Effect of Hydrogen on the Tensile Behavior of Austenitic Stainless Steels 316L Produced by Laser-Powder Bed Fusion
Abstract
1. Introduction
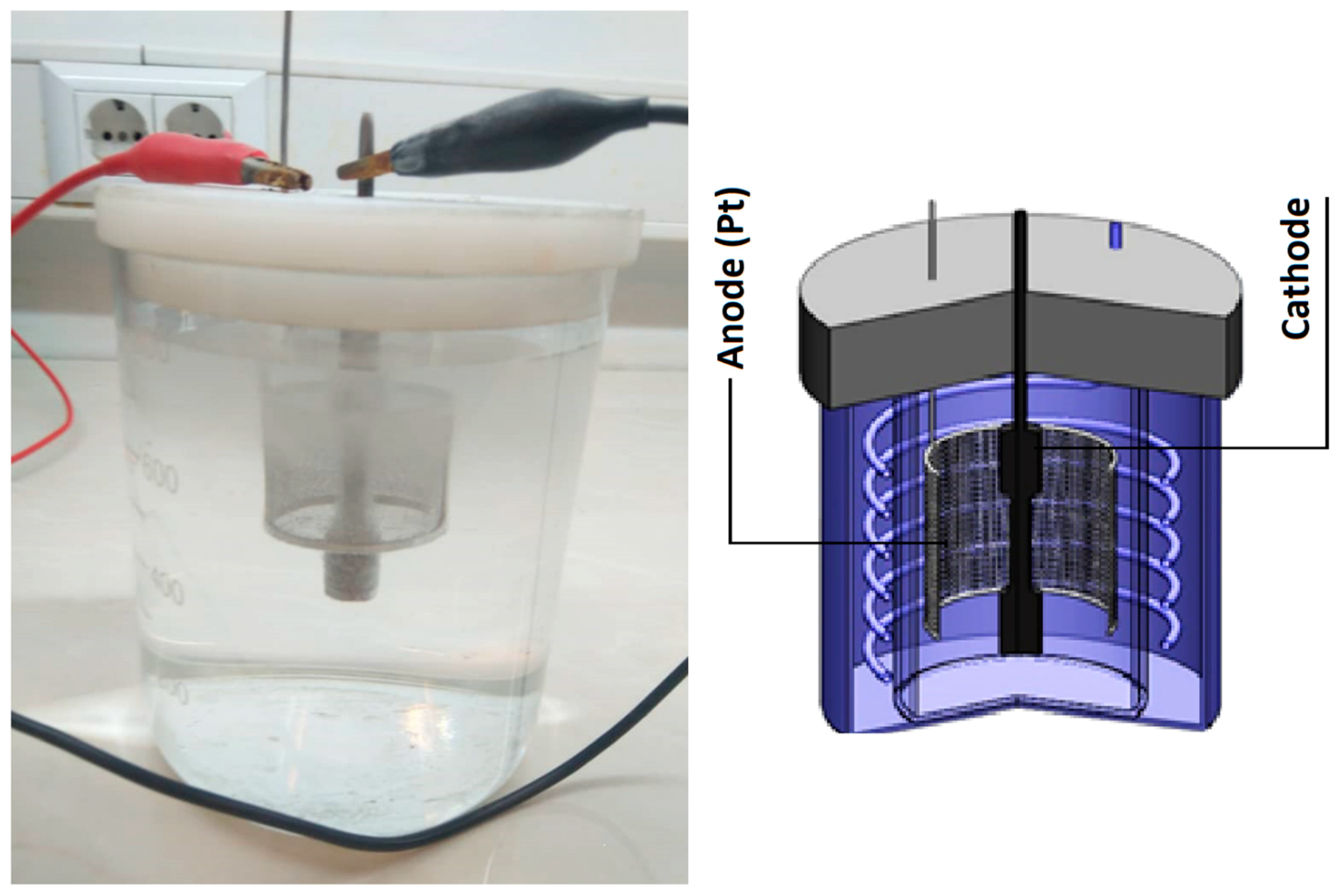
2. Materials and Methods
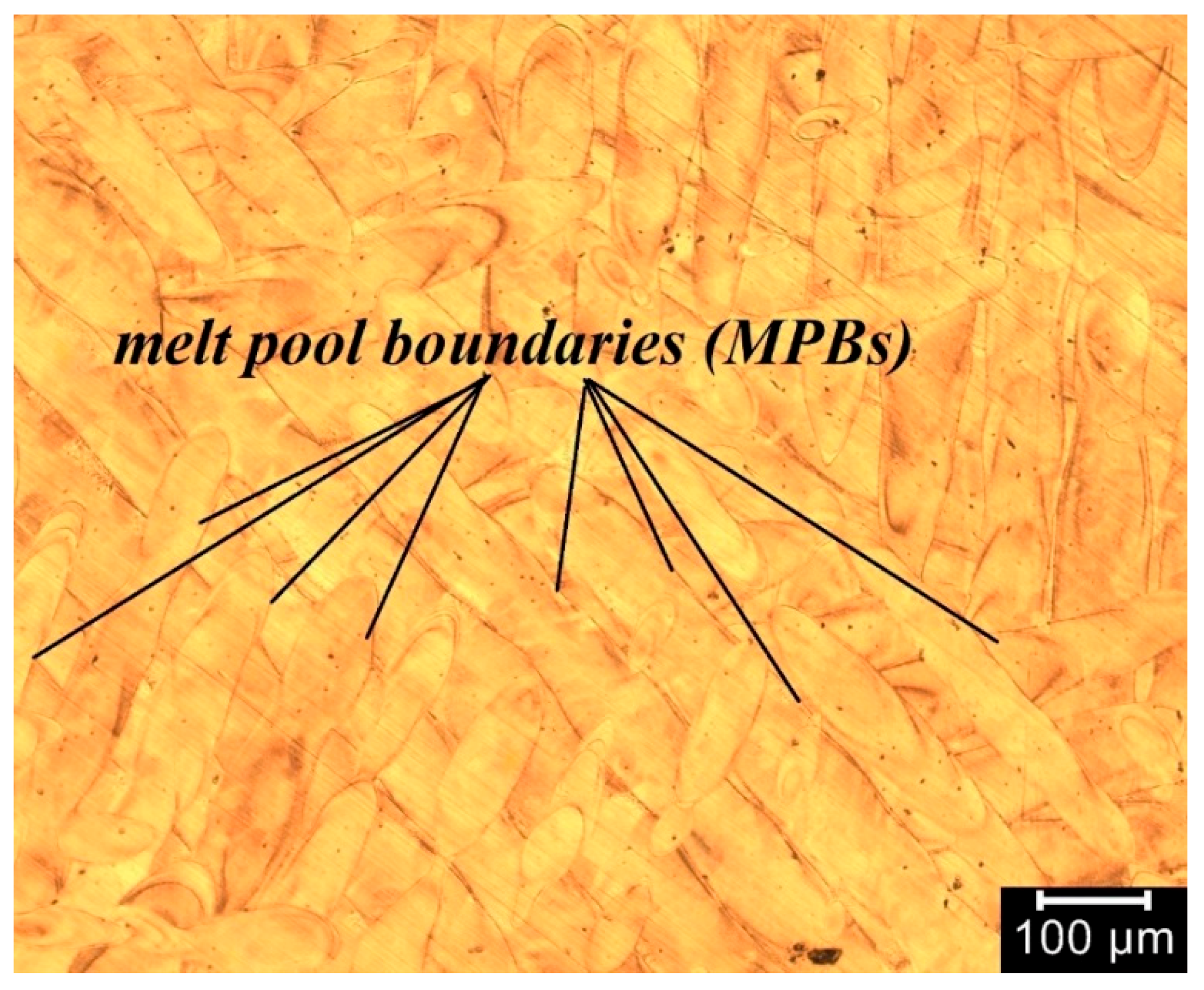
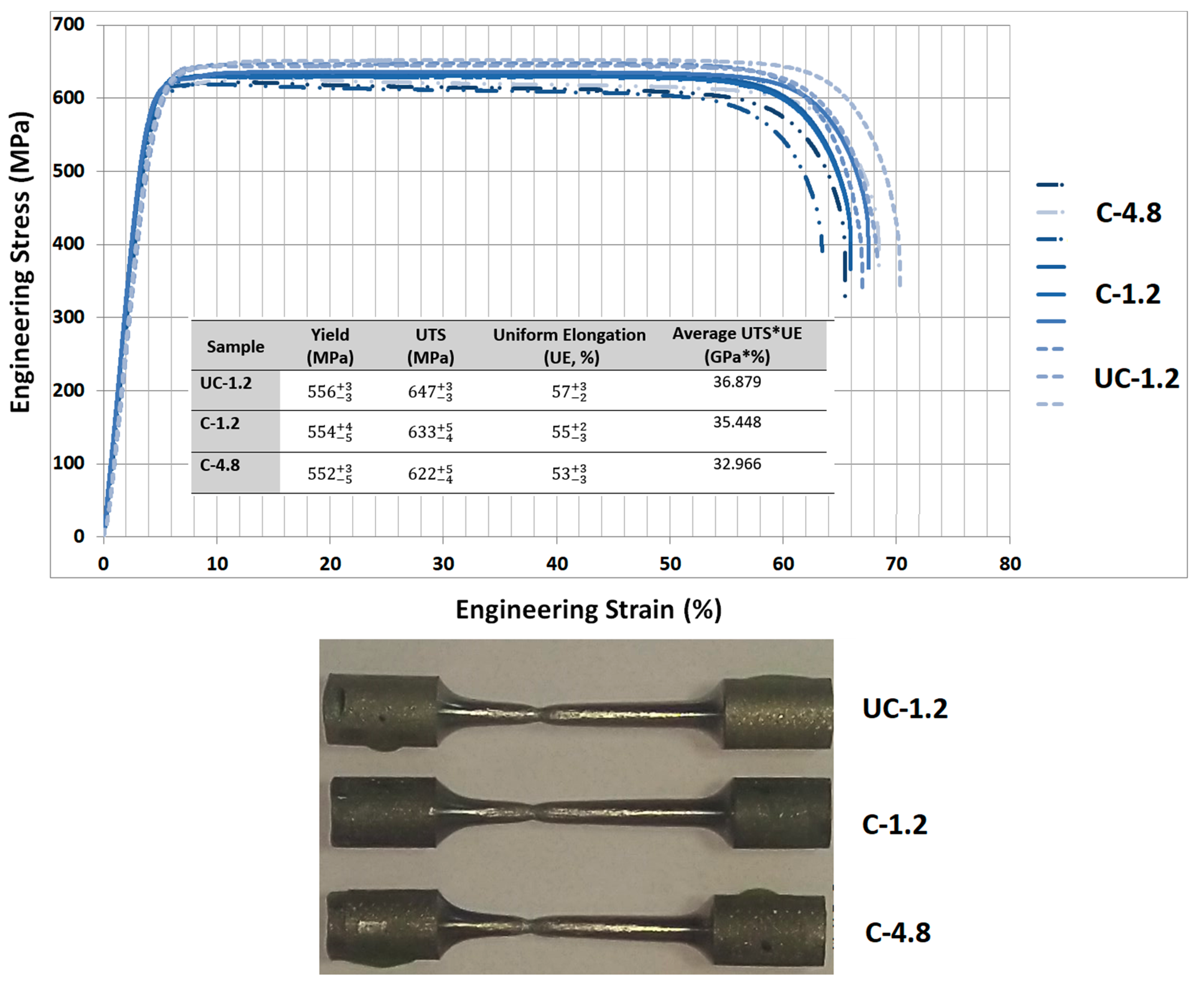
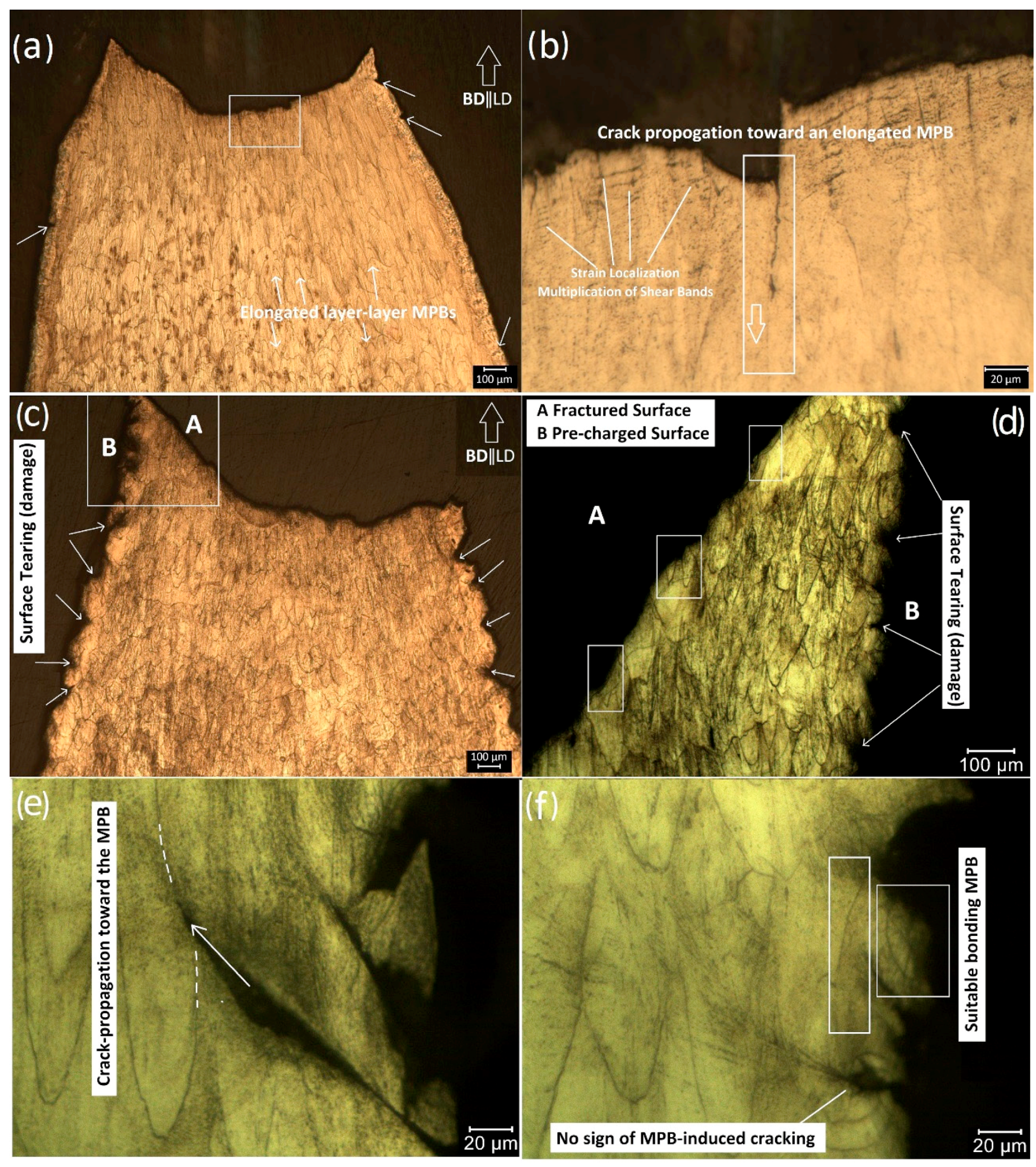
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbol | Meaning |
| 3D | 3-Dimensional |
| ASS | Austenitic Stainless Steels |
| CAD | Computer-Aided Design |
| ED | Energy Density |
| EDS | Energy Dispersive X-Ray Spectroscopy |
| EL | Elongation |
| UE | Uniform Elongation |
| HE | Hydrogen Embrittlement |
| HELP | Hydrogen-Enhanced Localized Plasticity |
| L-PBF | Laser-Powder Bed Fusion |
| L-PBFed | Laser-Powder Bed Fusioned |
| MPB | Melt Pool Boundary |
| OM | Optical microscopy |
| SEM | Scanning Electron Microscopy |
| SSs | Stainless Steels |
| UTS | Ultimate Tensile Strength |
| XRD | X-Ray Diffraction |
| YS | Yield Strength |
References
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components—Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Mostafaei, A.; Heidarzadeh, A.; Brabazon, D. Additive Manufacturing of Metal Matrix Composites; Elsvier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- He, J.; Chen, L.; Guo, Z.; Zhi, H.; Antonov, S.; Su, Y. A novel 13Cr austenitic stainless steel with excellent mechanical properties and high hydrogen embrittlement resistance via heterostructure and TRIP effects. Mater. Sci. Eng. A 2020, 793, 139835. [Google Scholar] [CrossRef]
- Cunningham, C.R.; Dhokia, V.; Shokrani, A.; Newman, S.T. Effects of in-process LN2 cooling on the microstructure and mechanical properties of type 316L stainless steel produced by wire arc directed energy deposition. Mater. Lett. 2021, 282, 128707. [Google Scholar] [CrossRef]
- Bertoli, U.S.; MacDonald, B.E.; Schoenung, J.M. Stability of cellular microstructure in laser powder bed fusion of 316L stainless steel. Mater. Sci. Eng. A 2019, 739, 109–117. [Google Scholar] [CrossRef]
- Li, Z.; Voisin, T.; McKeown, J.T.; Ye, J.; Braun, T.; Kamath, C.; King, W.E.; Wang, Y.M. Tensile properties, strain rate sensitivity, and activation volume of additively manufactured 316L stainless steels. Int. J. Plast. 2019, 120, 395–410. [Google Scholar] [CrossRef]
- Xiaobing, L.; Ming, G.; Haoze, L.; Weiwei, X.; Long, Z.; Lei, S.; Xiujuan, Z.; Yingche, M.; Kui, L. Effect of residual hydrogen content on the tensile properties and crack propagation behavior of a type 316 stainless steel. Int. J. Hydrogen Energy 2019, 44, 25054–25063. [Google Scholar] [CrossRef]
- Hu, X.; Lach, T.G.; Terrani, K.A. Deuterium permeation and retention in 316L Stainless Steel Manufactured by Laser Powder Bed Fusion. J. Nucl. Mater. 2021, 548, 152871. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Zhang, L.; Luo, H.; Li, R.; Wang, L.; Man, C.; Li, X. Superior resistance to hydrogen damage for selective laser melted 316L stainless steel in a proton exchange membrane fuel cell environment. Corros. Sci. 2020, 166, 108425. [Google Scholar] [CrossRef]
- Nicho, K.; Yokoyama, K.I. Marked Degradation of Tensile Properties Induced by Plastic Deformation after Interactions between Strain-Induced Martensite Transformation and Hydrogen for Type 316L Stainless Steel. Metals 2020, 10, 928. [Google Scholar] [CrossRef]
- Huang, S.; Ma, D.; Sheng, J.; Agyenim-Boateng, E.; Zhao, J.; Zhou, J. Effects of laser peening on tensile properties and martensitic transformation of AISI 316L stainless steel in a hydrogen-rich environment. Mater. Sci. Eng. A 2020, 788, 139543. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E.; Harris, Z.D.; Fuentes-Alonso, S.; Scully, J.R.; Burns, J.T. On the suitability of slow strain rate tensile testing for assessing hydrogen embrittlement susceptibility. Corros. Sci. 2020, 163, 108291. [Google Scholar] [CrossRef]
- Komatsu, A.; Fujinami, M.; Hatano, M.; Matsumoto, K.; Sugeoi, M.; Chiari, L. Straining-temperature dependence of vacancy behavior in hydrogen-charged austenitic stainless steel 316L. Int. J. Hydrogen Energy 2021, 46, 6960–6969. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, X.; Tao, X.; Su, Y.; Qiao, L. Effect of dislocation cell walls on hydrogen adsorption, hydrogen trapping and hydrogen embrittlement resistance. Corros. Sci. 2020, 166, 108428. [Google Scholar] [CrossRef]
- Park, J.-M.; Zhao, Y.; Voisin, T.; Lee, D.-H.; Komazaki, S.-I.; Ko, Y.; Kim, D.-I.; Suh, J.-Y.; Han, H.N.; Wang, Y.M.; et al. Hydrogen uptake and its influence in selective laser melted austenitic stainless steel: A nanoindentation study. Scr. Mater. 2021, 194, 113718. [Google Scholar] [CrossRef]
- Baek, S.-W.; Song, E.J.; Kim, J.H.; Jung, M.; Baek, U.B.; Nahm, S.H. Hydrogen embrittlement of 3-D printing manufactured austenitic stainless steel part for hydrogen service. Scr. Mater. 2017, 130, 87–90. [Google Scholar] [CrossRef]
- Lee, D.-H.; Sun, B.; Lee, S.; Ponge, D.; Jägle, E.A.; Raabe, D. Comparative study of hydrogen embrittlement resistance between additively and conventionally manufactured 304L austenitic stainless steels. Mater. Sci. Eng. A 2021, 803, 140499. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Y.; Zhang, Q.; Ge, J.; Ji, S.; Li, W.; Liang, J. Microstructural heterogeneity of AlSi10Mg alloy lattice structures fabricated by selective laser melting: Phenomena and mechanism. J. Alloys Compd. 2020, 833, 155071. [Google Scholar] [CrossRef]
- Shibayama, Y.; Hojo, T.; Akiyama, E. An Evaluation Method for Hydrogen Embrittlement of High Strength Steel Sheets Using U-bend Specimens. ISIJ Int. 2020. [Google Scholar] [CrossRef]
- Mertens, G.; Duprez, L.; de Cooman, B.C.; Verhaege, M. Advanced Materials Research. Trans. Tech. Publ. 2007, 15–17, 816–821. [Google Scholar]
- Takagi, S.; Toji, Y. Application of NH4SCN Aqueous Solution to Hydrogen Embrittlement Resistance Evaluation of Ultra-high Strength Steels. ISIJ Int. 2012, 52, 329–331. [Google Scholar] [CrossRef]
- Chatzidouros, E.V.; Traidia, A.; Devarapalli, R.S.; Pantelis, D.I.; Steriotis, T.A.; Jouiad, M. Effect of hydrogen on fracture toughness properties of a pipeline steel under simulated sour service conditions. Int. J. Hydrogen Energy 2018, 43, 5747–5759. [Google Scholar] [CrossRef]
- Park, I.-J.; Jung, J.-G.; Jo, S.Y.; Lee, S.-M.; Lee, Y.-K. The Effect of Pre-Strain on the Resistance to Hydrogen Embrittlement in 316L Austenitic Stainless Steel. Mater. Trans. 2014. [Google Scholar] [CrossRef]
- Valentini, R.; Tedesco, M.M.; Corsinovi, S.; Bacchi, L.; Villa, M. Investigation of mechanical tests for hydrogen embrittlement in automotive PHS steels. Metals 2019, 9, 934. [Google Scholar] [CrossRef]
- Gong, P.; Katzarov, I.; Nutter, J.; Paxton, A.T.; Wynne, B.; Rainforth, W.M. Hydrogen suppression of dislocation cell formation in micro and nano indentation of pure iron single crystals. Scr. Mater. 2021, 194, 113683. [Google Scholar] [CrossRef]
- Ajito, S.; Hojo, T.; Koyama, M.; Akiyama, E. Effects of Ammonium Thiocyanate and pH of Aqueous Solutions on Hydrogen Absorption into Iron under Cathodic Polarization. ISIJ Int. 2021. [Google Scholar] [CrossRef]
- Takashima, K.; Yoshioka, Y.; Yokoyama, K.I.; Funakawa, Y. Hydrogen Embrittlement Behavior of Ultra-high Strength Dual Phase Steel Sheet under Sustained Tensile-loading Test. ISIJ Int. 2018, 58, 173–178. [Google Scholar] [CrossRef]
- Kim, H.-J. Effects of Prior Austenite Grain Size on Hydrogen Delayed Fracture of Hot-Stamped Boron Martensitic Steel. Metall. Mater. Trans. A 2020, 51, 237–251. [Google Scholar] [CrossRef]
- Shibayama, Y.; Hojo, T.; Koyama, M.; Saitoh, H.; Shiro, A.; Yasuda, R.; Shobu, T.; Matsuno, T.; Akiyama, E. Effects of Stress and Plastic Strain on Hydrogen Embrittlement Fracture of a U-bent Martensitic Steel Sheet. ISIJ Int. 2021. [Google Scholar] [CrossRef]
- Ichiba, M.; Takai, K.; Sakai, J.I. Effects of Test Conditions on Corrosion Reactions and Hydrogen Absorption in Hydrogen Embrittlement Tests Using an Ammonium Thiocyanate Solution. ISIJ Int. 2016, 56, 397–404. [Google Scholar] [CrossRef]
- Sofinowski, K.A.; Raman, S.; Wang, X.; Gaskey, B.; Seita, M. Layer-wise engineering of grain orientation (LEGO) in laser powder bed fusion of stainless steel 316L. Addit. Manuf. 2021, 38, 101809. [Google Scholar] [CrossRef]
- Voisin, T.; Forien, J.-B.; Perron, A.; Aubry, S.; Bertin, N.; Samanta, A.; Baker, A.; Wang, Y.M. New insights on cellular structures strengthening mechanisms and thermal stability of an austenitic stainless steel fabricated by laser powder-bed-fusion. Acta Mater. 2021, 203, 116476. [Google Scholar] [CrossRef]
- Wang, Y.M.; Voisin, T.; McKeown, J.T.; Ye, J.; Calta, N.P.; Li, Z.; Zeng, Z.; Zhang, Y.; Chen, W.; Roehling, T.T.; et al. Additively manufactured hierarchical stainless steels with high strength and ductility. Nat. Mater. 2018, 17, 63–71. [Google Scholar] [CrossRef]
- Liu, L.; Ding, Q.; Zhong, Y.; Zou, J.; Wu, J.; Chiu, Y.-L.; Li, J.; Zhang, Z.; Yu, Q.; Shen, Z. Dislocation network in additive manufactured steel breaks strength-ductility trade-off. Mater. Today 2018, 21, 354–361. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Q.; Venezuela, J.; Zhang, M.; Atrens, A. Hydrogen influence on some advanced high-strength steels. Corros. Sci. 2017, 125, 114–138. [Google Scholar] [CrossRef]
- Cui, L.; Jiang, S.; Xu, J.; Peng, R.L.; Mousavian, R.T.; Moverare, J. Revealing relationships between microstructure and hardening nature of additively manufactured 316L stainless steel. Mater. Des. 2021, 198, 109385. [Google Scholar] [CrossRef]
- Koyama, M.; Akiyama, E.; Tsuzaki, K.; Raabe, D. Hydrogen-assisted failure in a twinning-induced plasticity steel studied under in situ hydrogen charging by electron channeling contrast imaging. Acta Mater. 2013, 61, 4607–4618. [Google Scholar] [CrossRef]
- Kazum, O.; Beladi, H.; Kannan, M.B. Hydrogen permeation in twinning-induced plasticity (TWIP) steel. Int. J. Hydrogen Energy 2018, 43, 22685–22693. [Google Scholar] [CrossRef]




| Chemical Compositions | ||||||||||
| Material | C | Mn | P | S | Cr | Mo | Ni | Si | Cu | Fe |
| L-PBFed SS316L | 0.025 | 0.76 | 0.009 | 0.010 | 17.30 | 2.30 | 12.90 | 0.642 | 0.026 | Bal. |
| Condition of Experiments | ||||||||||
| Sample | Strain Rate (S−1) | |||||||||
| Uncharged, UC-1.2 | 1.2 × 10−4 | |||||||||
| Charged, C-1.2 | 1.2 × 10−4 | |||||||||
| Charged, C-4.8 | 4.8 × 10−4 | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaleghifar, F.; Razeghi, K.; Heidarzadeh, A.; Taherzadeh Mousavian, R. Effect of Hydrogen on the Tensile Behavior of Austenitic Stainless Steels 316L Produced by Laser-Powder Bed Fusion. Metals 2021, 11, 586. https://doi.org/10.3390/met11040586
Khaleghifar F, Razeghi K, Heidarzadeh A, Taherzadeh Mousavian R. Effect of Hydrogen on the Tensile Behavior of Austenitic Stainless Steels 316L Produced by Laser-Powder Bed Fusion. Metals. 2021; 11(4):586. https://doi.org/10.3390/met11040586
Chicago/Turabian StyleKhaleghifar, Farzaneh, Khashayar Razeghi, Akbar Heidarzadeh, and Reza Taherzadeh Mousavian. 2021. "Effect of Hydrogen on the Tensile Behavior of Austenitic Stainless Steels 316L Produced by Laser-Powder Bed Fusion" Metals 11, no. 4: 586. https://doi.org/10.3390/met11040586
APA StyleKhaleghifar, F., Razeghi, K., Heidarzadeh, A., & Taherzadeh Mousavian, R. (2021). Effect of Hydrogen on the Tensile Behavior of Austenitic Stainless Steels 316L Produced by Laser-Powder Bed Fusion. Metals, 11(4), 586. https://doi.org/10.3390/met11040586






