Author Contributions
Conceptualization, H.Y.S., D.-Q.F., and A.A.; software, D.-Q.F.; validation, H.Y.S., D.-Q.F., and A.A.; formal analysis, H.Y.S., D.-Q.F., and A.A.; investigation, H.Y.S., D.-Q.F., and A.A.; resources, H.Y.S.; data curation, D.-Q.F. and A.A.; writing—original draft preparation, H.Y.S., D.-Q.F., and A.A.; writing—review and editing, H.Y.S.; visualization, D.-Q.F. and A.A.; supervision, H.Y.S.; project administration, H.Y.S.; funding acquisition, H.Y.S. All authors have read and agreed to the published version of the manuscript.
Disclaimer
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Figure 1.
A schematic diagram of a possible direct steelmaking process based on flash ironmaking technology (FIT).
Figure 2.
The Utah laboratory flash ironmaking reactor (I.D. 0.19 m and length 2.13 m).
Figure 3.
The pilot plant with a flash reactor installed at the University of Utah.
Figure 4.
Schematic diagram of the pilot flash reactor (PFR).
Figure 5.
Schematic diagrams for (a) preheat burner and (b) main burner. NG stands for natural gas and Ox for oxygen.
Figure 6.
The controlling screen for the human machine interface (HMI).
Figure 7.
Sketches of possible configurations of flash ironmaking reactors.
Figure 8.
Distribution of the powder feeding ports on the roof of the reactor.
Figure 9.
Burner configuration.
Figure 10.
Reactor wall structure (unit in m).
Figure 11.
Velocity vector field in the plane passing through the centers of two powder feeding ports: (
a) design No. 1 and (
b) design No. 3 under operating conditions listed in
Table 5 (unit in m/s).
Figure 12.
Particle number density (particles/cm
3) in the plane passing through the centers of two powder feeding ports: (
a) design No. 1 and (
b) design No. 3 under operating conditions listed in
Table 5.
Figure 13.
Temperature (in K) distribution in the plane passing through the centers of two powder feeding ports: (
a) design No. 1 and (
b) design No. 3 under operating conditions listed in
Table 5.
Figure 14.
Species distribution in the plane passing through the centers of two powder feeding ports of design No. 5: (a) H2, (b) H2O, and (c) CO.
Figure 15.
Species distribution in the plane passing through the centers of two powder feeding ports of design No. 6: (a) H2, (b) H2O, and (c) CO.
Figure 16.
Distribution of the burners on the roof of the reactor. The large openings are burners and the small openings are powder feeding ports.
Figure 17.
Burner configuration and dimension (unit in m).
Figure 18.
Velocity field in the plane passing through (a) the centers of two opposite burners and (b) the centers of two opposite powder feeder ports (unit in m/s).
Figure 19.
Particle number density (particles/cm3) in the plane passing through the centers of two opposite powder feeding ports.
Figure 20.
Velocity vector field in the plane passing through (a) the centers of two opposite burners and (b) the centers of two opposite powder feeder ports (unit in m/s).
Figure 21.
Species distribution in the plane passing through the centers of two opposite burners: (a) H2 and (b) H2O.
Figure 22.
Species distribution in the plane passing through the centers of two opposite burners: (a) CO and (b) CO2.
Figure 23.
Schematic representation of the industrial reactor.
Figure 24.
Schematic representation of the burner.
Figure 25.
Meshing of the top section for a quarter of Reactor 2.
Figure 26.
Gas velocity magnitude in m/s where the right vertical line represents the axis of symmetry: (a) Reactor 1 and (b) Reactor 2.
Figure 27.
Contours of gas temperature (in K) where the right vertical line represents the axis of symmetry: (a) Reactor 1 and (b) Reactor 2.
Figure 28.
Contours of H2 content in mole fraction where the right vertical line represents the axis of symmetry: (a) Reactor 1 and (b) Reactor 2.
Figure 29.
Contours of CO content in mole fraction where the right vertical line represents the axis of symmetry: (a) Reactor 1 and (b) Reactor 2.
Figure 30.
Contours of H2O vapor content in mole fraction where the right vertical line represents the axis of symmetry: (a) Reactor 1 and (b) Reactor 2.
Figure 31.
Contours of CO2 content in mole fraction where the right vertical line represents the axis of symmetry: (a) Reactor 1 and (b) Reactor 2.
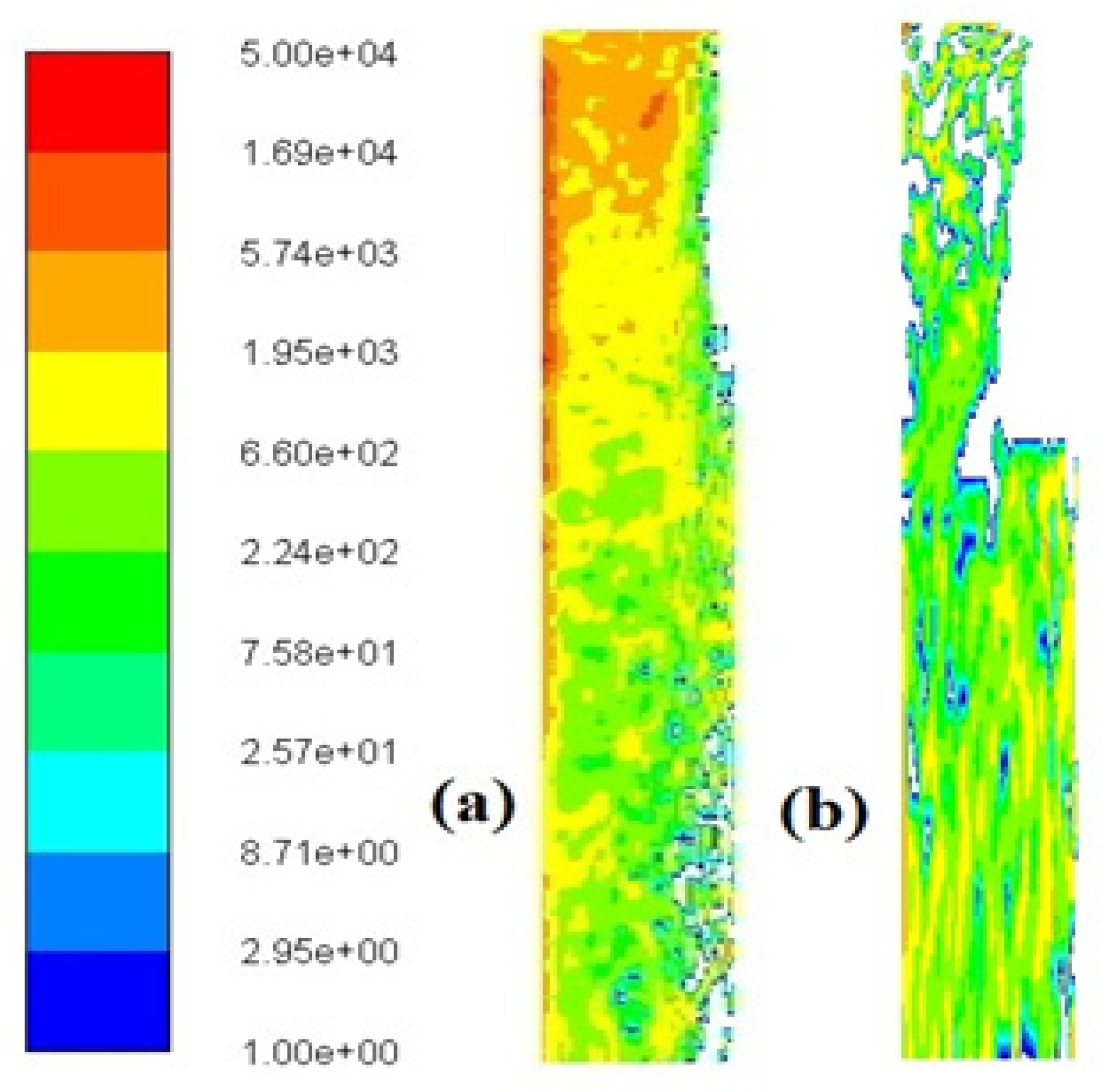
Figure 32.
Number density in particles/cm3 where the right vertical line represents the axis of symmetry: (a) Reactor 1 and (b) Reactor 2.
Figure 33.
Mass weighted average mass fraction of metallic iron in particles: (a) Reactor 1 and (b) Reactor 2.
Figure 34.
Mass weighted average particle temperature (in K): (a) Reactor 1 and (b) Reactor 2.
Table 1.
Kinetic parameters for reduction of magnetite concentrate by individual component gases [
16,
17,
20,
21].
| Reducing Gas, j | Temperature Range | | Ej (kJ/mol) | mj | nj | sj |
|---|
| H2 | 1423–1623 K | atm−1∙s−1 | 196 | 1 | 1 | 0 |
| 1623–1873 K | atm−1·s−1·µm | 180 | 1 | 1 | 1 |
| CO | 1423–1623 K | atm−1∙s−1 | 451 | 1 | 0.5 | 0 |
| 1623–1873 K | atm−1·s−1·µm | 88 | 1 | 0.5 | 1 |
Table 2.
The results of runs performed in the pilot flash reactor (PFR).
Inner Wall Temperature
(°C) | Magnetite Concentrate Feeding Rate (kg/h) | Gas Flow Rate * | H2 EDF † | Nominal Residence Time (s) | RD ††
(%) |
|---|
| Main Burner |
|---|
| NG (SLPM) ** | O2 (SLPM) |
|---|
| 1200–1130 | 5.0 | 404 | 321 | 0.76 | 12.5 | 65 |
| 1290–1220 | 1.8 | 410 | 293 | 0.84 | 12.0 | 79 |
| 1290–1210 | 2.9 | 410 | 293 | 0.96 | 12.0 | 82 |
| 1290–1230 | 2.5 | 358 | 270 | 1.00 | 13.3 | 83 |
| 1290–1240 | 3.5 | 512 | 327 | 1.07 | 10.2 | 76 |
| 1330–1230 | 4.7 | 330 | 200 | 1.36 | 15.3 | 89 |
| 1330–1230 | 4.5 | 330 | 200 | 1.44 | 15.3 | 87 |
| 1330–1230 | 5.2 | 500 | 290 | 3.00 | 10.6 | 80 |
| 1330–1230 | 4.3 | 500 | 290 | 3.00 | 10.6 | 82 |
| 1355–1260 | 5.5 | 235 | 190 | 0.03 | 18.3 | 7 |
| 1350–1300 | 4.0 | 255 | 209 | 0.15 | 17.0 | 49 |
| 1350–1270 | 4.5 | 275 | 212 | 0.20 | 16.2 | 31 |
| 1340–1280 | 5.0 | 280 | 209 | 0.21 | 16.2 | 37 |
| 1350–1290 | 4.6 | 280 | 230 | 0.50 | 15.6 | 80 |
| 1400–1300 | 6.3 | 300 | 240 | 0.82 | 14.4 | 88 |
| 1400–1300 | 5.0 | 330 | 200 | 1.51 | 14.6 | 100 |
| 1415–1350 | 4.5 | 220 | 191 | 0.07 | 18.0 | 18 |
| 1410–1360 | 4.0 | 240 | 195 | 0.33 | 17.1 | 32 |
| 1410–1330 | 5.0 | 295 | 221 | 0.50 | 14.7 | 66 |
| 1410–1330 | 6.0 | 300 | 210 | 0.70 | 14.9 | 74 |
| 1410–1320 | 5.0 | 300 | 210 | 0.82 | 14.9 | 82 |
Table 3.
Dimension of reactors with one-burner.
| D1 (m) | D2 (m) | H1 (m) | H2 (m) | Preheat Temp. (°C) | Designed Product Temp. (°C) | Design No. |
|---|
| 4.0 | 2.0 | 12.0 | 6.0 | 600 | 1300 | 1 |
| 4.0 | 2.0 | 10.0 | 6.0 | 1000 | 1300 | 2 |
| 6.0 | 2.0 | 6.0 | 6.3 | 600 | 1300 | 3 |
| 6.0 | 2.0 | 6.0 | 5.0 | 1000 | 1300 | 4 |
| 4.0 | – | 13.0 | – | 1000 | 1600 | 5 |
| 6.0 | – | 9.0 | – | 1000 | 1600 | 6 |
Table 4.
Wall material properties.
| Wall Material | Thermal Conductivity
(W·m−1·K−1) | Density
(kg·m−3) | Specific Heat
(J·kg−1·K−1) |
|---|
| Refractory | | 2890 | 0.2965 T + 362 |
| Insulation | | 1081 | 714 |
| Steel shell | 50 | 7850 | 470 |
Table 5.
Operating conditions for solid product with input gases preheated to 600 °C.
| Feed | Flow Rate (kg/s) | Preheat Temp. (°C) |
|---|
| Natural Gas | 1.15 | 600 |
| Recycled H2 | 0.43 | 600 |
| Oxygen | 2.19 | 600 |
| N2 (Carrier Gas) | 0.07 | 25 |
| Concentrate | 5.20 | 25 |
Table 6.
Operating conditions for solid product with input gases preheated to 1000 °C.
| Feed | Flow Rate (kg/s) | Preheat Temp. (°C) |
|---|
| Natural Gas | 0.91 | 1000 |
| Recycled H2 | 0.36 | 1000 |
| Oxygen | 1.54 | 1000 |
| N2 (Carrier Gas) | 0.07 | 25 |
| Concentrate | 5.20 | 25 |
Table 7.
Operating conditions for molten product with input gases preheated to 1000 °C.
| Feed | Flow Rate (kg/s) | Preheat Temp. (°C) |
|---|
| Natural Gas | 0.91 | 1000 |
| Recycled H2 | 0.36 | 1000 |
| Oxygen | 1.54 | 1000 |
| N2 (Carrier Gas) | 0.07 | 25 |
| Concentrate | 5.20 | 25 |
Table 8.
Heat loss and heat generated from partial combustion.
| Design No. | Heat Loss (MW) | Heat Generated (MW) | Sensible Heat of Input Gases from Preheating (MW) | Percentage (%) |
|---|
| 1 | 0.72 | 19.26 | 6.82 | 2.76 |
| 2 | 0.55 | 12.31 | 9.92 | 2.47 |
| 3 | 0.62 | 19.26 | 6.82 | 2.38 |
| 4 | 0.45 | 12.31 | 9.92 | 2.02 |
| 5 | 0.68 | 20.58 | 13.6 | 1.99 |
| 6 | 0.61 | 20.58 | 13.6 | 1.79 |
Table 9.
Operating conditions of the two industrial reactors.
| Parameter | Reactor 1 | Reactor 2 |
|---|
| Target production of iron in million t/y. | 0.3 | 1.0 |
| Feed of the magnetite concentrate in million t/y. | 0.415 | 1.38 |
| Natural gas feeding rate in m3/s | 17.5 | 45.8 |
| Oxygen feeding rate in m3/s | 13.2 | 34.7 |
| Expected excess of hydrogen at full reduction (EDF) | 0.3 | 0.3 |
Table 10.
The dimensions of the industrial reactors.
| Parameter | Definition | Reactor 1 (m) | Reactor 2 (m) |
|---|
| H | Height | 35.0 | 35.0 |
| D1 | Inner diameter | 7.0 | 12.0 |
| D2 | Diameter of powder feeder (4 feeders) | 0.05 | 0.30 |
| D3 | Inner diameter of the oxygen inlet 1 | 0.02 | 0.02 |
| D4 | Outer diameter of the oxygen inlet 1 | 0.26 | 0.8 |
| D5 | Outer diameter of the natural gas inlet | 0.44 | 1.6 |
| D6 | Outer diameter of the oxygen inlet 2 | 0.51 | 1.8 |
Table 11.
Average gas composition and metallization degree at reactor exit.
| Reactor | T (K) | H2 | CO | CO2 | H2O | Metallization (%) |
|---|
| 1 | 1519 | 40.2 | 26.3 | 5.9 | 24.1 | 91.2 |
| 2 | 1578 | 39.9 | 26.6 | 5.7 | 24.8 | 91.4 |
Table 12.
Heat generated from the combustion of natural gas, heat loss from the walls, and percentage heat loss.
| Reactor | Heat Generated (MW) | Heat Loss (MW) | Percent Heat Loss |
|---|
| 1 | 89.8 | 1.8 | 2.0 |
| 2 | 327 | 3.8 | 1.2 |