2897. [Google Scholar] [CrossRef]
- Liang, Y.F.; Shang, S.L.; Wang, J.; Wang, Y.; Ye, F.; Lin, J.P.; Chen, G.L.; Liu, Z.K. First-Principles calculations of photon and thermodynamic properties of Fe-Si compounds. Intermetallics 2011, 19, 1374–1384. [Google Scholar] [CrossRef]
- Wu, J.; Chong, X.Y.; Jiang, Y.H.; Feng, J. Stability, electronic structure, mechanical and thermodynamic properties of Fe-Si binary compounds. J. Alloys Compd. 2017, 693, 859–870. [Google Scholar] [CrossRef]
- Shen, B.; He, Y.; Wang, Z.; Yu, L.; Jiang, Y.; Gao, H. Reactive synthesis of porous FeSi intermetallic compound. J. Alloys Compd. 2020, 826, 154227. [Google Scholar] [CrossRef]
- Schmitt, A.L.; Bierman, M.J.; Schmeisser, D.; Himpsel, F.L.; Jin, S. Synthesis and properties of single-crystal FeSi nanowires. Nano Lett. 2006, 6, 1617–1621. [Google Scholar] [CrossRef]
- Sen, S.; Gogurla, N.; Banerji, P.; Guha, P.K.; Pramanik, P. Synthesis and characterization of β-phase iron silicide nano-particles by chemical reduction. Mater. Sci. Eng. B 2015, 200, 28–39. [Google Scholar] [CrossRef]
- Meng, Q.S.; Fan, W.H.; Chen, R.X.; Munir, Z.A. Thermoelectric properties of nanostructured FeSi2 prepared by field-activated and pressure-assisted reactive sintering. J. Alloys Compd. 2010, 492, 303–306. [Google Scholar] [CrossRef]
- Qu, X.; Lü, S.; Hu, J.; Meng, Q. Microstructure and thermoelectric properties of β-FeSi2 ceramics fabricated by hot-pressing and spark plasma sintering. J. Alloys Compd. 2011, 509, 10217–10221. [Google Scholar] [CrossRef]
- Merzhanov, A.G. Combustion processes that synthesize materials. J. Mater. Process. Technol. 1996, 56, 222–241. [Google Scholar] [CrossRef]
- Mossino, P. Some aspects in self-propagating high-temperature synthesis. Ceram. Int. 2004, 30, 311–332. [Google Scholar] [CrossRef]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-Propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2017, 62, 203–239. [Google Scholar] [CrossRef]
- Yeh, C.L.; Wang, H.J.; Chen, W.H. A comparative study on combustion synthesis of Ti-Si compounds. J. Alloys Compd. 2008, 450, 200–207. [Google Scholar] [CrossRef]
- Bertolino, N.; Anselmi-Tamburini, U.; Maglia, F.; Spinolo, G.; Munir, Z.A. Combustion synthesis of Zr-Si intermetallic compounds. J. Alloys Compd. 1999, 288, 238–248. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H. A comparative study on combustion synthesis of Nb-Si compounds. J. Alloys Compd. 2006, 425, 216–222. [Google Scholar] [CrossRef]
- Yeh, C.L.; Wang, H.J. A comparative study on combustion synthesis of Ta-Si compounds. Intermetallics 2007, 15, 1277–1284. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chen, W.H. Combustion synthesis of MoSi2 and MoSi2-Mo5Si3 composites. J. Alloys Compd. 2007, 438, 165–170. [Google Scholar] [CrossRef]
- Binnewies, M.; Milke, E. Thermochemical Data of Elements and Compounds; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2002. [Google Scholar]
- Merzhanov, A.G. Solid flames: Discoveries, concepts, and horizons of cognition. Combust. Sci. Technol. 1994, 98, 307–336. [Google Scholar] [CrossRef]
- Gras, C.; Gaffet, E.; Bernard, F.; Niepce, J.C. Enhancement of self-sustaining reaction by mechanical activation: Case of an Fe-Si system. Mater. Sci. Eng. A 1999, 264, 94–107. [Google Scholar] [CrossRef]
- Gras, C.; Bernsten, N.; Bernard, F.; Gaffet, E. The mechanically activated combustion reaction in the Fe–Si system: In situ time-resolved synchrotron investigations. Intermetallics 2002, 10, 271–282. [Google Scholar] [CrossRef]
- Gras, C.; Zink, N.; Bernard, F.; Gaffet, E. Assisted self-sustaining combustion reaction in the Fe–Si system: Mechanical and chemical activation. Mater. Sci. Eng. A 2007, 456, 270–277. [Google Scholar] [CrossRef]
- Zakeri, M.; Rahimipour, M.R.; Sadrnezhad, S.K. In situ synthesis of FeSi-Al2O3 nanocomposite powder by mechanical alloying. J. Alloys Compd. 2010, 492, 226–230. [Google Scholar] [CrossRef]
- Wang, L.L.; Munir, Z.A.; Maximov, Y.M. Thermite reactions: Their utilization in the synthesis and processing of materials. J. Mater. Sci. 1993, 28, 3693–3708. [Google Scholar] [CrossRef]
- Liang, Y.H.; Wang, H.Y.; Yang, Y.F.; Zhao, R.Y.; Jiang, Q.C. Effect of Cu content on the reaction behaviors of self-propagating high-temperature synthesis in Cu-Ti-B4C system. J. Alloys Compd. 2008, 462, 113–118. [Google Scholar] [CrossRef]
- Yeh, C.L.; Ke, C.Y. Intermetallic/ceramic composites synthesized from Al–Ni–Ti combustion with B4C addition. Metals 2020, 10, 873. [Google Scholar] [CrossRef]
- Acker, J.; Bohmhammel, K.; van den Berg, G.J.K.; van Miltenburg, J.C.; Kloc, C. Thermodynamic properties of iron silicides FeSi and α-FeSi2. J. Chem. Thermodyn. 1999, 31, 1523–1536. [Google Scholar] [CrossRef]
- Yeh, C.L.; Lin, J.Z. Combustion synthesis of Cr-Al and Cr-Si intermetallics with Al2O3 additions from Cr2O3-Al and Cr2O3-Al-Si reaction systems. Intermetallics 2013, 33, 126–133. [Google Scholar] [CrossRef]
- Varma, A.; Rogachev, A.S.; Mukasyan, A.S.; Hwang, S. Combustion synthesis of advanced materials: Principals and applications. Adv. Chem. Eng. 1998, 24, 79–225. [Google Scholar]
- Yeh, C.L.; Ke, C.Y. Combustion synthesis of FeAl-Al2O3 composites with TiB2 and TiC additions via metallothermic reduction of Fe2O3 and TiO2. Trans. Nonferrous Met. Soc. China 2020, 30, 2510–2517. [Google Scholar] [CrossRef]
- Bradley, D.; Matthews, K.J. Measurement of high gas temperatures with fine wire thermocouples. J. Mech. Eng. Sci. 1968, 10, 299–305. [Google Scholar] [CrossRef]
- Holman, J.P. Experimental Methods for Engineers, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Yeh, C.L.; Chen, Y.C. Formation of Mo5Si3/Mo3Si-MgAl2O4 composites via self-propagating high-temperature synthesis. Molecules 2020, 25, 83. [Google Scholar] [CrossRef]
- Fan, R.H.; Lü, H.L.; Sun, K.N.; Wang, W.X.; Yi, X.B. Kinetics of thermite reaction in Al-Fe2O3 system. Thermochim. Acta 2006, 440, 129–131. [Google Scholar] [CrossRef]
- Schneider, H.; Schreuer, J.; Hildmann, B. Structure and properties of mullite—A review. J. Eur. Ceram. Soc. 2008, 28, 329–344. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeh, C.-L.; Chen, K.-T. Synthesis of FeSi-Al2O3 Composites by Autowave Combustion with Metallothermic Reduction. Metals 2021, 11, 258. https://doi.org/10.3390/met11020258
Yeh C-L, Chen K-T. Synthesis of FeSi-Al2O3 Composites by Autowave Combustion with Metallothermic Reduction. Metals. 2021; 11(2):258. https://doi.org/10.3390/met11020258
Chicago/Turabian StyleYeh, Chun-Liang, and Kuan-Ting Chen. 2021. "Synthesis of FeSi-Al2O3 Composites by Autowave Combustion with Metallothermic Reduction" Metals 11, no. 2: 258. https://doi.org/10.3390/met11020258
APA StyleYeh, C.-L., & Chen, K.-T. (2021). Synthesis of FeSi-Al2O3 Composites by Autowave Combustion with Metallothermic Reduction. Metals, 11(2), 258. https://doi.org/10.3390/met11020258






