Thermal Conductivity of As-Cast and Annealed Mg-RE Binary Alloys
Abstract
:1. Introduction
2. Experimental Procedure
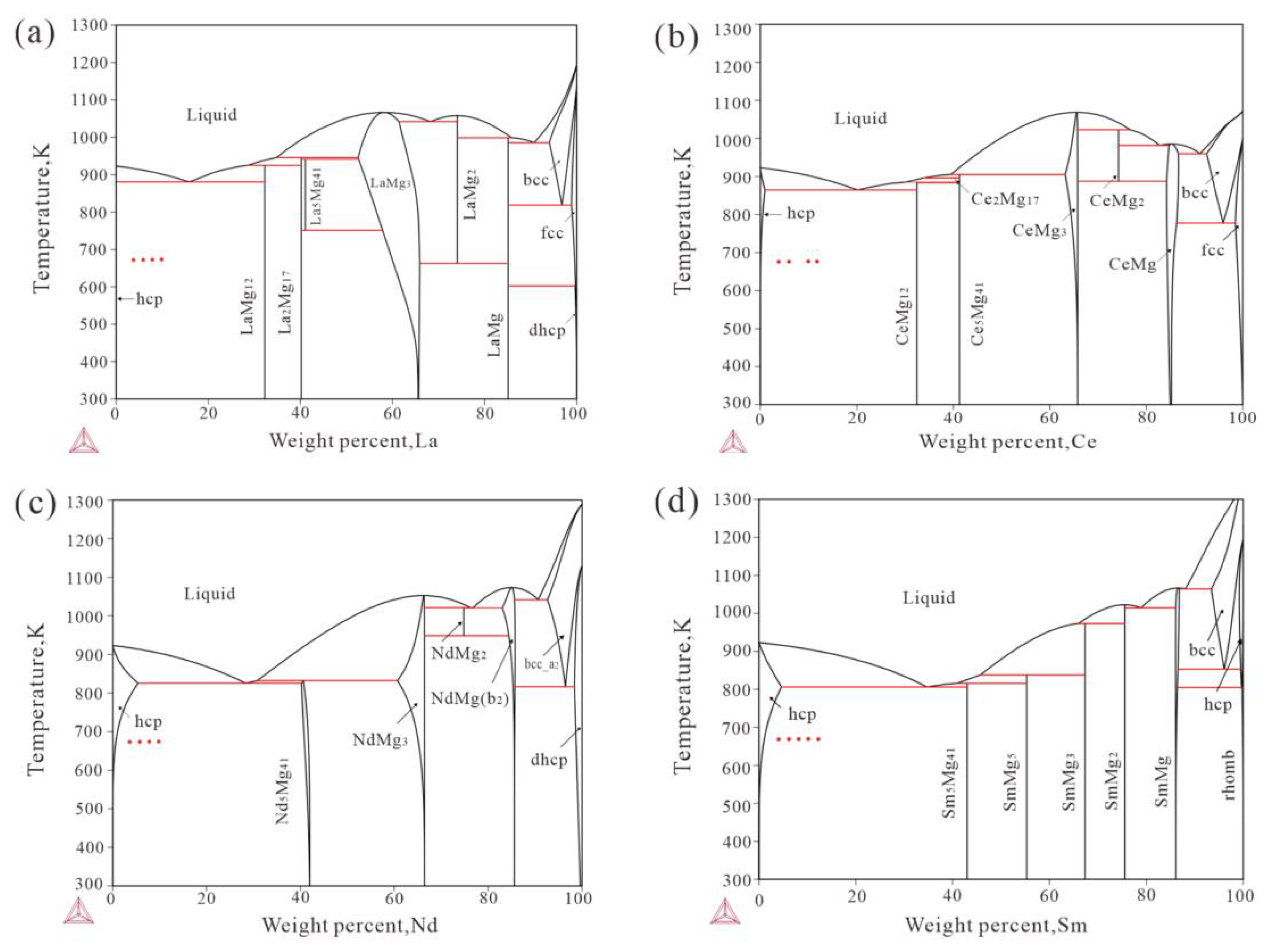
3. Results
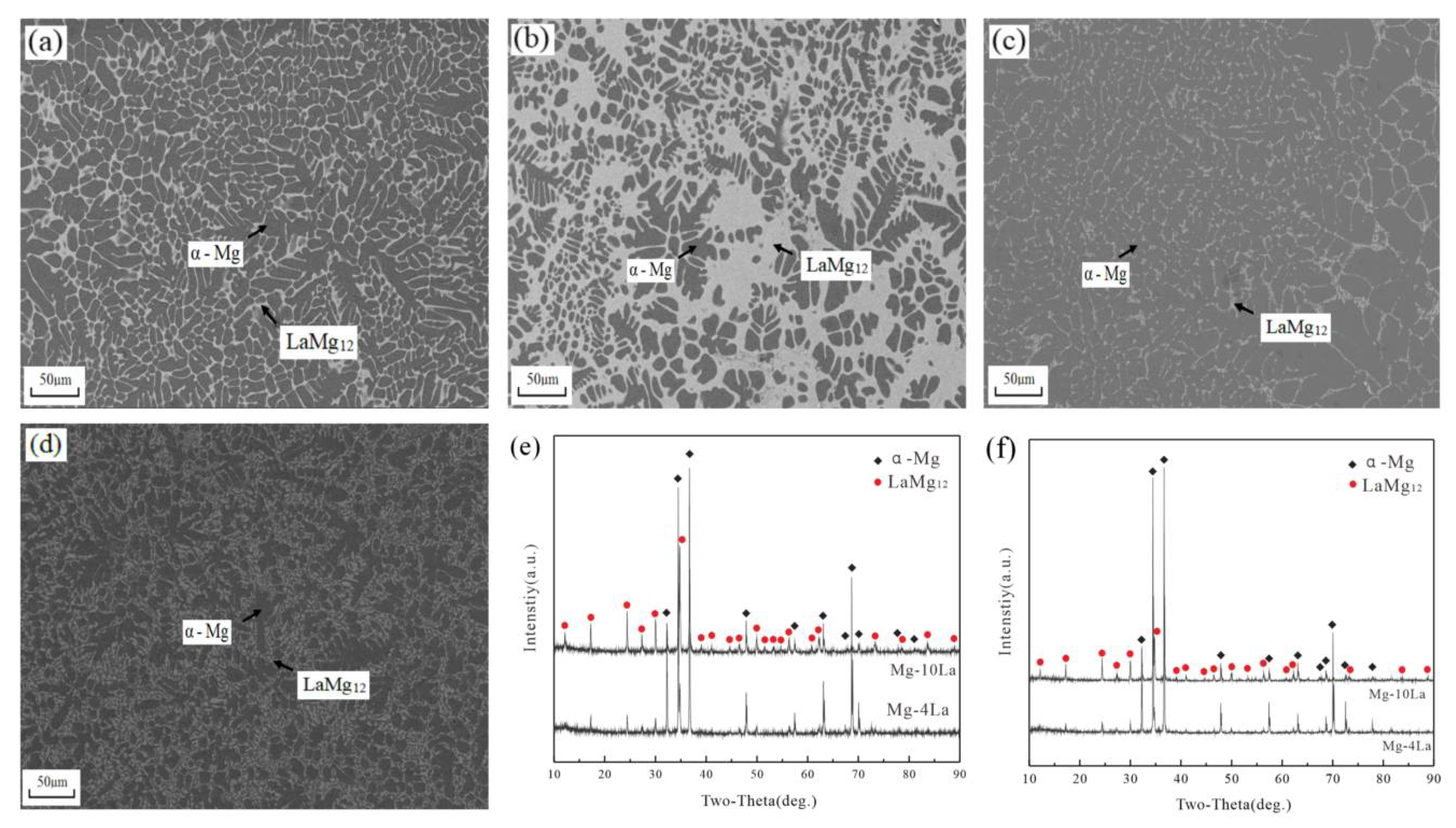
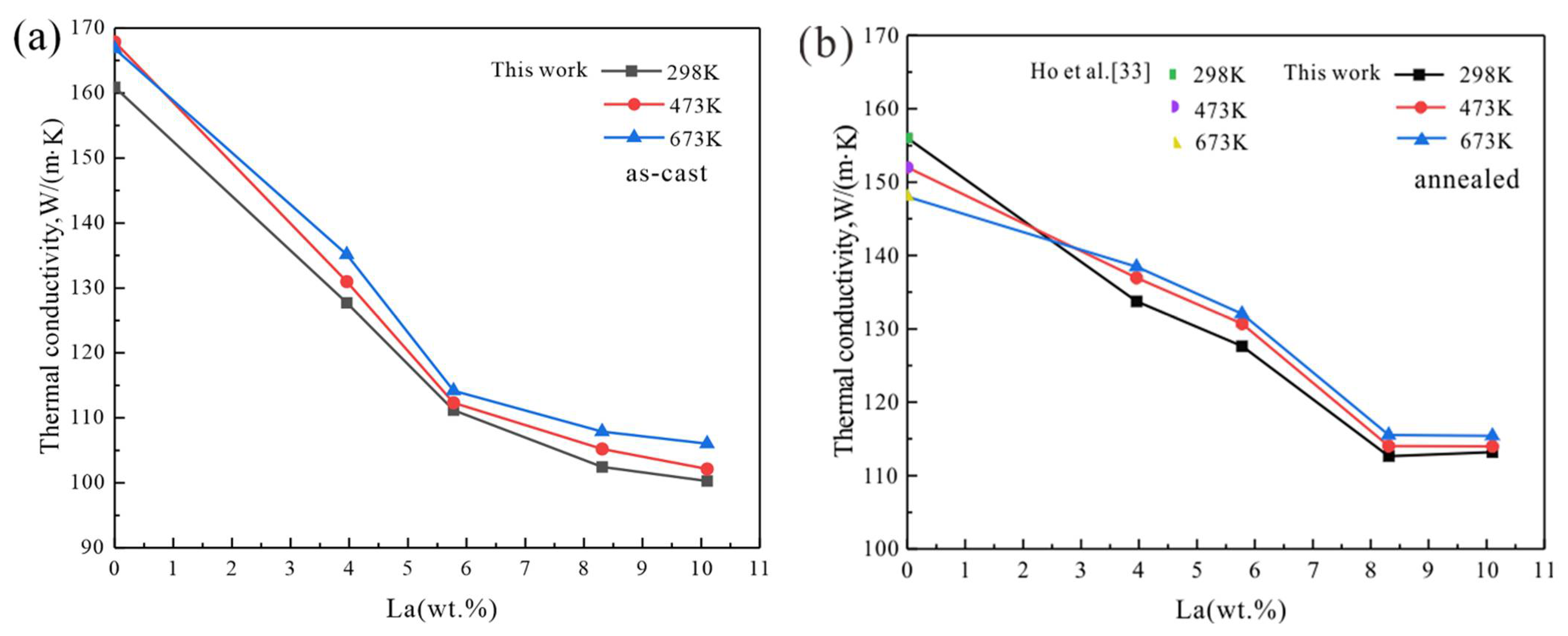
3.1. Mg-La Alloys
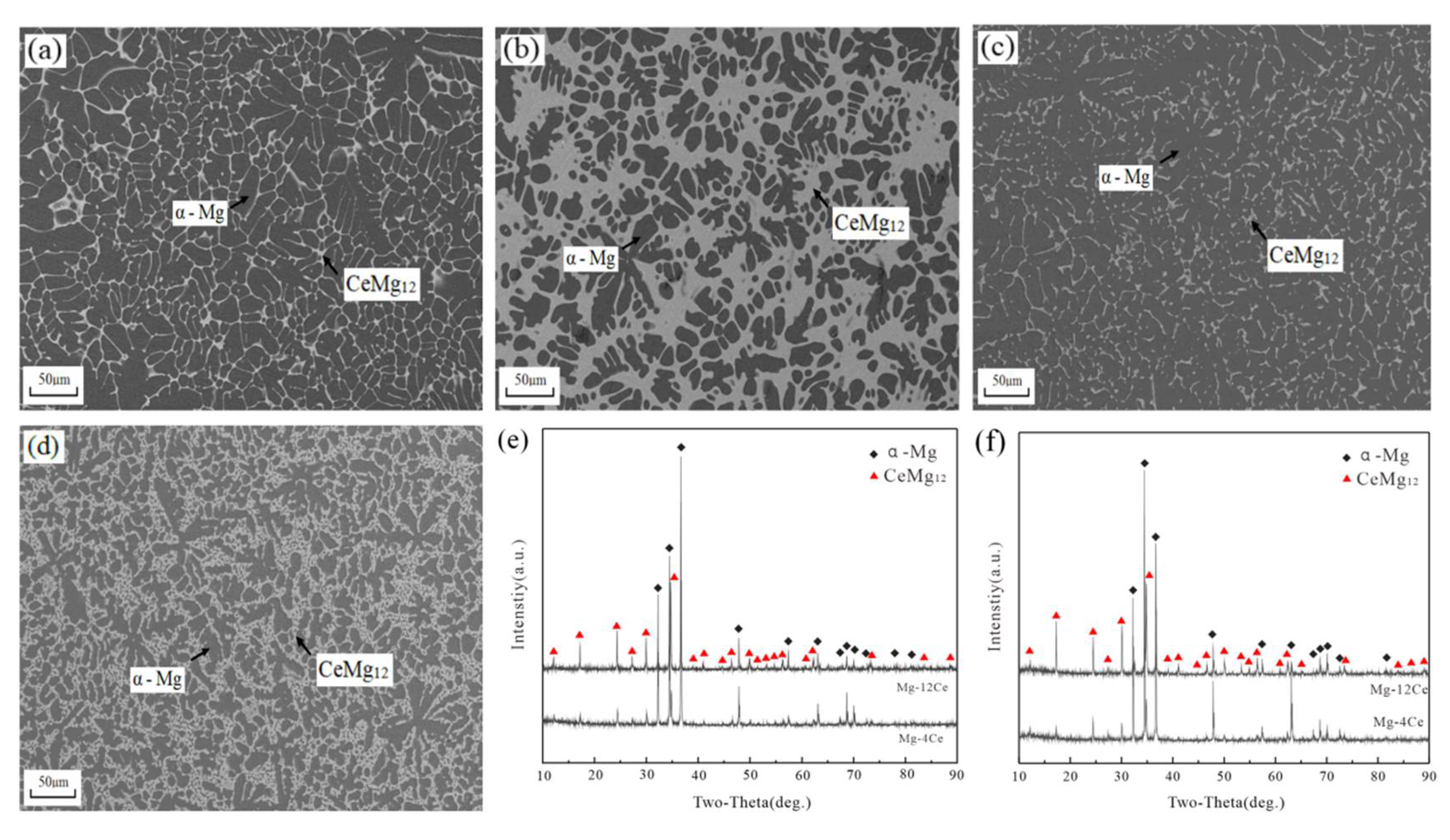
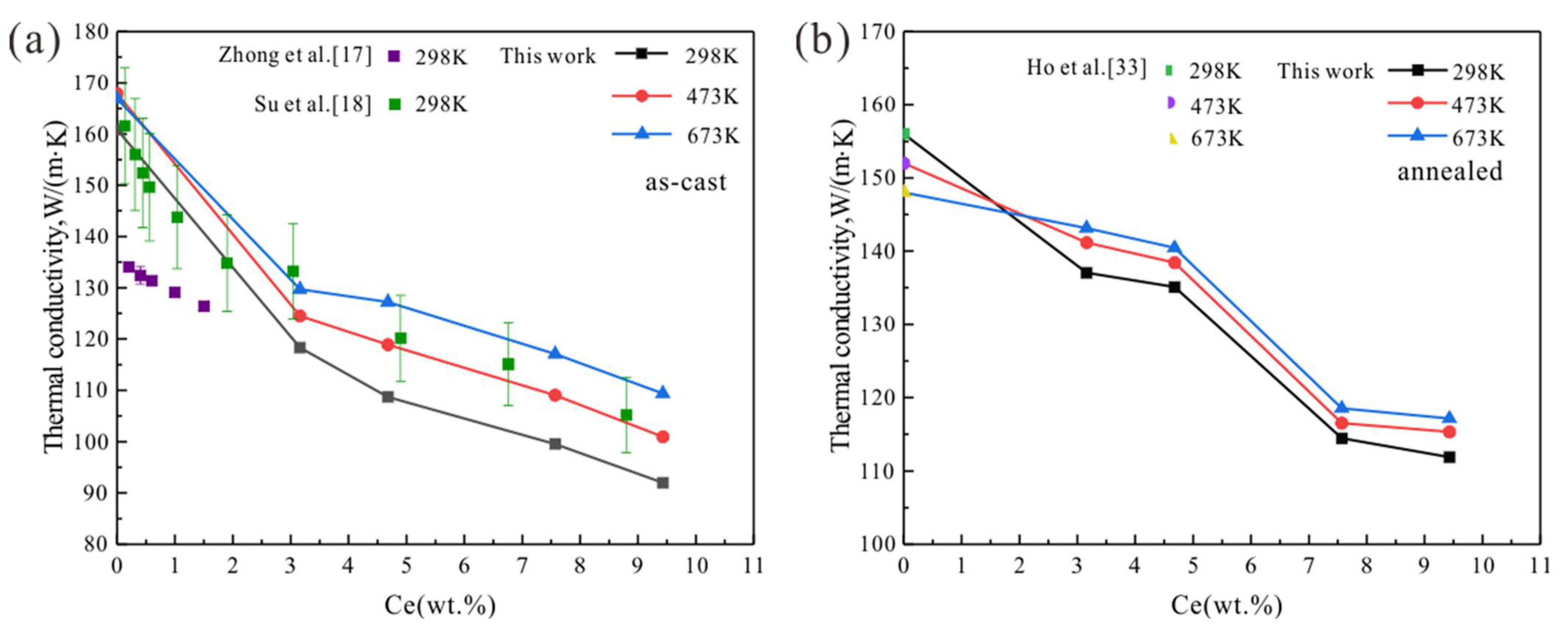
3.2. Mg-Ce Alloys
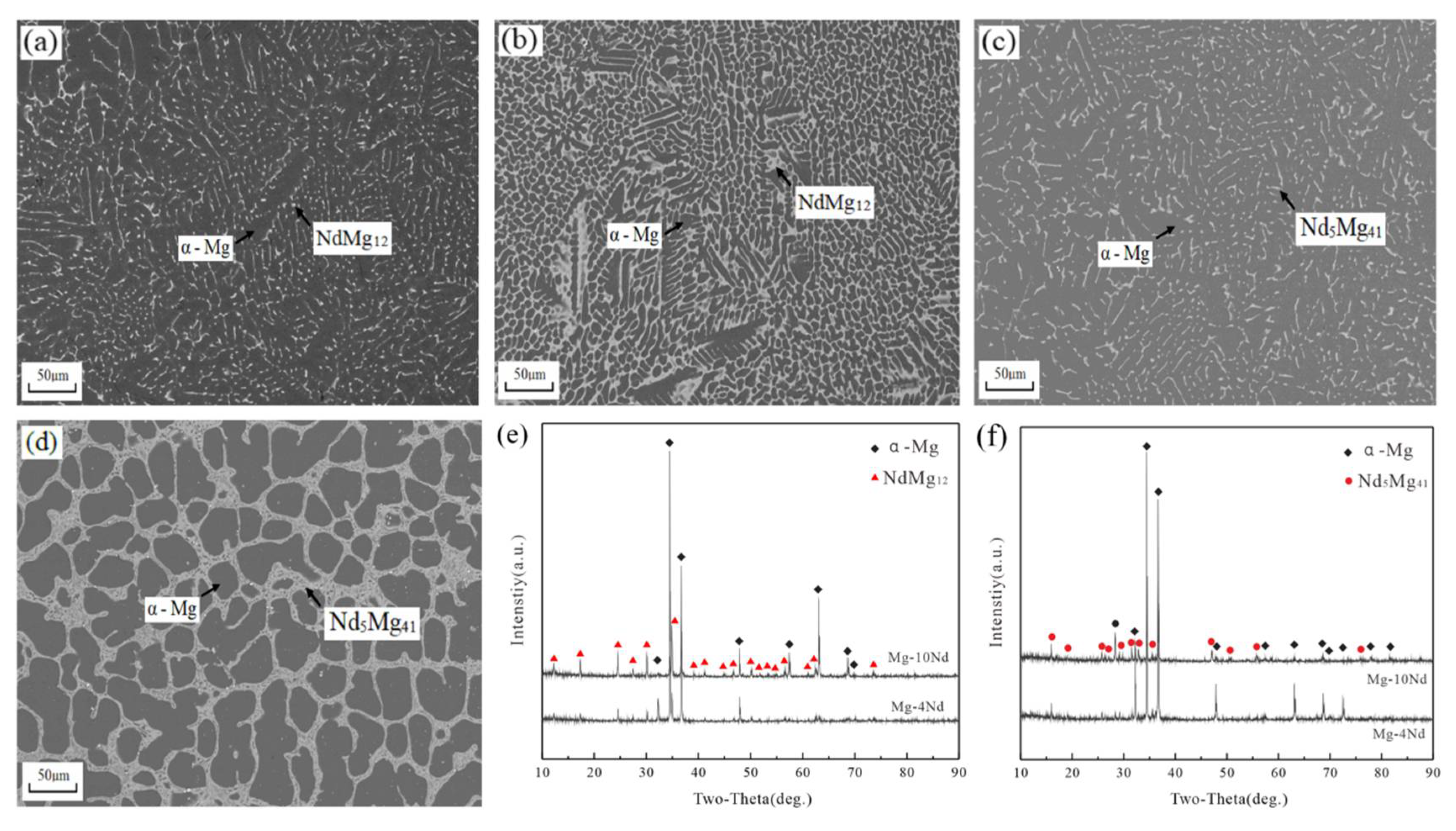
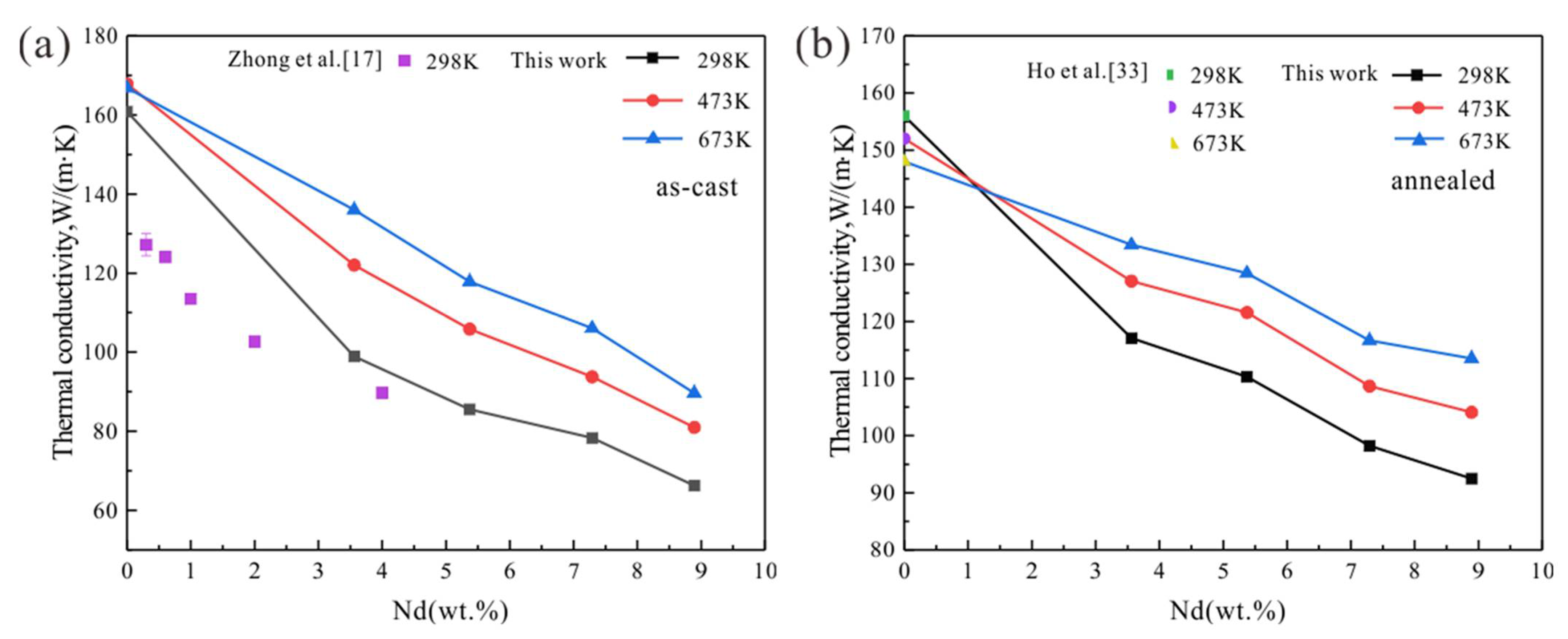
3.3. Mg-Nd Alloys
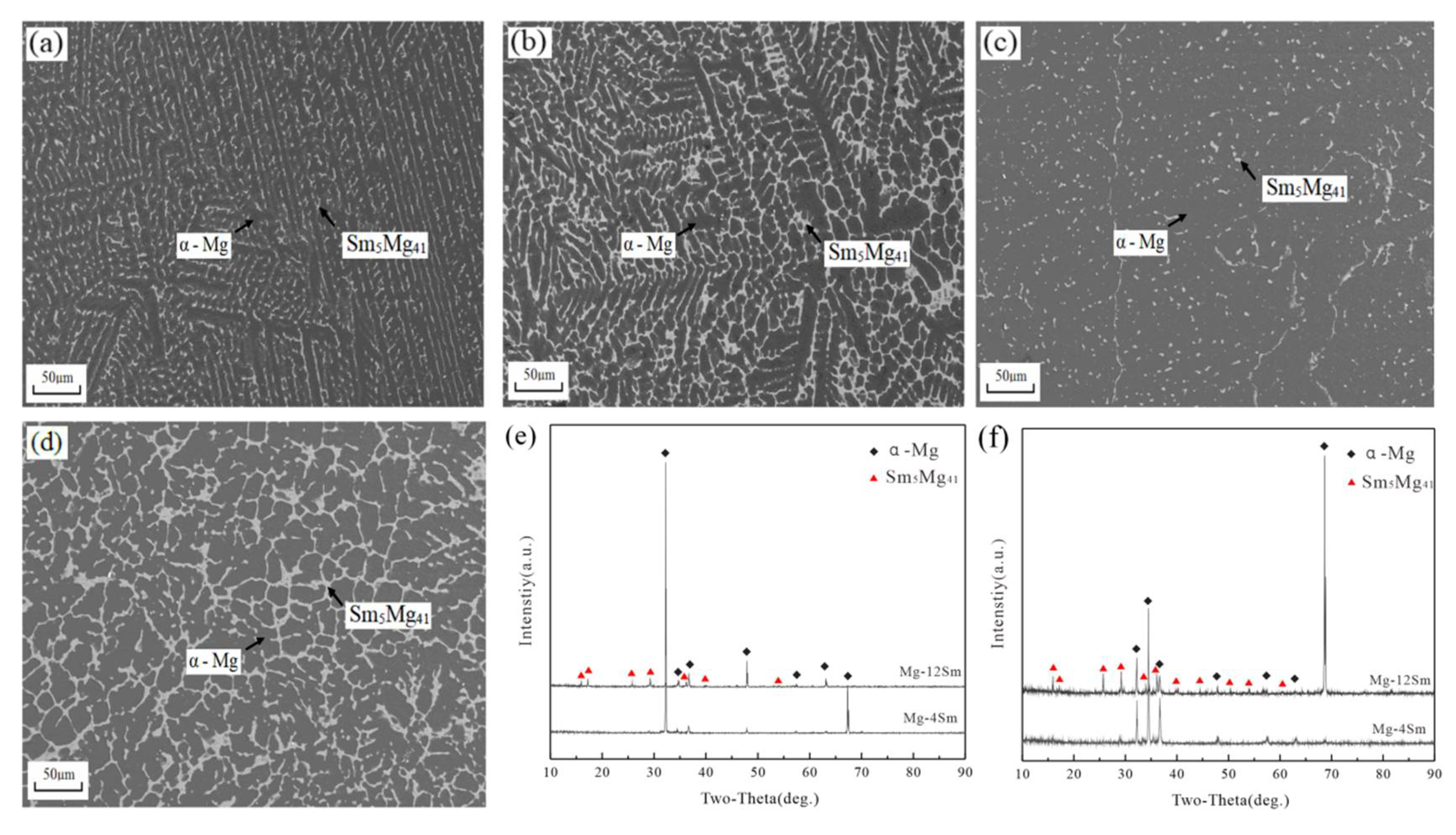
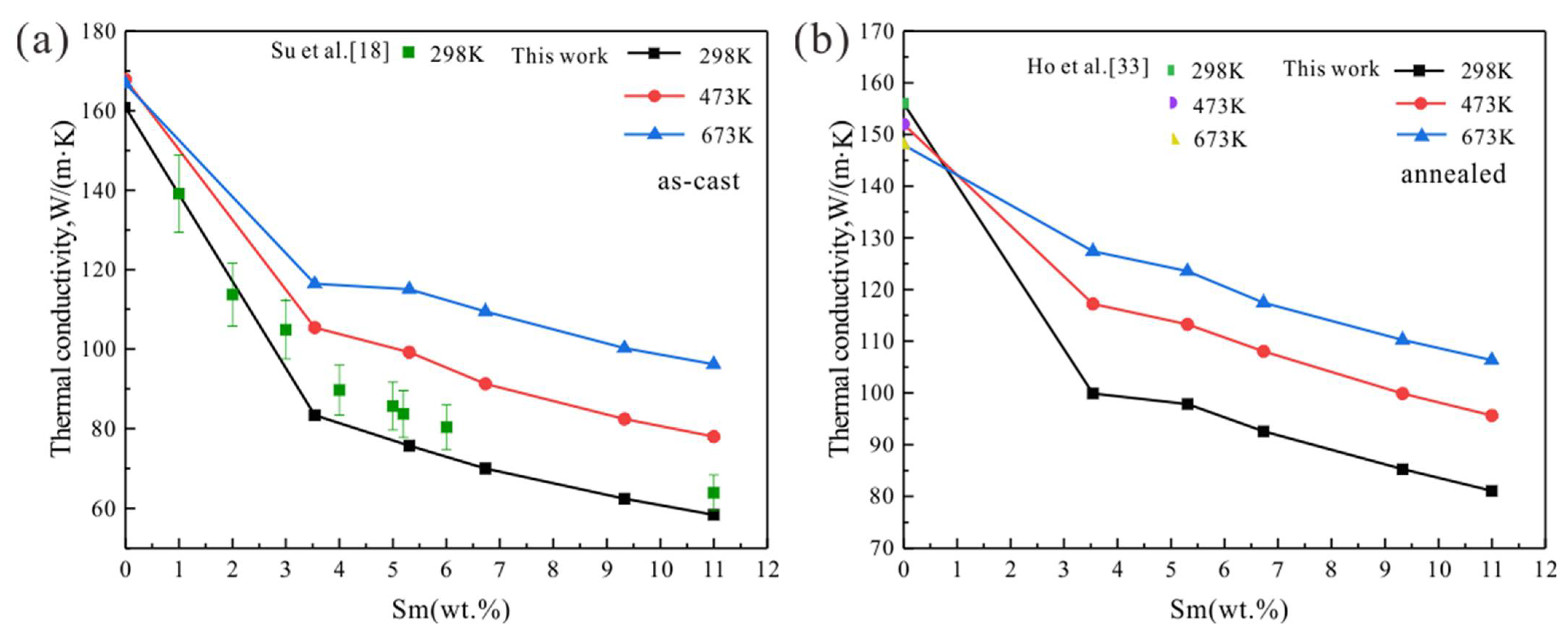
3.4. Mg-Sm Alloys
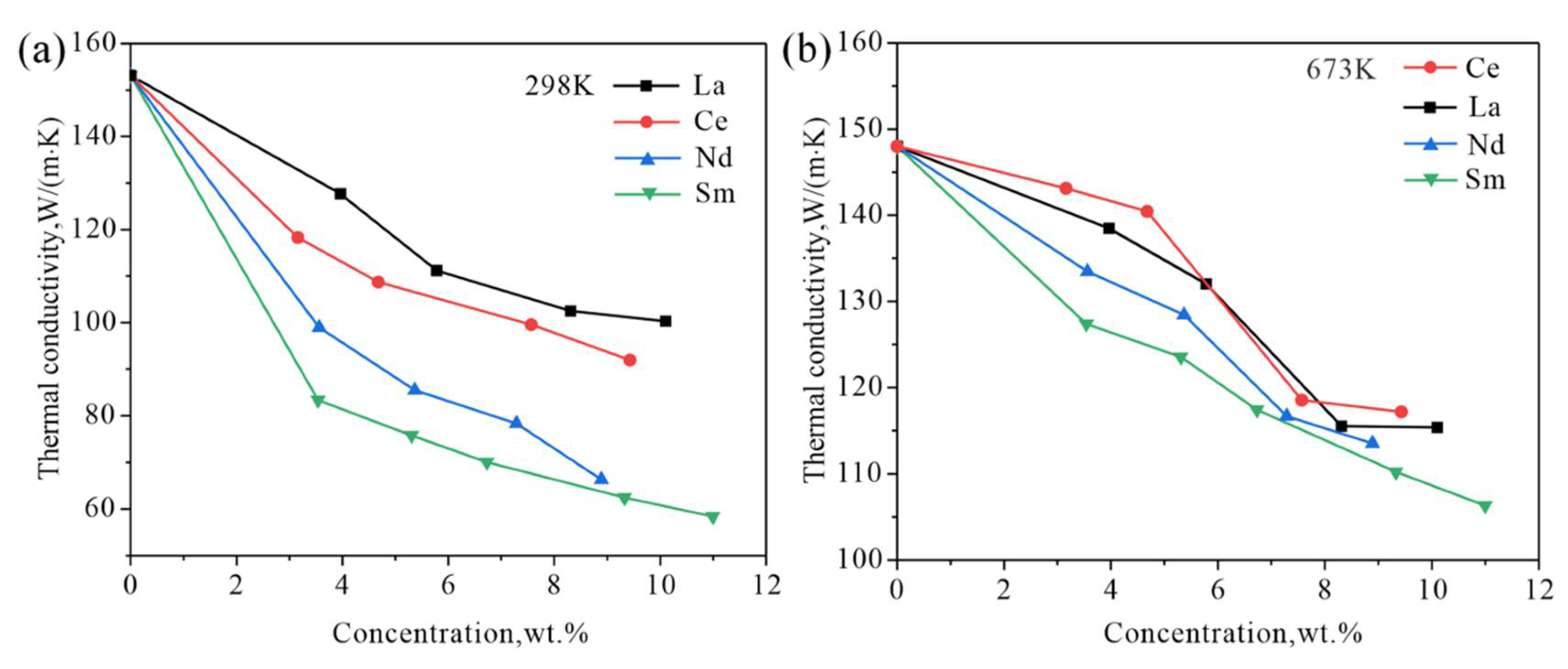
4. Discussion
4.1. Temperature Dependence
4.2. Effect of Homogenization Treatment
4.3. Effect of RE Elements
5. Conclusions
- (1)
- Thermal conductivity of the studied Mg-RE alloys decreases with RE addition, and increases with increasing temperature.
- (2)
- The annealed Mg-RE alloys featured higher thermal conductivity than the as-cast alloys at the same composition and temperature. Homogenization treatment can increase the thermal conductivity.
- (3)
- The solid solubility of Sm in α-Mg is the largest, and Mg-Sm alloy has the lowest thermal conductivity under the same conditions. The reduction of thermal conductivity caused by the addition of Nd and Sm, which show observable solid solubility in α-Mg, is significantly greater than the addition of La and Ce with negligible solid solubility in α-Mg.
Author Contributions
Funding
Conflicts of Interest
References
- Imandoust, A.; Barrett, C.D.; Al-Samman, T.; Inal, K.A.; EI Kadiri, H. A review on the effect of rare-earth elements on texture evolution during processing of magnesium alloys. J. Mater. Sci. 2016, 52, 1–29. [Google Scholar] [CrossRef]
- Suh, B.C.; Sasaki, T.; Nakata, T.; Kamado, S.; Hono, K. Effect of Ca on the microstructure, texture and mechanical properties in Mg–Zn–Mn based alloy. Magnes. Technol. 2017, 525–531. [Google Scholar] [CrossRef]
- Straumal, A.; Mazilkin, I.; Tzoy, K.; Straumal, B.; Bryła, K.; Baranchikov, A.; Eggeler, G. Bulk and Surface Low Temperature Phase Transitions in the Mg-Alloy EZ33A. Metals 2020, 10, 1127. [Google Scholar] [CrossRef]
- Rudajevová, A.; Buch, F.V.; Mordike, B.L. Thermal diffusivity and thermal conductivity of MgSc alloys. J. Alloys Compd. 1999, 292, 27–30. [Google Scholar] [CrossRef]
- Zhang, C.; Du, Y.; Liu, S.H.; Liu, Y.L.; Sundman, B. Thermal conductivity of the Al-Cu-Mg-Si alloys: Experimental measurement and CALPHAD modeling. Thermochim. Acta 2016, 635, 8–16. [Google Scholar] [CrossRef]
- Terada, Y.; Ohkubo, K.; Nakagawa, K.; Mohri, T.; Suzuki, T. Thermal conductivity of B2-type aluminides and titanides. Intermetallics 1995, 3, 347–355. [Google Scholar] [CrossRef]
- Peet, M.J.; Hasan, H.S.; Bhadeshia, H. Prediction of thermal conductivity of steel. Int. J. Heat Mass Tran. 2011, 54, 2602–2608. [Google Scholar] [CrossRef] [Green Version]
- Risegari, L.; Barucci, M.; Olivieri, E.; Pasca, E.; Ventura, G. Measurement of the thermal conductivity of copper samples between 30 and 150 mK. Cryogenics 2004, 44, 875–878. [Google Scholar] [CrossRef]
- Yang, W.S.; Xiu, Z.Y.; Chen, G.Q.; Wu, G.H. Microstructure and thermal conductivity of submicron Si3N4 reinforced 2024Al composite. Trans. Nonferrous Met. Soc. China 2009, 19, 378–381. [Google Scholar] [CrossRef]
- Gao, X.; Nie, J.F. Structure and thermal stability of primary intermetallic particles in an Mg–Zn casting alloy. Scr. Mater. 2007, 57, 655–658. [Google Scholar] [CrossRef]
- Yamasaki, M.; Kagao, S.; Kawamura, Y.; Yoshimura, K. Thermal diffusivity and conductivity of supercooled liquid in Zr41Ti14Cu12Ni10Be23 metallic glass. Appl. Phys. Lett. 2004, 84, 4653–4655. [Google Scholar] [CrossRef]
- Salter, J.A.M.; Charsley, P. The effect of grain size on the lattice thermal conductivity of copper aluminium alloys. Phys. Stat. Sol. 1967, 21, 357–368. [Google Scholar] [CrossRef]
- Pan, H.; Pan, F.; Yang, R.; Peng, J.; Zhao, C.; She, J.; Gao, Z.; Tang, A. Thermal and electrical conductivity of binary magnesium alloys. J. Mater. Sci. 2014, 49, 3107–3124. [Google Scholar] [CrossRef]
- Lyu, S.Y.; Li, G.D.; Hu, T.; Zheng, R.X.; Xiao, W.L.; Ma, C.L. A new cast Mg-Y-Sm-Zn-Zr alloy with high hardness. Mater. Lett. 2018, 217, 79–82. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Yang, L.; Dai, J.; Guo, G.L.; Liu, Z. Effect of Ca and Sr on microstructure and compressive creep property of Mg-4Al-RE alloys. Mater. Sci. Eng. A 2014, 610, 309–314. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, Y.; Huang, S.; Wei, Y.Y.; Xi, X.F.; Cai, K.Y.; Pan, F.S. Effects of Y on the microstructure, mechanical and bio-corrosion properties of Mg-Zn-Ca bulk metallic glass. J. Mater. Sci. Technol. 2014, 30, 1255–1261. [Google Scholar] [CrossRef]
- Zhong, L.P.; Peng, J.; Sun, S.; Wang, Y.J.; Lu, Y.; Pan, F.S. Microstructure and thermal conductivity of as-cast and as-solutionized Mg–Rare earth binary Alloys. J. Mater. Sci. Technol. 2017, 33, 1240–1248. [Google Scholar] [CrossRef]
- Su, C.Y.; Li, D.J.; Luo, A.A.; Ying, T.; Zeng, X.Q. Effect of solute atoms and second phases on the thermal conductivity of Mg-RE alloys: A quantitative study. J. Alloys Compd. 2018, 747, 431–437. [Google Scholar] [CrossRef]
- Peng, J.; Zhong, L.P.; Wang, Y.J.; Lu, Y.; Pan, F.S. Effect of extrusion temperature on the microstructure and thermal conductivity of Mg–2.0Zn–1.0Mn–0.2Ce alloys. Mater. Des. 2015, 87, 914–919. [Google Scholar] [CrossRef]
- Zhong, L.P.; Peng, J.; Li, M.; Wang, Y.J.; Lu, Y.; Pan, F.S. Effect of Ce addition on the microstructure, thermal conductivity and mechanical properties of Mg–0.5Mn alloys. J. Alloys Compd. 2016, 661, 402–410. [Google Scholar] [CrossRef]
- Mills, K.C. Recommended values of thermophysical properties for selected commercial alloys. Aircr. Eng. Aerosp. Technol. 2002, 74, 492–494. [Google Scholar]
- Touloukian, Y.S.; Buyco, E.H. Specific heat-metallic elements and alloys. In Thermophysical Properties of Matter—The TPRC Data Series; Touloukian, Y.S., Ho, C.Y., Eds.; Plenum Publishing Corp: New York, NY, USA, 1970; Volume 4, pp. 1–740. [Google Scholar]
- Leitner, J.; Voňka, P.; Sedmidubský, D.; Svoboda, P. Application of Neumann-Kopp rule for the estimation of heat capacity of mixed oxides. Thermochim. Acta 2010, 497, 7–13. [Google Scholar] [CrossRef]
- Rudajevová, A.; Lukác, P. Comparison of the thermal properties of AM20 and AS21 magnesium alloys. Mater. Sci. Eng. A 2005, 397, 16–21. [Google Scholar] [CrossRef]
- Du, Y.; Liu, S.H.; Zhang, L.J.; Xu, H.H.; Zhao, D.D.; Wang, A.J. An overview on phase equilibria and thermodynamic modeling in multicomponent Al alloys: Focusing on the Al-Cu-Fe-Mg-Mn-Ni-Si-Zn system. Calphad 2011, 35, 427–445. [Google Scholar] [CrossRef]
- Guo, C.P.; Du, Z.M.; Li, C.R. A thermodynamic description of the Ce–La–Mg system. Int. J. Mater. Res. 2010, 101, 1424–1431. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S.H.; Du, Y. Experimental investigation and thermodynamic modeling of the La–Mg system. J. Alloys Compd. 2016, 663, 279–288. [Google Scholar] [CrossRef]
- Qi, H.Y.; Huang, G.X.; Bo, H.; Xu, G.L.; Liu, L.B.; Jin, Z.P. Thermodynamic description of the Mg–Nd–Zn ternary system. J. Alloys Compd. 2011, 509, 3274–3281. [Google Scholar] [CrossRef]
- Guo, C.P.; Du, Z.M.; Li, C.R. A thermodynamic description of the Gd–Mg–Sm system. Calphad 2010, 34, 90–97. [Google Scholar] [CrossRef]
- Blumm, J. Measuring thermal conductivity. Ceram. Ind. 2002, 152, 53–59. [Google Scholar]
- Konyashin, I.; Lachmann, F.; Ries, B.; Mazilkin, A.A.; Straumal, B.B.; Kübel, C.; Llanes, L.; Baretzky, B. Strengthening zones in the Co matrix of WC–Co cemented carbides. Scr. Mater. 2014, 83, 17–20. [Google Scholar] [CrossRef]
- Mazilkin, I.; Tsoy, K.; Straumal, A.; Rodin, A.; Baretzky, B. Grain boundary wetting of different types of grain boundaries in the Cu-Ag system. Mater. Lett. 2020, 272, 127730. [Google Scholar] [CrossRef]
- Ho, C.Y.; Powell, R.W.; Liley, P.E. Thermal conductivity of the elements: A comprehensive review. J. Phys. Chem. Ref. Data 1974, 1, 1–796. [Google Scholar]
- Zhai, C.; Luo, Q.; Cai, Q.; Guan, R.G.; Li, Q. Thermodynamically analyzing the formation of Mg12Nd and Mg41Nd5 in Mg-Nd system under a static magnetic field. J. Alloys Compd. 2019, 773, 202–209. [Google Scholar] [CrossRef]
- Huang, L.; Liu, S.H.; Du, Y.; Zhang, C. Thermal conductivity of the Mg–Al–Zn alloys: Experimental measurement and CALPHAD modeling. Calphad 2018, 62, 99–108. [Google Scholar] [CrossRef]
- White, G.K. The thermal conductivity of gold at low temperatures. Proc. Phys. Soc. Sect. A 1953, 66, 559–564. [Google Scholar] [CrossRef]
- Ying, T.; Chi, H.; Zheng, M.; Li, Z.T.; Uher, C. Low-temperature electrical resistivity and thermal conductivity of binary magnesium alloys. Acta. Mater. 2014, 80, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Eivani, A.R.; Ahmed, H.; Zhou, J.; Duszczyk, J. Correlation between electrical resistivity, particle dissolution, precipitation of dispersoids, and recrystallization behavior of AA7020 aluminum alloy. Metall. Mater. Trans. 2009, 40, 2435–2446. [Google Scholar] [CrossRef] [Green Version]
| Nominal Composition of Allys (wt.%) | Actual Composition of RE (wt.%) | Actual Composition of RE (at.%) | Mg |
|---|---|---|---|
| Mg-4La | 3.96 | 0.71 | Balance |
| Mg-6La | 5.78 | 1.04 | |
| Mg-8La | 8.31 | 1.50 | |
| Mg-10La | 10.10 | 1.82 | |
| Mg-4Ce | 3.16 | 0.55 | |
| Mg-6Ce | 4.68 | 0.82 | |
| Mg-10Ce | 7.57 | 1.32 | |
| Mg-12Ce | 9.43 | 1.65 | |
| Mg-4Nd | 3.56 | 0.61 | |
| Mg-6Nd | 5.37 | 0.91 | |
| Mg-8Nd | 7.29 | 1.24 | |
| Mg-10Nd | 8.89 | 1.51 | |
| Mg-4Sm | 3.54 | 0.58 | |
| Mg-6Sm | 5.31 | 0.88 | |
| Mg-8Sm | 6.73 | 1.11 | |
| Mg-10Sm | 9.33 | 1.54 | |
| Mg-12Sm | 11.00 | 1.82 |
| Element | Atomic Number | Valence | Atomic Radius/nm | Maximum Solid Solubility wt.%/at.% in Mg |
|---|---|---|---|---|
| Mg | 12 | +2 | 0.1602 | — |
| La | 57 | +3 | 0.1877 | 5.55 × 10−7 ≈ 0 |
| Ce | 58 | +3 | 0.1824 | 0.52/0.09 |
| Nd | 60 | +3 | 0.1821 | 3.63/0.63 |
| Sm | 62 | +3 | 0.1804 | 5.83/0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Liu, S.; Huang, L.; Wang, D.; Du, Y.; Chu, M. Thermal Conductivity of As-Cast and Annealed Mg-RE Binary Alloys. Metals 2021, 11, 554. https://doi.org/10.3390/met11040554
Guo H, Liu S, Huang L, Wang D, Du Y, Chu M. Thermal Conductivity of As-Cast and Annealed Mg-RE Binary Alloys. Metals. 2021; 11(4):554. https://doi.org/10.3390/met11040554
Chicago/Turabian StyleGuo, Huiyun, Shuhong Liu, Lei Huang, Deqing Wang, Yong Du, and Mingqiang Chu. 2021. "Thermal Conductivity of As-Cast and Annealed Mg-RE Binary Alloys" Metals 11, no. 4: 554. https://doi.org/10.3390/met11040554







