Performance Assessment of Magnesium Anodes Manufactured by Sintering Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Steel Used
2.2. Fabrication of Anodes
2.3. Electrochemical Tests
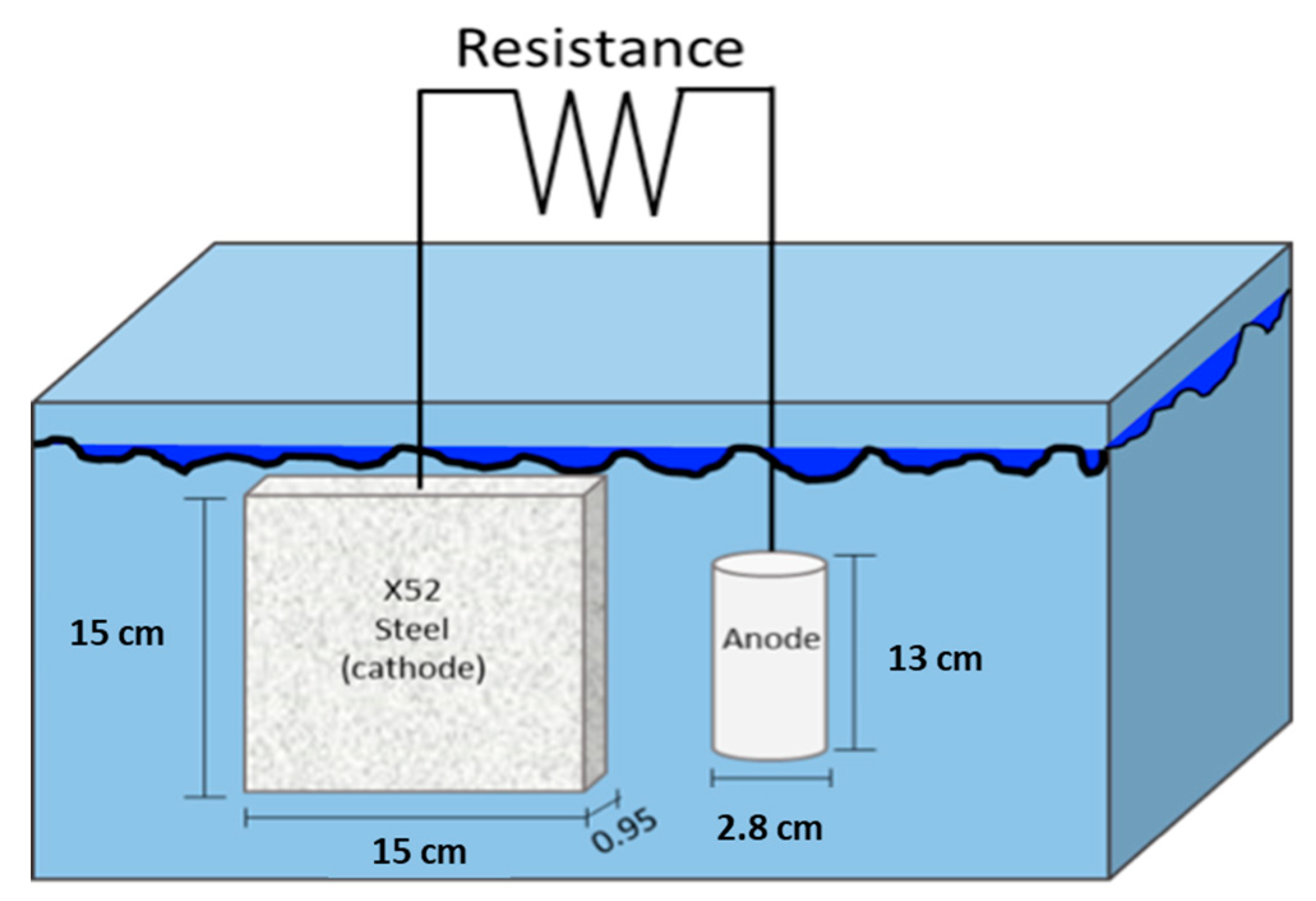
2.4. Cathodic Protection System
3. Results and Discussion
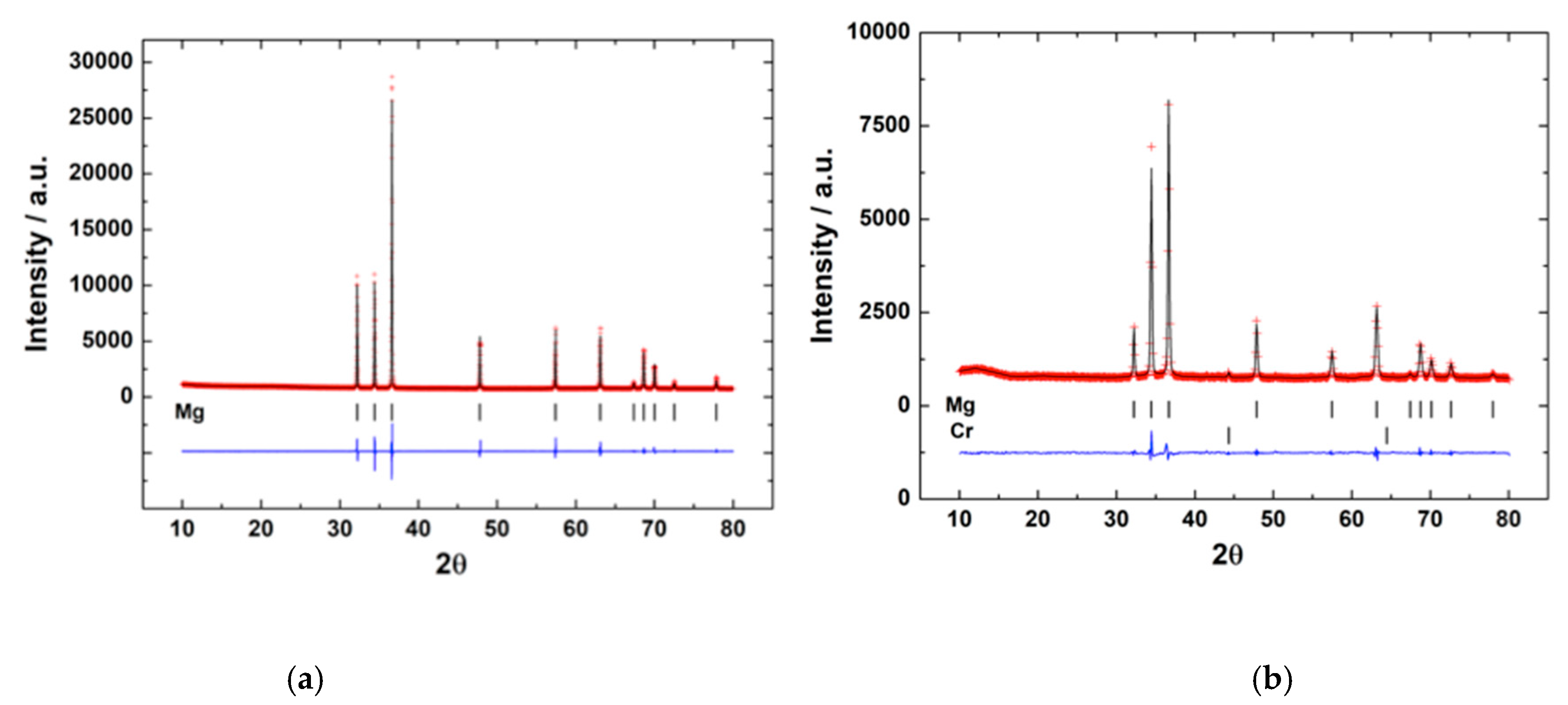
3.1. Microstructure of Mg Anodes
3.2. Electrochemical Tests
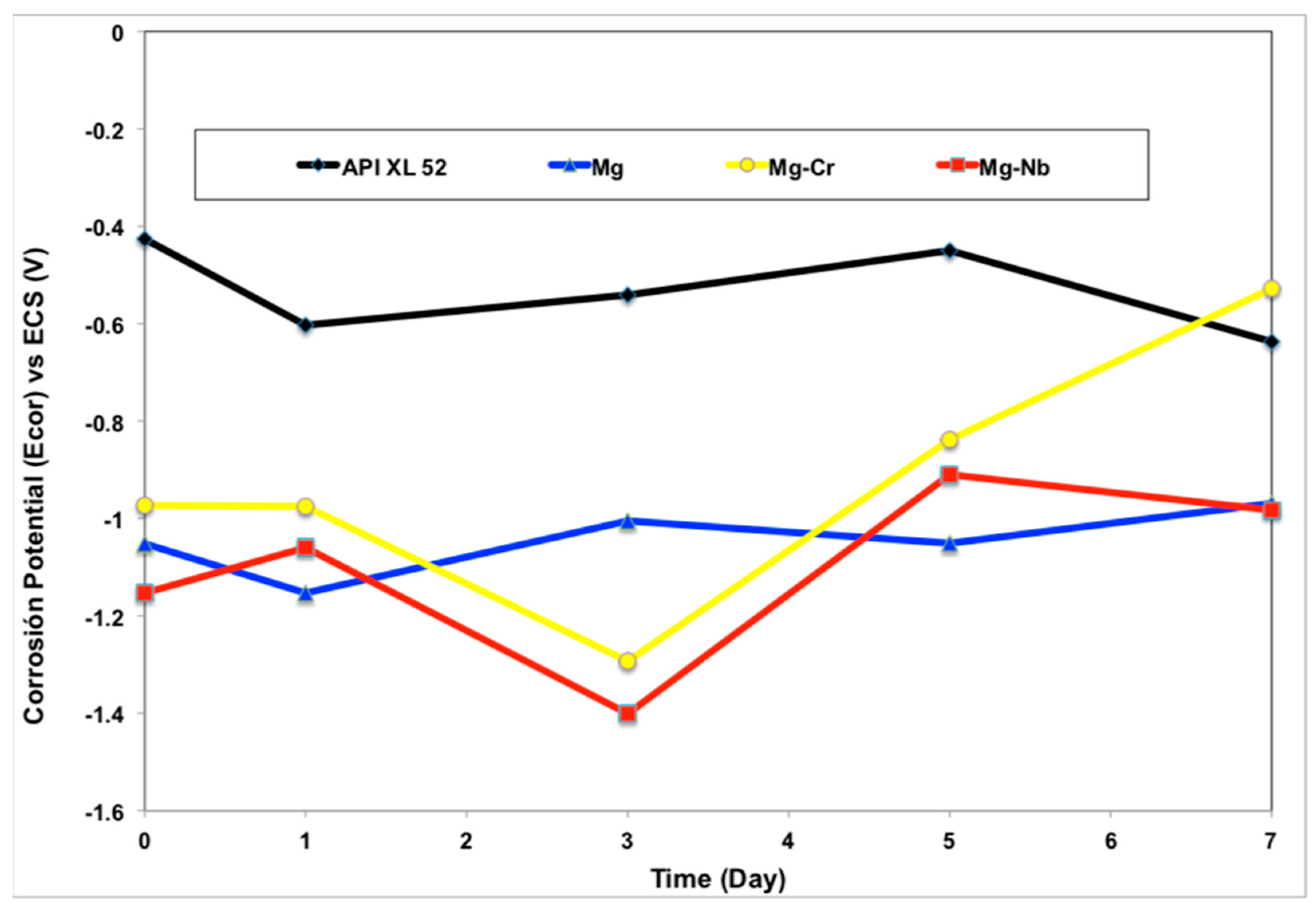
3.2.1. Corrosion Potential (Ecorr)
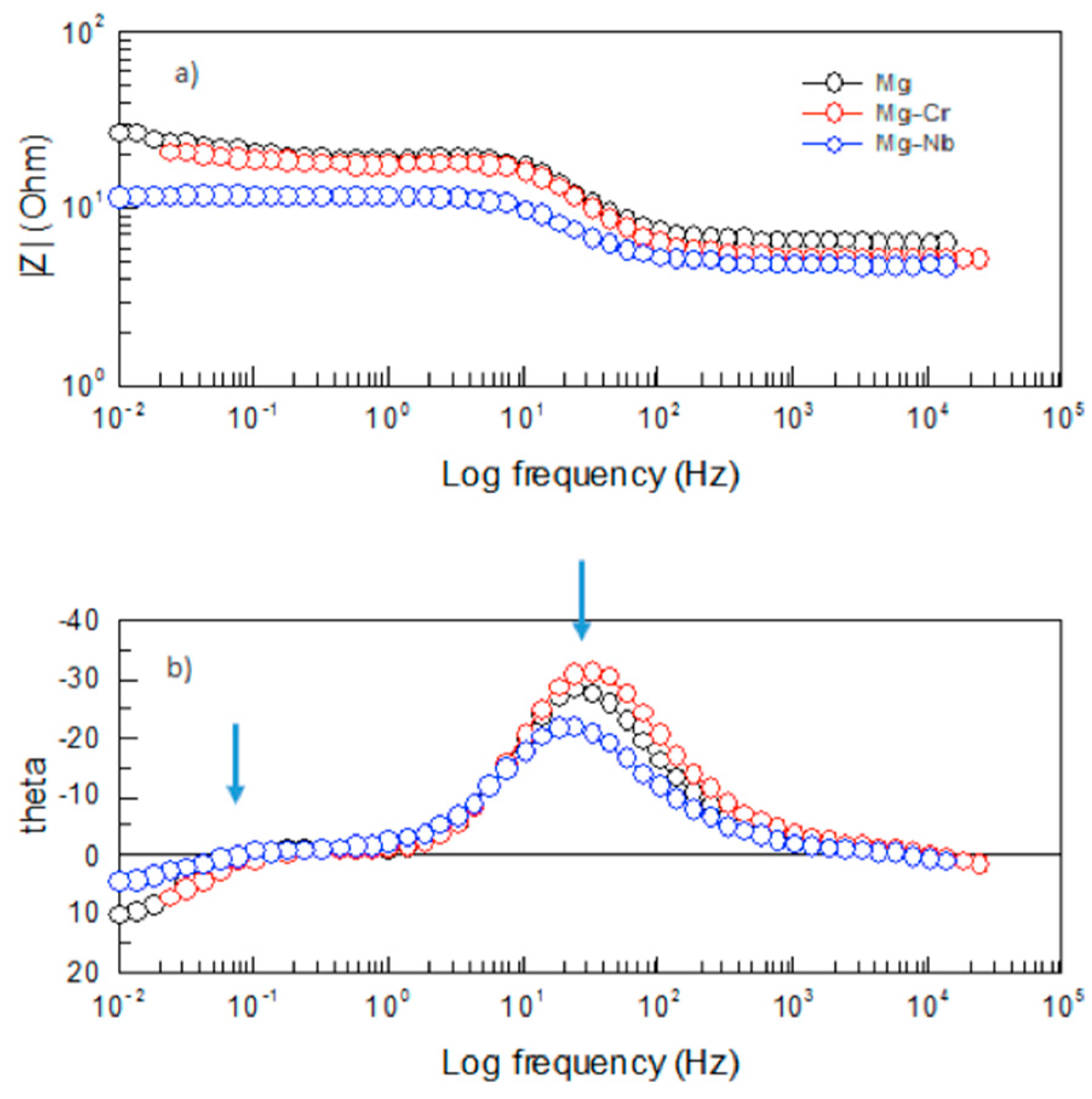
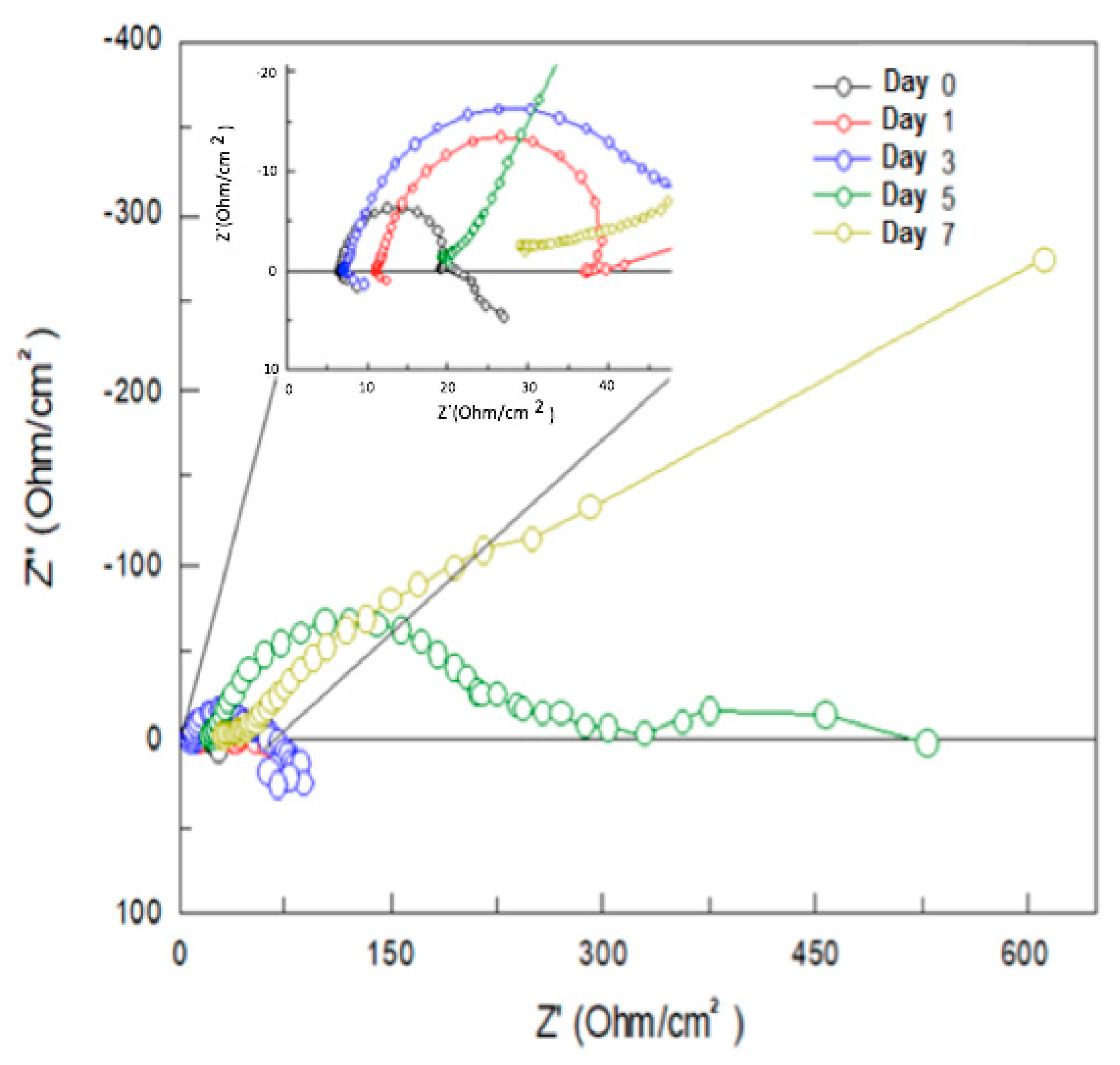
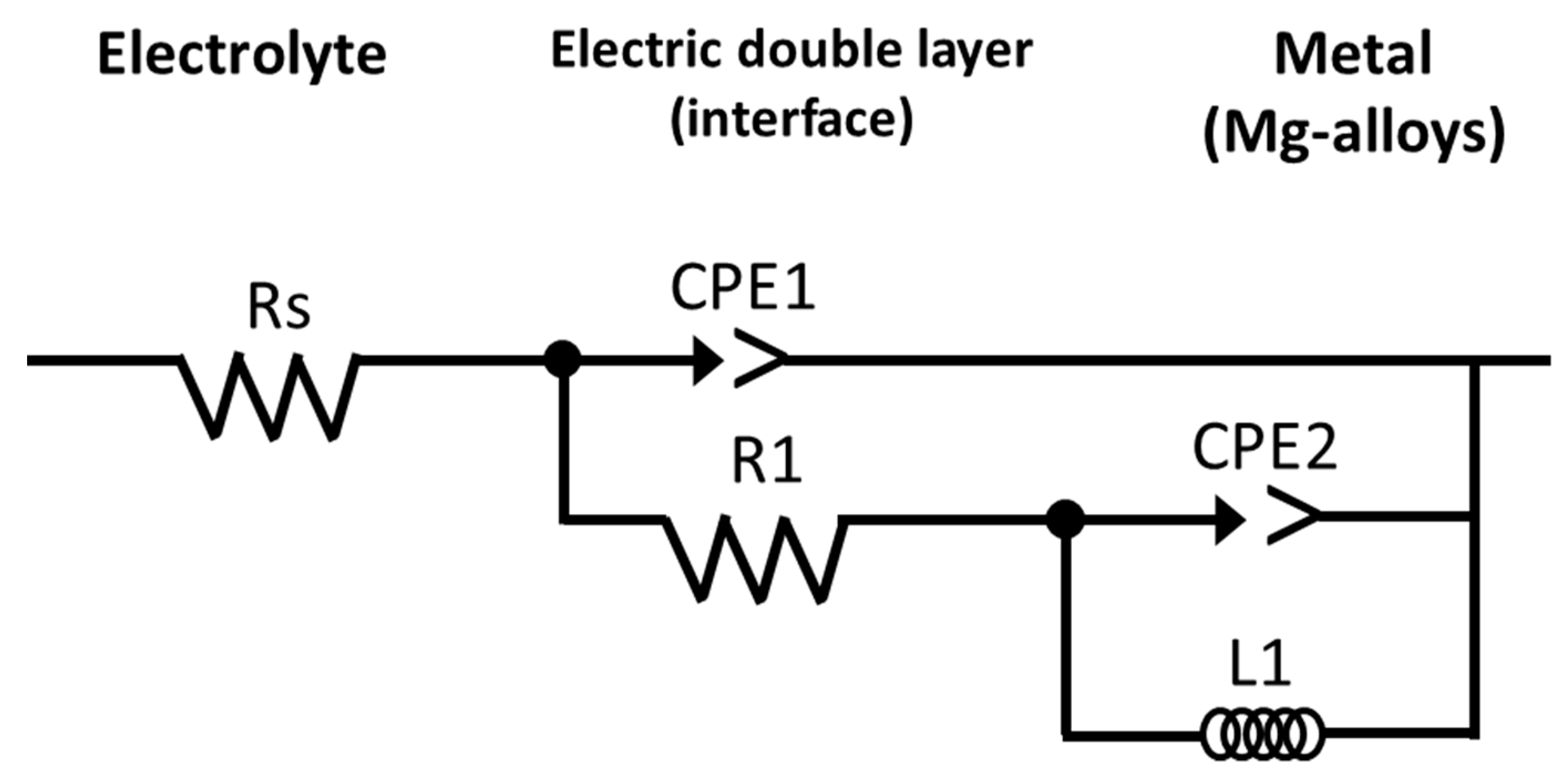
3.2.2. Evaluation of X52 Steel and Mg-Anodes by Electrochemical Impedance Spectroscopy (EIS)
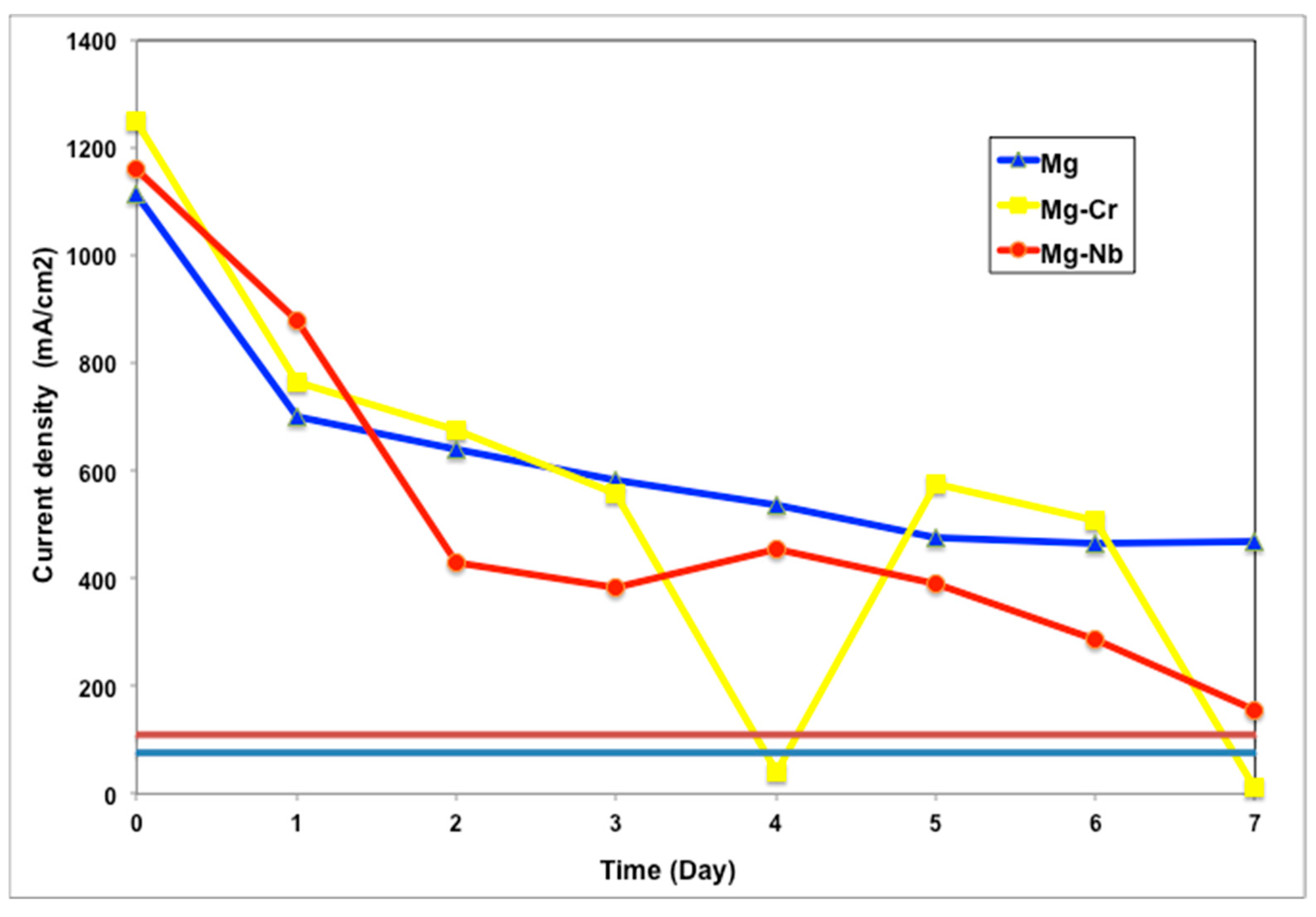
3.2.3. Analysis of the Cathodic Protection System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, Y.; Liu, L.; Yin, L.; Wang, R.; Li, X. Effect of lanthanum on microstructure and electrochemical corrosion behavior of Mg-6Al-5Pb alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 2623–2631. [Google Scholar]
- Wang, N.; Wang, R.; Peng, C.; Feng, Y.; Chen, B. Effect of hot rolling and subsequent annealing on electrochemical discharge behavior of AP65 magnesium alloy as anode for seawater activated battery. Corr. Sci. 2012, 64, 17–27. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Peng, C.; Feng, Y. Enhancement of the discharge performance of AP65 magnesium alloy anodes by hot extrusion. Corros. Sci. 2014, 81, 85–95. [Google Scholar] [CrossRef]
- Jugović, B.; Gvozdenović, M.; Stevanović, J.; Trišović, T.; Grgur, B. Corrosion behavior of magnesium, aluminum and zinc as anodic materials in chloride based electrolytes for use in primary and secondary electrochemical power sources. Mater. Des. 2009, 30, 3291–3294. [Google Scholar] [CrossRef]
- Çiçek, B.; Sun, Y. A study on the mechanical and corrosion properties of lead added magnesium alloys. Mater. Des. 2012, 37, 369–372. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Shinohara, T. Corrosion behavior of AZ91 magnesium alloy in dilute NaCl solutions. Mater. Des. 2010, 31, 857–863. [Google Scholar] [CrossRef]
- Boby, A.; Srinivasan, A.; Pillai, U.T.S.; Pai, B.C. Mechanical characterization and corrosion behavior of newly designed Sn and Y added AZ91 alloy. Mater. Des. 2015, 88, 871–879. [Google Scholar] [CrossRef]
- Shi, Y.; Peng Ch Feng, Y.; Wang, R.; Wang, N. Microstructure and electrochemical corrosion behavior of extruded Mg–Al–Pb–La alloy as anode for seawater-activated battery. Mater. Des. 2017, 124, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Meng, G.; Shao, Y.; Cui, Z.; Wang, F. Corrosion of hot extrusion AZ91 magnesium alloy. Part II: Effect of rare earth element neodymium (Nd) on the corrosion behavior of extruded alloy. Corros. Sci. 2011, 53, 2934–2942. [Google Scholar] [CrossRef]
- Meng, J.; Sun, W.; Tian, Z.; Qiu, X.; Zhang, D. Corrosion performance of magnesium (Mg) alloys containing rare-earth (RE) elements. In Corrosion Prevention of Magnesium Alloys; Woodhead Publishing: Cambridge, UK, 2013; pp. 38–60. [Google Scholar]
- Zakowski, K. Studying the effectiveness of a modernized cathodic protection system for an offshore platform. Anti Corros. Methods Mater. 2011, 58, 167–172. [Google Scholar] [CrossRef]
- Narozny, M.; Zakowski, K.; Darowicki, K. Method of sacrificial anode transistor driving in cathodic protection system. Corros. Sci. 2014, 88, 275–279. [Google Scholar] [CrossRef]
- Wang, R.C.; Li, Q.; Wang, N.G.; Peng, C.Q.; Feng, Y. Effect of lithium on the discharge and corrosion behavior of Mg-3 wt.% Al-alloy as the anode for seawater activated battery. J. Mater. Eng. Perform. 2018, 27, 6552–6563. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; He, L. Effect of adding different contents of mercury to magnesium on discharge and corrosion performances of magnesium anode sheet. Russ. J. Electrochem. 2011, 47, 900–907. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Banjade, D.R. Galvanic Corrosion of Magnesium Coupled to Steel at High Cathode-to-Anode Area Ratios. Master’s Thesis, Brigham Young University, Provo, UT, USA, 2015. [Google Scholar]
- Tang, D.; Du, Y.; Li, X.; Liang, Y.; Lu, M. Effect of alternating current on the performance of magnesium sacrificial anode. Mater. Des. 2016, 9, 133–145. [Google Scholar] [CrossRef]
- Kalisvaart, P.; Luber, E.; Fritzsche, H.; Mitlin, D. Effect of alloying magnesium with chromium and vanadium on hydrogenation kinetics studied with neutron reflectometry. Chem. Comm. 2011, 47, 4294–4296. [Google Scholar] [CrossRef] [Green Version]
- Mosaner, P.; Bazzanella, N.; Bonelli, M.; Checchetto, M.; Miotello, A. Mg:Nb films produced by pulsed laser deposition for hydrogen storage. Mater. Sci. Eng. B 2004, 108, 33–37. [Google Scholar] [CrossRef]
- De Castro, J.F.R.; Santos, S.F.; Costa, A.L.M.; Yavari, A.R.; Botta, W.J.F.; Ishikawa, T.T. Structural characterization and dehydrogenation behavior of Mg–5 at.% Nb nano-composite processed by reactive milling. J. Alloys Compd. 2004, 376, 251–256. [Google Scholar] [CrossRef]
- Shanthi, M.; Jayaramanavar, P.; Vyas, V.; Seenivasan, D.V.S.; Gupta, M. Effect of niobium particulate addition on the microstructure and mechanical properties of pure magnesium. J. Alloys Compd. 2012, 513, 202–207. [Google Scholar] [CrossRef]
- Staišiūnas, L.; Leinartas, K.; Samulevičienė, M.; Miečinskas, P.; Grigucevičienė, A.; Juškėnas, R.; Juzeliūnas, E. Electrochemical and structural characterization of sputter-deposited Mg–Nb and Mg–Nb–Al–Zn alloy films. J Solid State Electroch. 2013, 17, 1649–1656. [Google Scholar] [CrossRef]
- Shang, C.X.; Bououdina, M.; Guo, Z.X. Structural stability of mechanically alloyed (Mg+10Nb) and (MgH2+10Nb) powder mixtures. J. Alloys Compd. 2003, 349, 217–223. [Google Scholar] [CrossRef]
- Martinez-Garcia, A.; Navarro-Mtz, A.K.; Reguera, E.; Valera-Zaragoza, M.; Morales-Serna, J.A.; Juarez-Arellano, E.A. Fabrication of ball-milled MgO-Mg(OH)2-hydromagnesite composites and evaluation as an air-stable hydrogen storage material. Int. J. Hydrogen Energy 2020, 45, 12949–12960. [Google Scholar] [CrossRef]
- Abidin, N.I.Z.; Atrens, A.D.; Martin, D.; Atrens, A. Corrosion of high purity Mg, Mg2Zn0.2Mn, ZE41 and AZ91 in Hank’s solution at 37 °C. Corros. Sci. 2011, 53, 3542–3556. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Likhanova, N.V.; Nava, N.; Olivares-Xometl, O.; Domínguez-Aguilar, M.A.; Arellanes-Lozada, P.; Lijanova, I.V.; Arriola-Morales, J.; Lartundo-Rojas, L. Corrosion evaluation of pipeline steel API 5L X52 in partially deaerated produced water with high chloride content. Int. J. Electrochem. Sci. 2018, 13, 7949–7967. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Mostafa, A.; Medraj, M. Essential Magnesium Alloys Binary Phase Diagrams and Their Thermochemical Data. J. Mater. 2014, 2014, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Pelton, A.D.; Degterov, S.A.; Eriksson, G.; Robelin, C.; Dessureault, Y. The modified quasichemical model I—Binary Journal of Materials solutions. Metall. Mater. Trans. B 2000, 31, 651–659. [Google Scholar] [CrossRef]
- Altobelli, R.; Costa, I.; Faria, D.L.A.D. Characterization of corrosion products formed on steels in the first months of atmospheric exposure. Mater. Res. 2003, 6, 403–408. [Google Scholar]
- Qu, Q.; Wang, L.; Li, L.; He, Y.; Yang, M.; Ding, Z. Effect of the fungus, Aspergillus Niger, on the corrosion behaviour of AZ31B magnesium alloy in artificial seawater. Corros. Sci. 2015, 98, 249–259. [Google Scholar] [CrossRef]
- Wei, D.; Wang, J.; Wang, H.; Liu, Y.; Lib, S.; Li, D. Anti-corrosion behaviour of super wetting structured surfaces on Mg-9Al-1Zn magnesium alloy. Appl. Surf. Sci. 2019, 483, 1017–1026. [Google Scholar] [CrossRef]
- Williams, G.; Birbilis, N.; McMurray, H.N. The source of hydrogen evolved from a magnesium anode. Electrochem. Commun. 2013, 36, 1–5. [Google Scholar] [CrossRef]
- Frankel, G.; Samaniego, A.; Birbilis, N. Evolution of hydrogen at dissolving magnesium surfaces. Corros. Sci. 2013, 70, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Gabbardo, A.; Viswanathan, G.; Frankel, G. Application of 2D pit growth method to Mg thin films: Part II. Salt film and hydrogen evolution. J. Electrochem. Soc. 2019, 166, C3266–C3274. [Google Scholar] [CrossRef]
- Fajardo, S.; Bosch, J.; Frankel, G. Anomalous hydrogen evolution on AZ31, AZ61 and AZ91 magnesium alloys in unbuffered sodium chloride solution. Corros. Sci. 2019, 146, 163–171. [Google Scholar] [CrossRef]
- Frankel, G.; Fajardo, S.; Lynch, B.M. Introductory lecture on corrosion chemistry: A focus on anodic hydrogen evolution on Al and Mg. Faraday Discuss. 2015, 180, 11–33. [Google Scholar] [CrossRef]
- Chen, Y.K.; An, Z.; Chen, M. Competition mechanism study of Mg+H2O and MgO+H2O reaction. In Proceedings of the 6th Asia Conference on Mechanical and Materials Engineering (ACMME 2018) IOP Conference Series: Materials Science and Engineering, Seoul, Korea, 15–18 June 2018; pp. 1–5. [Google Scholar]
- He, B.; Lu, C.; Han, P.; Bai, X. Short-term electrochemical corrosion behavior of pipeline steel. Eng. Fail. Anal. 2016, 59, 410–418. [Google Scholar] [CrossRef]
- Guadarrama-Muñoz, F.; Mendoza-Flores, J.; Duran-Romero, R.; Genesca, J. Electrochemical study on magnesium anodes in NaCl and CaSO4–Mg(OH)2 aqueous solutions. Electrochim. Acta. 2006, 51, 1820–1830. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization 1. A theoretical analysis of the shape of polarization curves. Int. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Kun, Y.U.; Huang, Q.; Jun, Z.; DAI, Y.L. Electrochemical properties of magnesium alloy anodes discharged in seawater. Trans. Nonferrous Met. Soc. China 2012, 22, 2184–2190. [Google Scholar]
- Feliú, S.; Samaniego, A.; Bermudez, E.; Abdelsam El-Hadad, A.; Llorente, I.; Galván, J.C. Effect of native oxide film on commercial magnesium alloys substrates and carbonate conversion coating growth and corrosion resistance. Materials 2014, 7, 2534–2560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feliú, S.; García-Galván, F.R.; Llorente, I.; Diaz, L.; Simancas, J. Influence of hydrogen bubbles adhering to the exposed surface on the corrosion rate of magnesium alloys AZ31 and AZ61 in sodium chloride solution. Mater. Corros. 2017, 68, 651–663. [Google Scholar] [CrossRef]
- Wen, L.; Yu, K.; Xiong, H.; Dai, Y.; Yang, S.; Qiao, X.; Teng, F.; Fan, S. Composition optimization and electrochemical properties of Mg-Al-Pb-(Zn) alloys as anodes for seawater activated battery. Electrochim. Acta 2016, 194, 40–51. [Google Scholar] [CrossRef]
- Diaz, L.; Garcia, F.R.; Llorente, I.; Jimenez, A.; Galvan, J.C.; Feliu, S. Effect of heat treatment of magnesium alloy substrates on corrosion resistance of a hybrid organic–inorganic sol–gel film. RSC Adv. 2015, 5, 105735–105746. [Google Scholar] [CrossRef]
- NRF-047-PEMEX-2014-Diseño, instalación y mantenimiento de los sistemas de protección catódica (Design, Installation and Maintenance of Cathodic Protection Systems, Reference Standard). Available online: https://www.academia.edu/36140282/NRF_047_PEMEX_2014_Anodos_y_tierras_fisicas (accessed on 5 November 2020).







| C | Mn | Si | P | S | Cu | Cr | Ni | Nb | V | Ti | Al | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.08 | 1.05 | 0.26 | 0.019 | 0.003 | 0.019 | 0.02 | 0.02 | 0.041 | 0.054 | 0.002 | 0.038 | Bal. |
| Anode | Immersion Time (Days) | Rs (Ohms.cm2) | R1 (Ohms.cm2) | CPE (Farads) |
|---|---|---|---|---|
| Mg | 0 | 6.45 | 13.24 | 0.000793 |
| Mg-Cr | 0 | 5.22 | 11.15 | 0.000031 |
| Mg-Nb | 0 | 6.45 | 13.24 | 0.001155 |
| Mg | 1 | 11.11 | 7.97 | 0.000204 |
| Mg-Cr | 1 | 16.38 | 39.96 | 0.000124 |
| Mg-Nb | 1 | 5.23 | 16.01 | 0.000256 |
| Mg | 3 | 7.38 | 43.87 | 0.000814 |
| Mg-Cr | 3 | 48.84 | 105.5 | 0.001627 |
| Mg-Nb | 3 | 32.47 | 47.19 | 0.005315 |
| Mg | 5 | 20.21 | 233.8 | 0.000586 |
| Mg-Cr | 5 | 126.31 | 790 | 0.005412 |
| Mg-Nb | 5 | 13.45 | 331.4 | 0.013894 |
| Mg | 7 | 32.93 | 220.5 | 0.013752 |
| Mg-Cr | 7 | 125 | 858.4 | 0.005583 |
| Mg-Nb | 7 | 17.93 | 920 | 0.019321 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanmiguel-May, J.A.; López-Alcantara, R.; Juárez-Arellano, E.A.; Pérez-Quiroz, J.T.; Contreras, A.; Pérez-López, T. Performance Assessment of Magnesium Anodes Manufactured by Sintering Process. Metals 2021, 11, 406. https://doi.org/10.3390/met11030406
Sanmiguel-May JA, López-Alcantara R, Juárez-Arellano EA, Pérez-Quiroz JT, Contreras A, Pérez-López T. Performance Assessment of Magnesium Anodes Manufactured by Sintering Process. Metals. 2021; 11(3):406. https://doi.org/10.3390/met11030406
Chicago/Turabian StyleSanmiguel-May, Judith A., Ruth López-Alcantara, Erick A. Juárez-Arellano, José T. Pérez-Quiroz, Antonio Contreras, and Tezozomoc Pérez-López. 2021. "Performance Assessment of Magnesium Anodes Manufactured by Sintering Process" Metals 11, no. 3: 406. https://doi.org/10.3390/met11030406
APA StyleSanmiguel-May, J. A., López-Alcantara, R., Juárez-Arellano, E. A., Pérez-Quiroz, J. T., Contreras, A., & Pérez-López, T. (2021). Performance Assessment of Magnesium Anodes Manufactured by Sintering Process. Metals, 11(3), 406. https://doi.org/10.3390/met11030406







